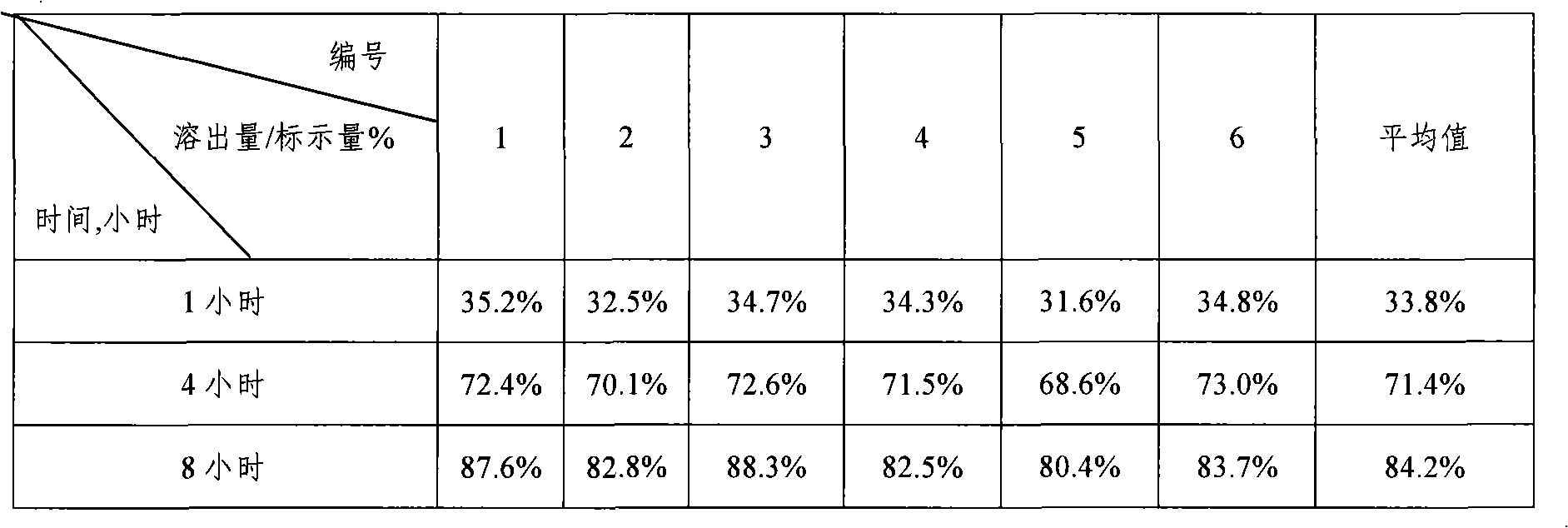
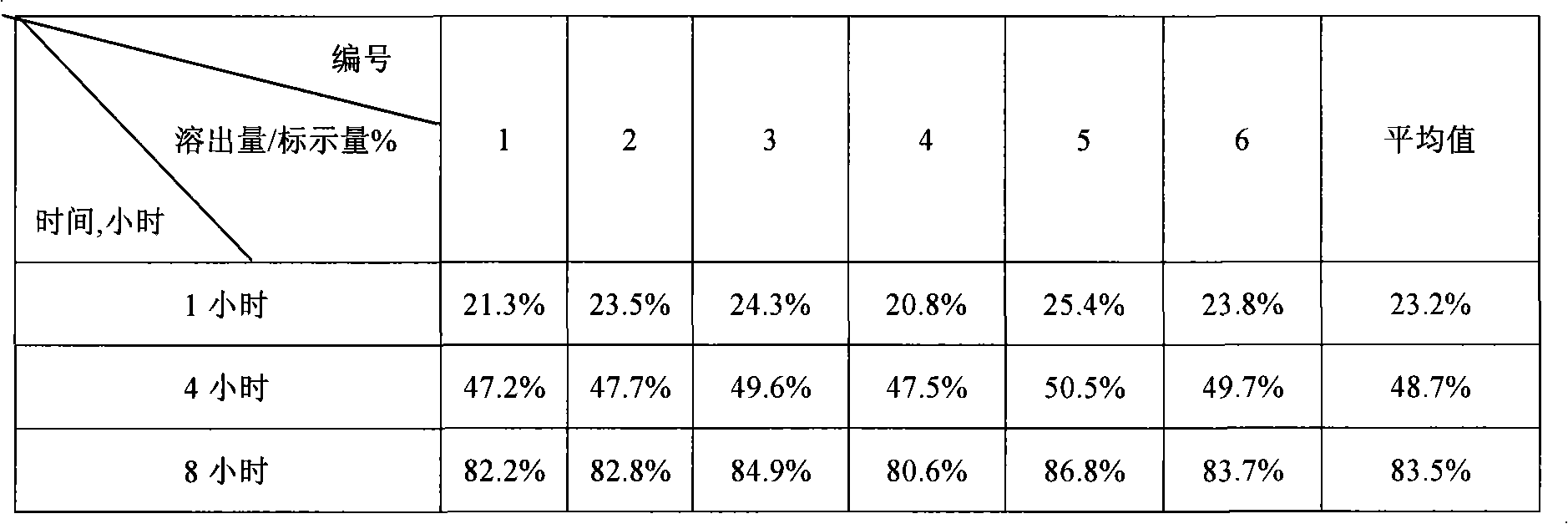
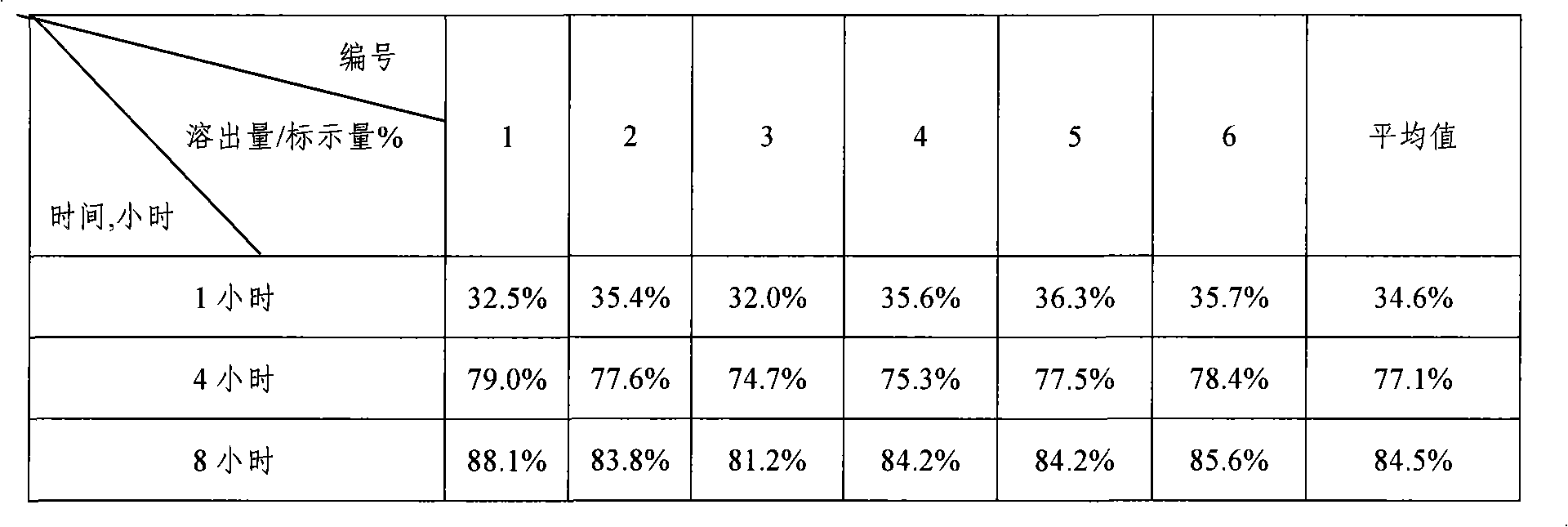
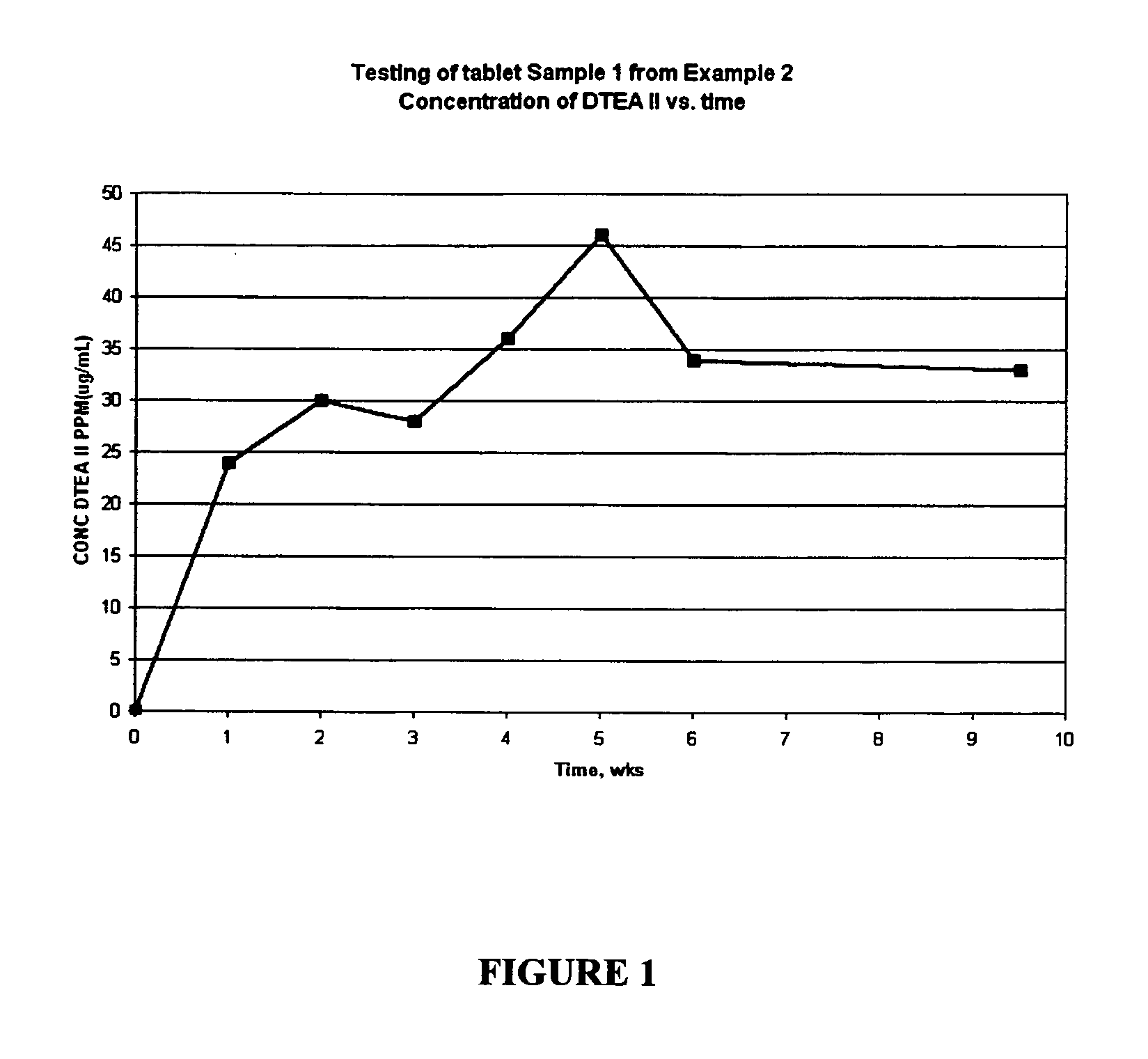
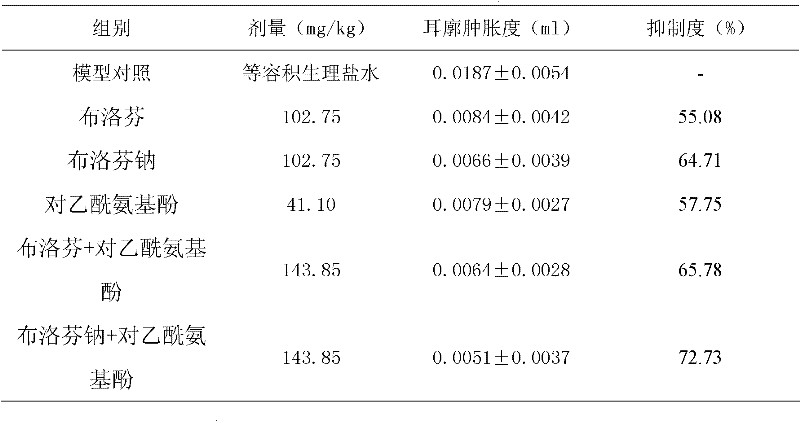
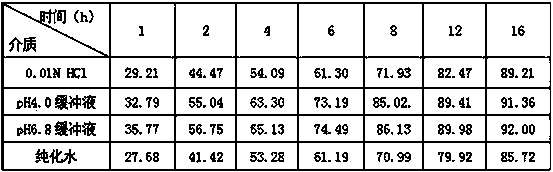
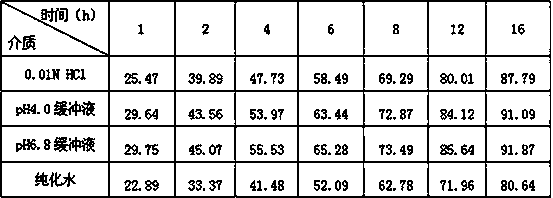
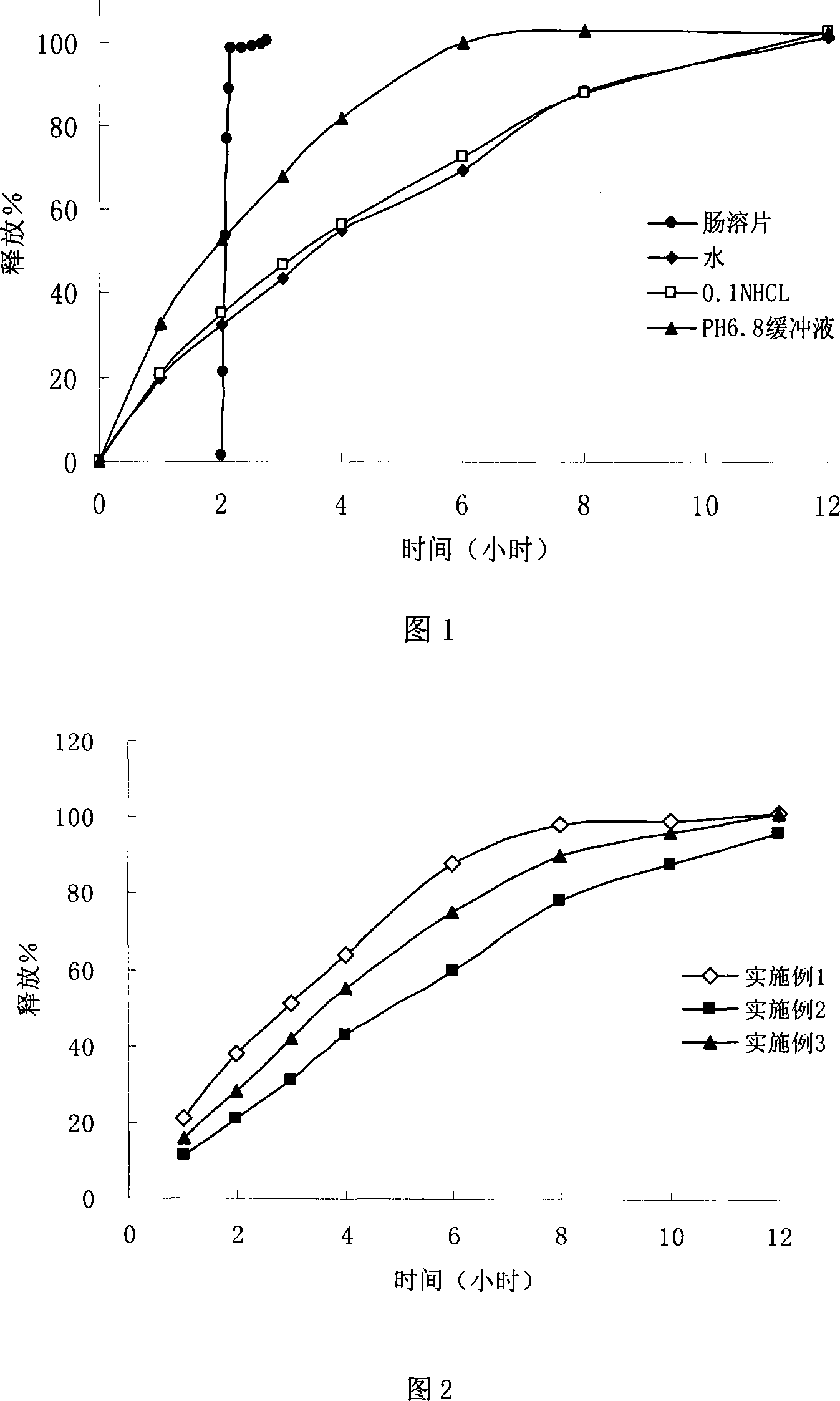
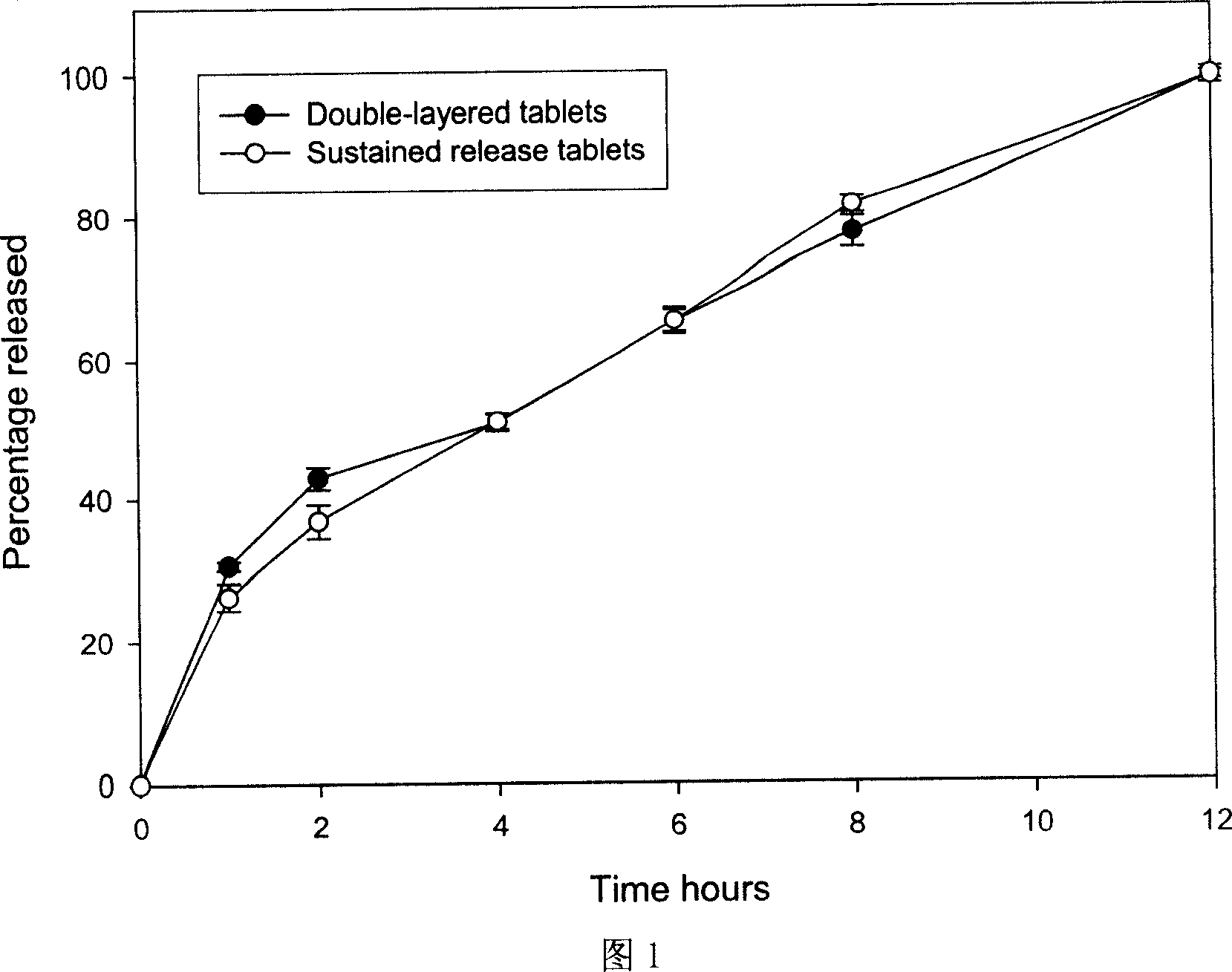
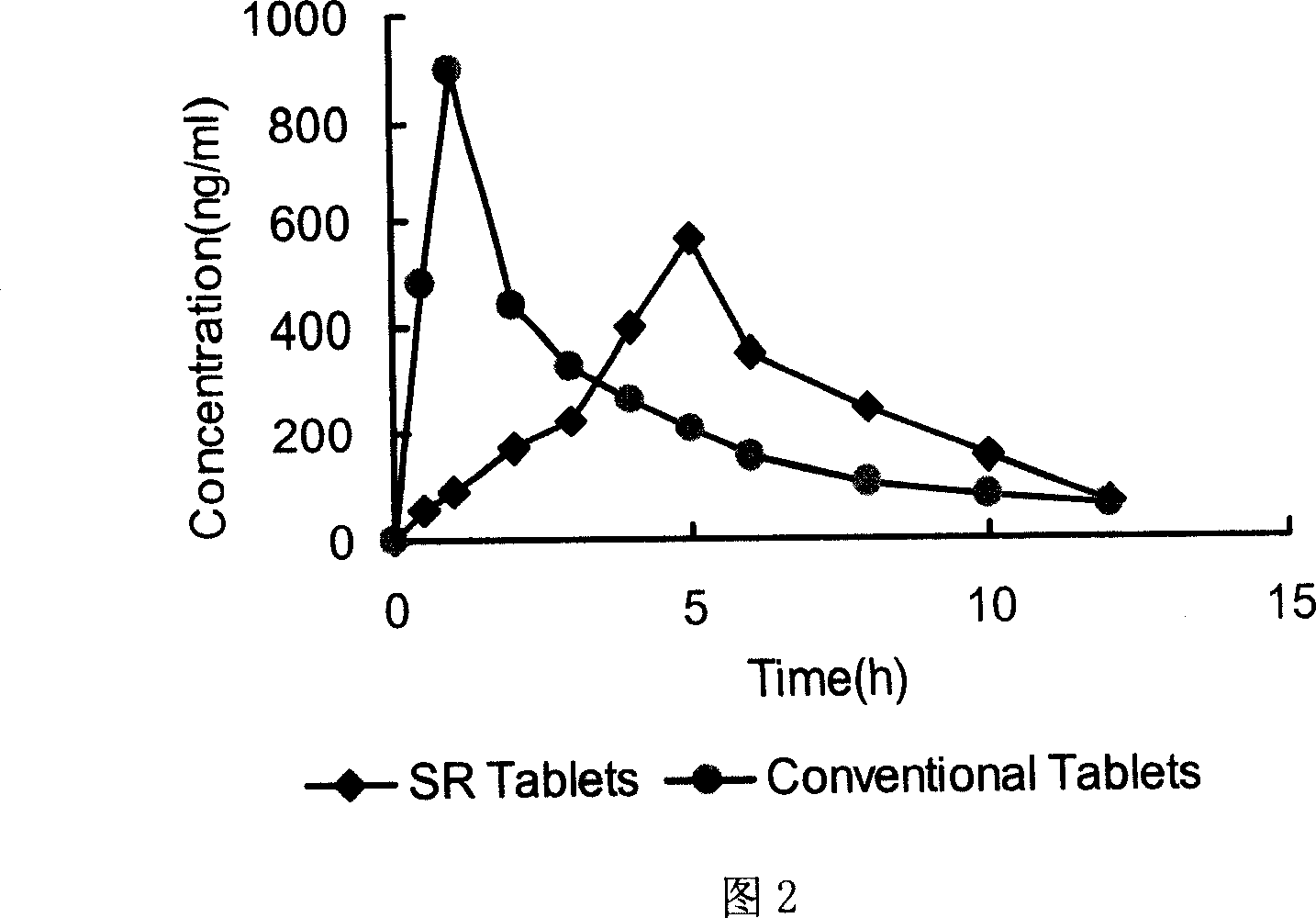
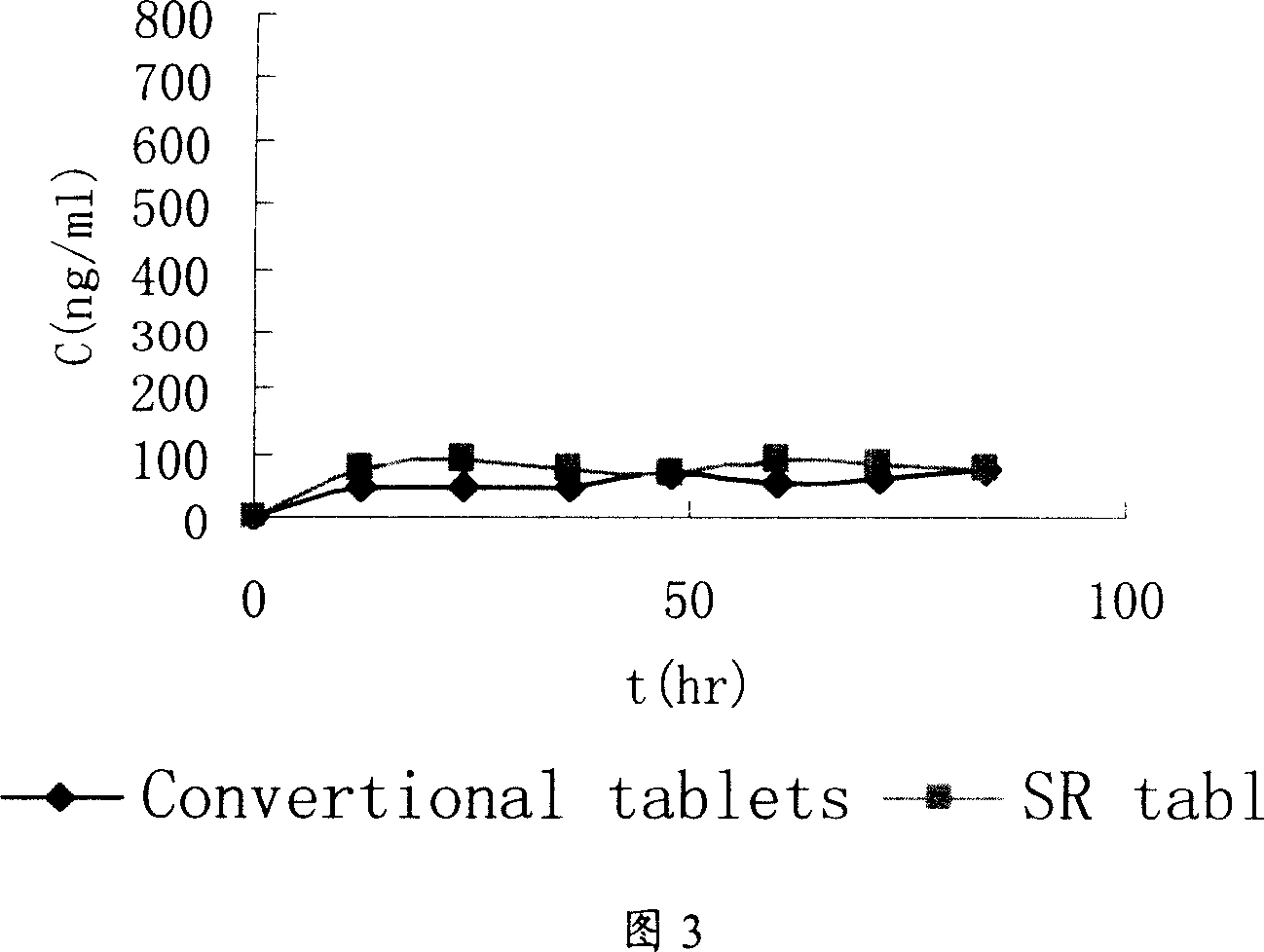
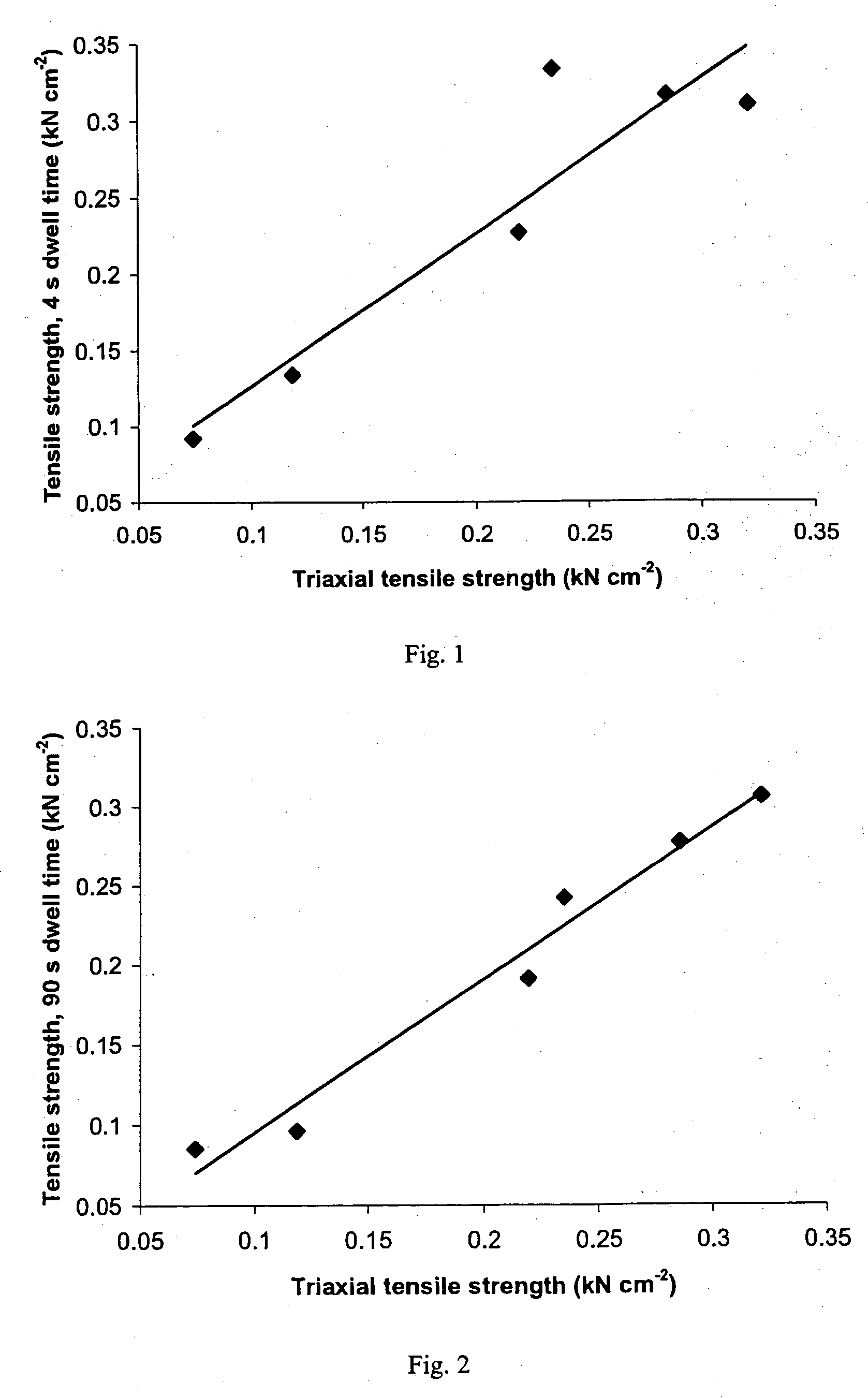
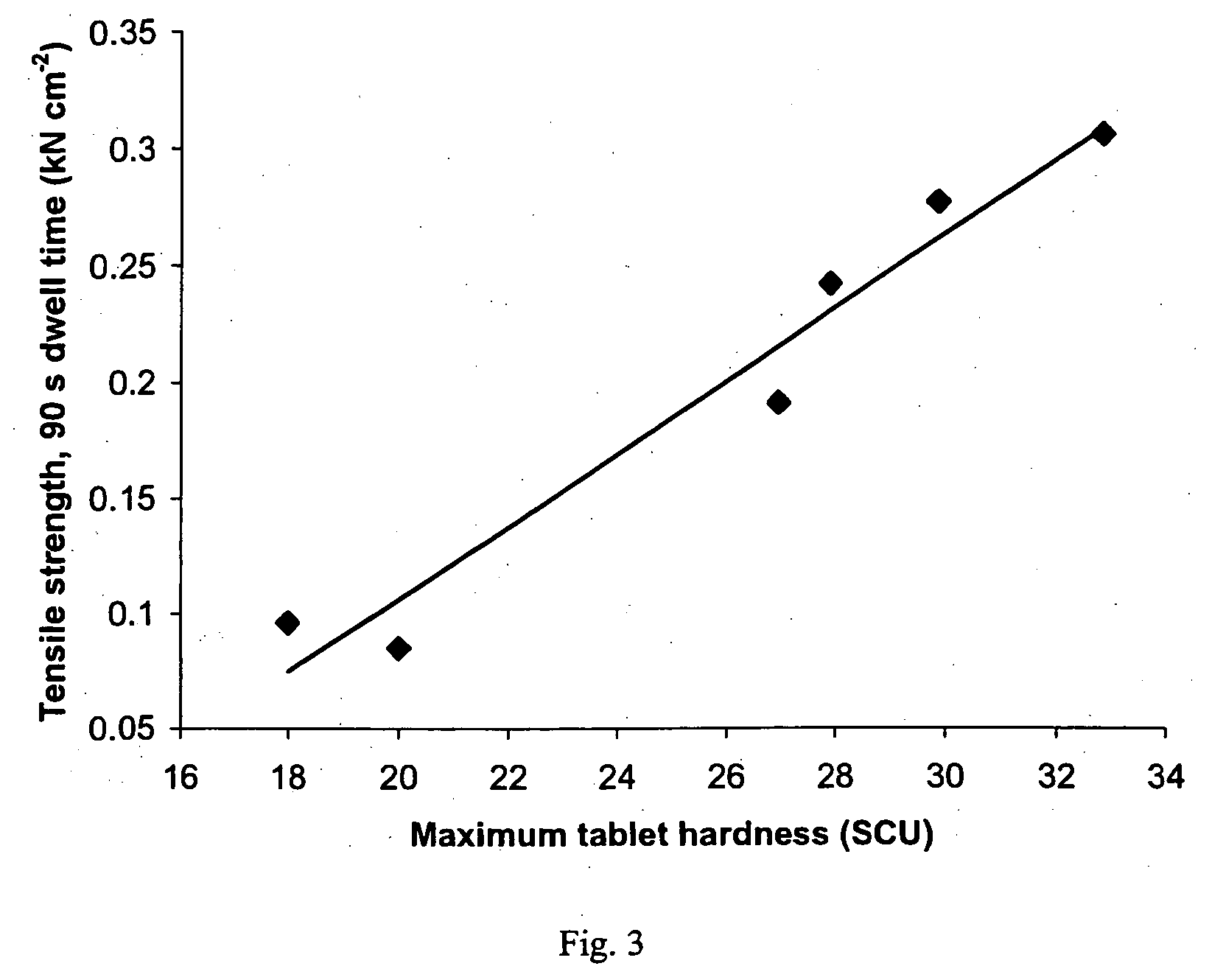
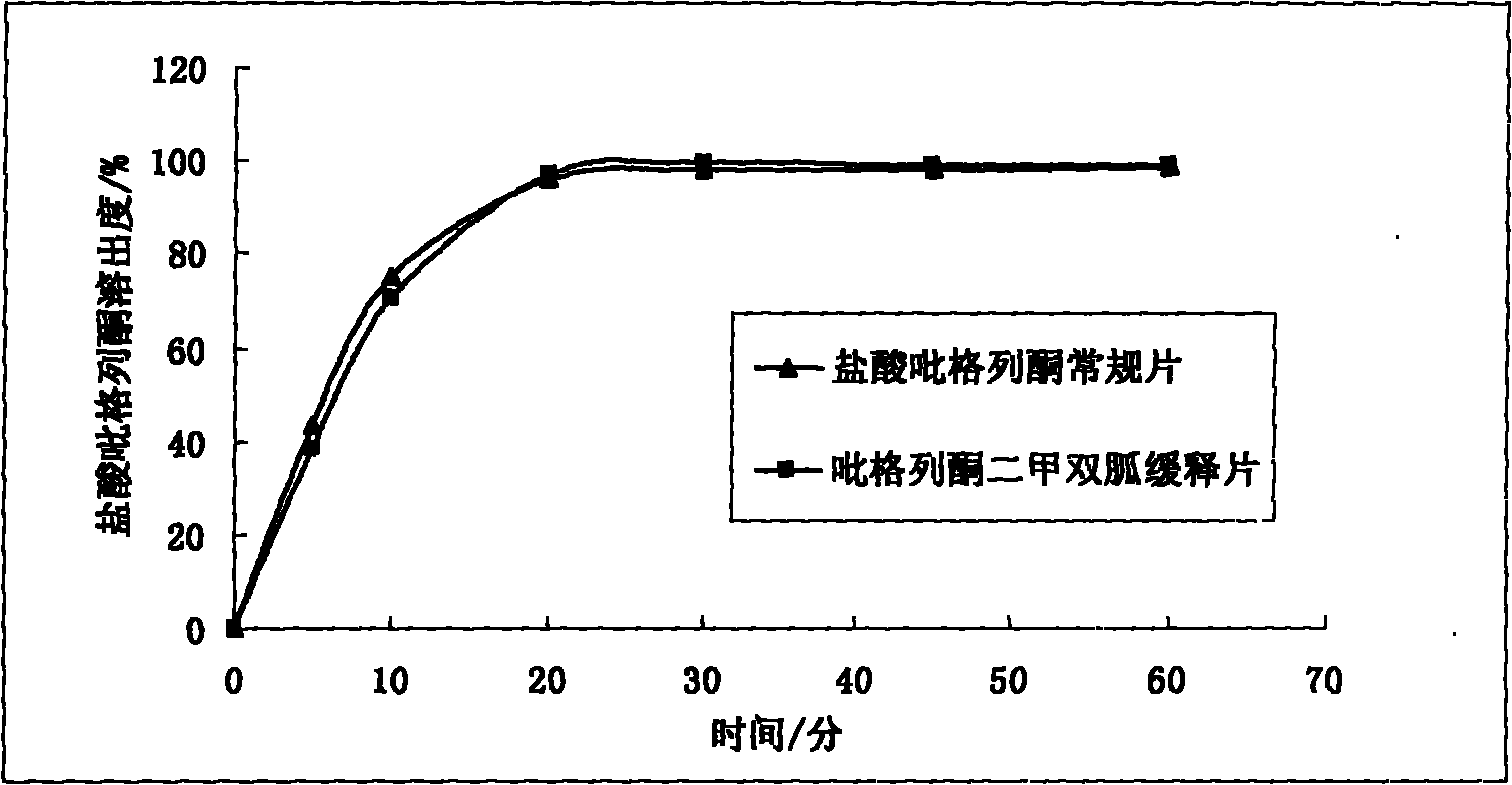
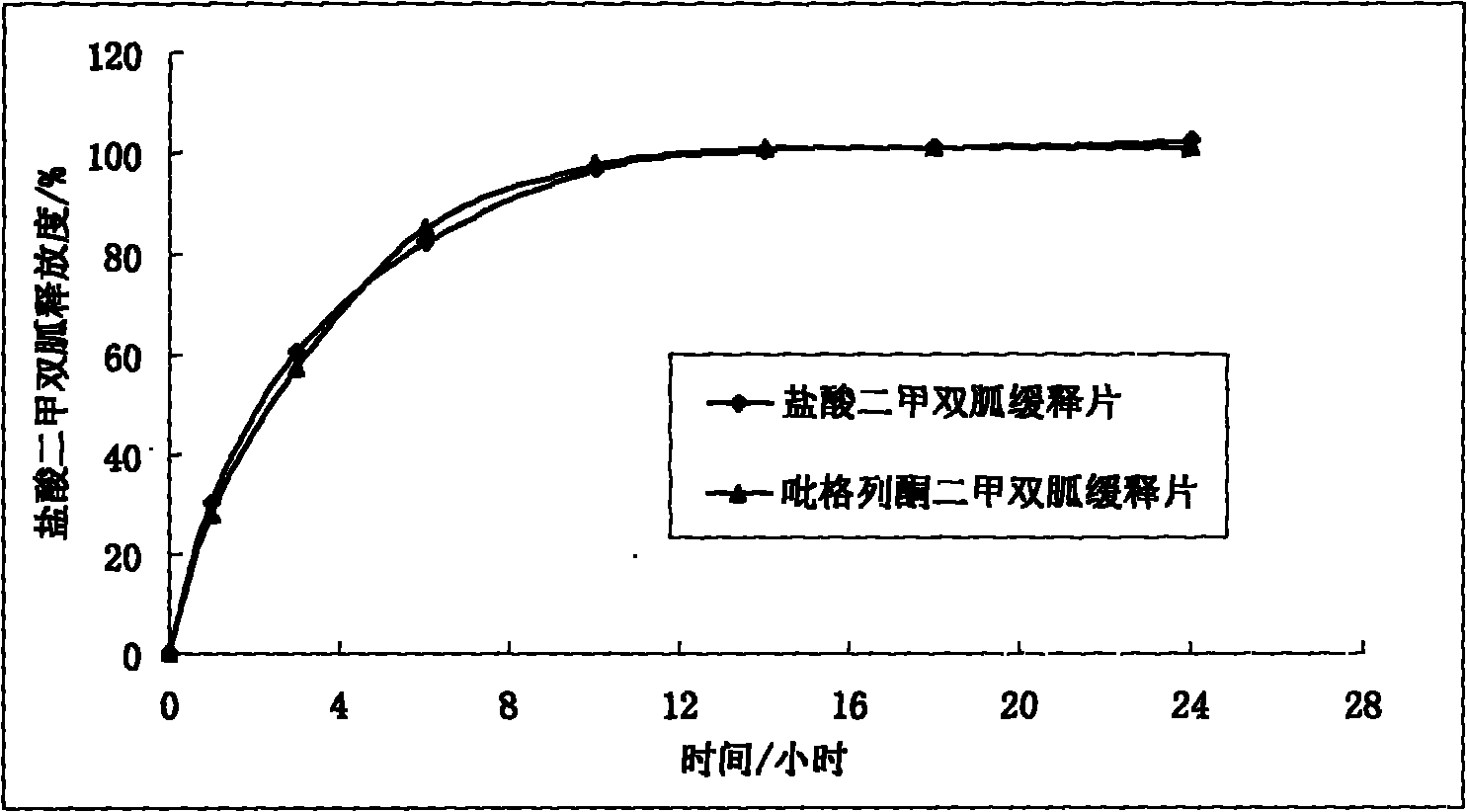
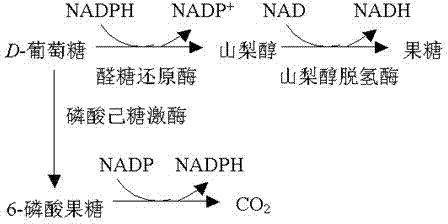
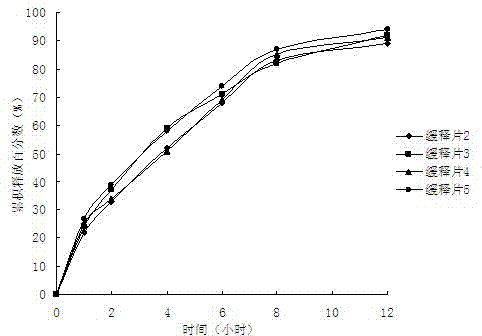
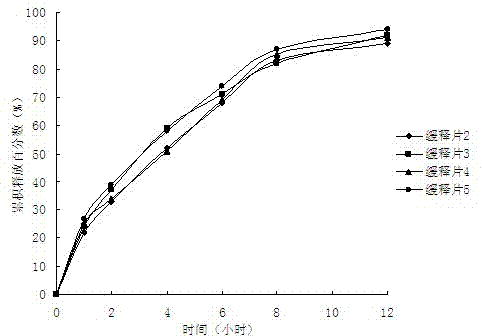
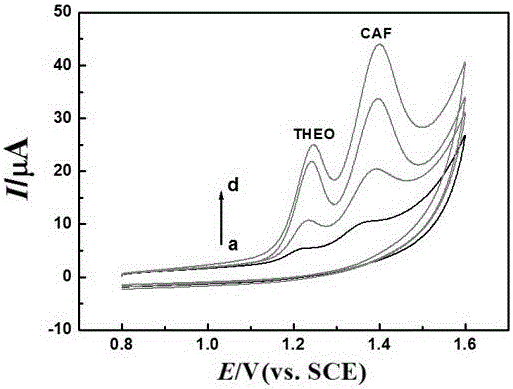
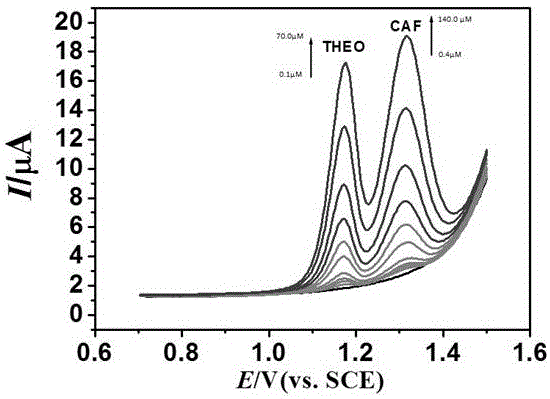
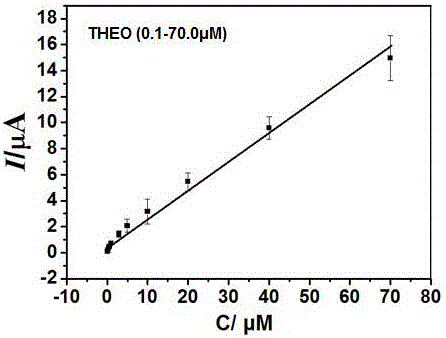
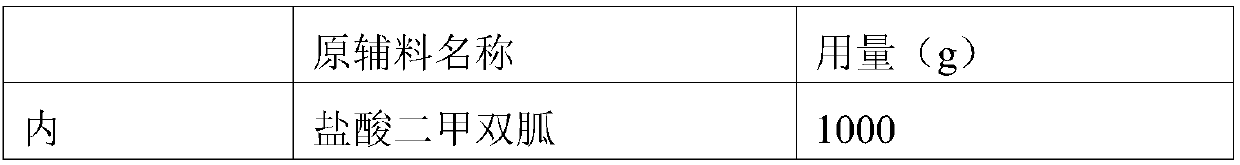
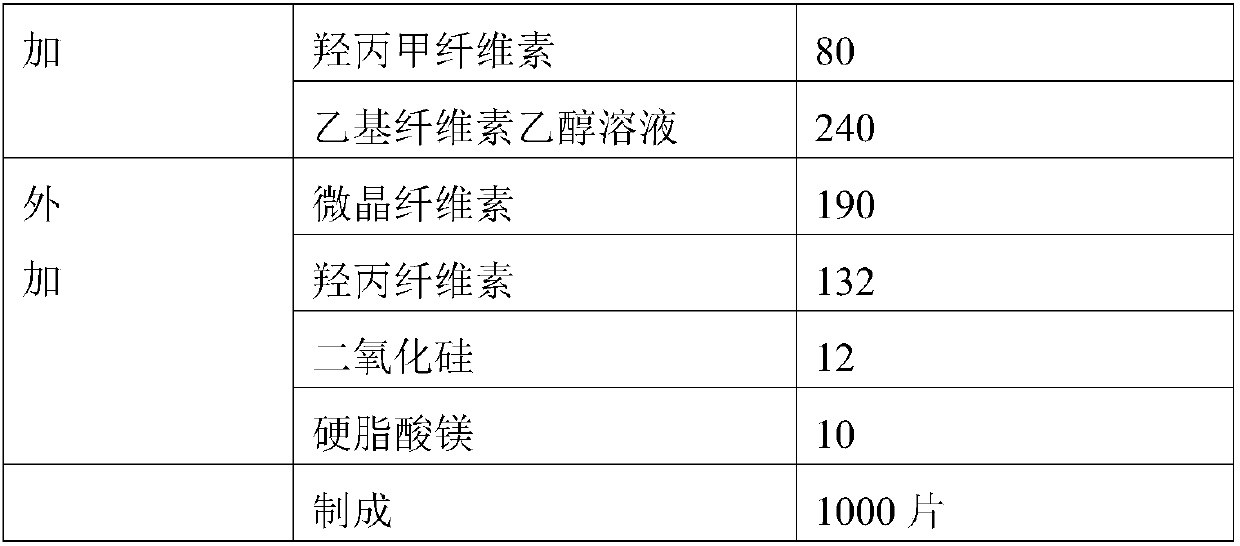
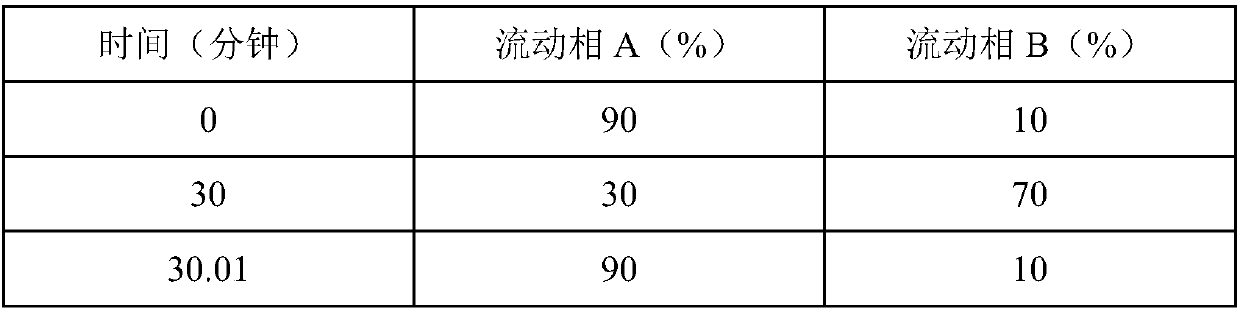
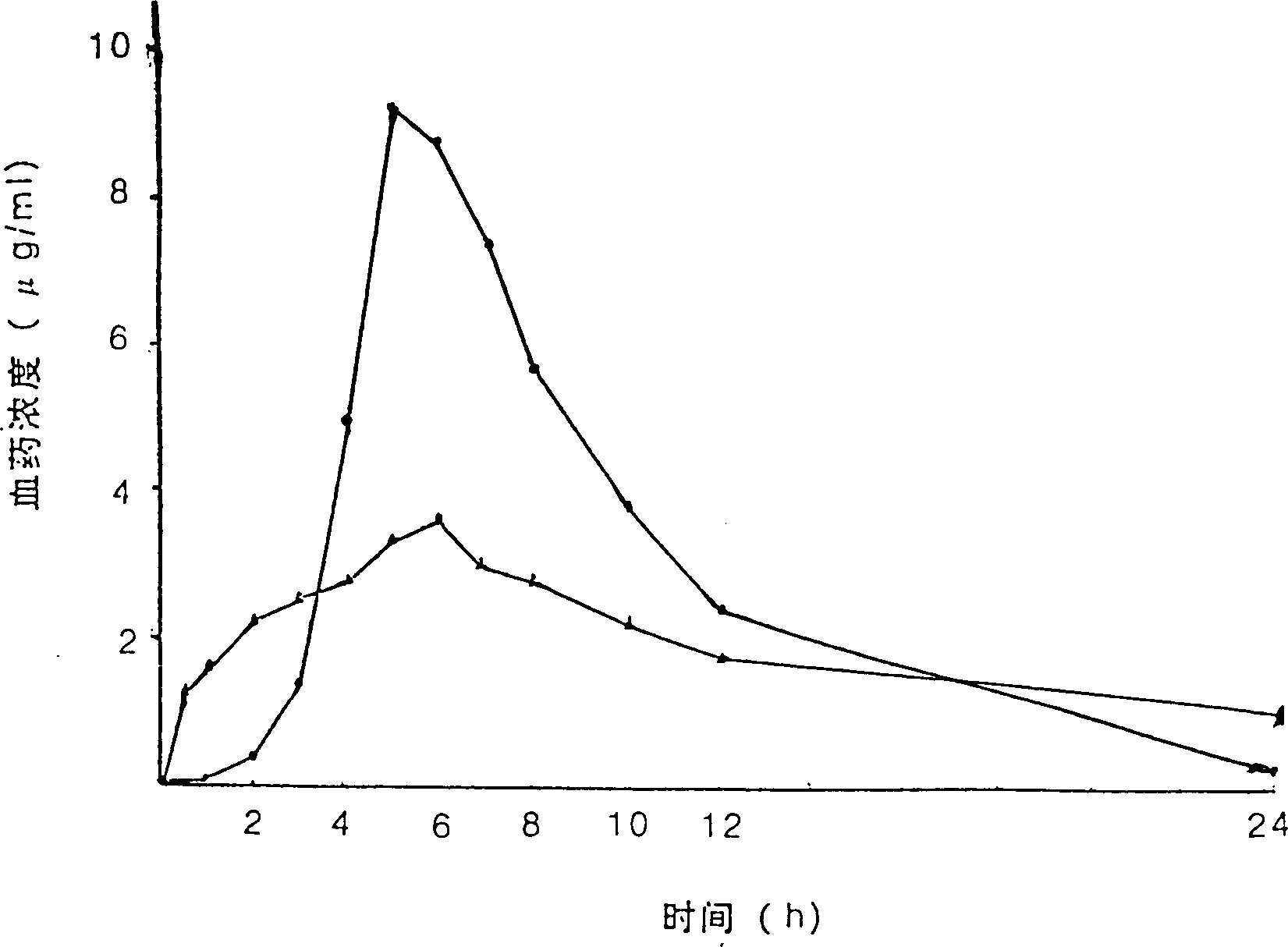
Patents
Literature
337 results about "Prolonged-release tablet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral medicine for treating cardio-cerebral vascular disease and preparation process thereof
InactiveCN1502337AReduce the number of daily dosesMedication convenienceOrganic active ingredientsPharmaceutical delivery mechanismOral medicineDisease
The present invention discloses a slowly-released oral medicine preparation which is made up by using breviscapine as main raw material and has the functions of promoting blood circulation and removing blood stasis, removing obstruction in the channels to relieve pain for curing angiocardiopathy and cerebrovascular disease with obvious therapeutic effect and its preparation method. Said slowly-released tablet (by one tablet) contains 60-120 mg of breviscapine, 20-75 mg of diluent, 50-150 mg of filling agent and 10-50 mg of slow release material. Said invention also provides its preparation method and concrete steps.
Owner:CHENGDU LIST PHARMA
Double-layer sustained release tablets for compensating iron and preparation thereof
ActiveCN101322778AEasy to prepareImprove nutritional statusHeavy metal active ingredientsOrganic active ingredientsSustained Release TabletNutritional status
The invention relates to a double-layer sustained release tablet for supplementing iron, the double-layer sustained release tablet consists of a sustained release layer and a quick release layer laminating with each other at the weight ratio of 1:0.25-1:4; the sustained release layer consists of an iron-containing compound, a sustained release framework, an adhesive, a lubricant and a filler; the quick release layer consists of a traditional Chinese medicine for enriching the blood and / or invigorating vital energy, a filler, a disintegrating agent, an adhesive and a lubricant; the quick release layer can also be added with vitamins. In the double-layer sustained release tablet, by arranging the iron-containing compound in the sustained release layer and the traditional Chinese medicine in the quick release layer, the iron element is released slowly and uniformly, which increases the absorption rate, avoids gastrointestinal reaction caused by high-content iron; meanwhile the double-layer sustained release tablet supplements iron element and the traditional Chinese medicine which has the functions of enriching the blood and invigorating vital energy, thus fully improving the nutritional status caused by iron deficiency anemia and improving iron supplementing effect.
Owner:BEIJING COMPETITOR SPORTS SCI & TECH
Slow release tablet composition for treating industrial water systems
ActiveUS20110073802A1Improve propertiesMaintain activityBiocideAntifouling/underwater paintsBiodispersanHardness
The present invention provides for a slow release composition, preferably as a tablet, for industrial water systems, under an alkaline pH or high water hardness, using as an active ingredient a multifunctional amine of Formula (I) and a hydrophilic polymer, and other components such as a compression agent, in one composition as a biocide, biostat, biodispersant, deposit penetrant aid, organic deposit remover, detergent, surfactant, antifoam, scale inhibitor, scale remover, and a corosion inhibitor. The composition is used in multiple water cycles, can remove, disperse or inhibit fouling. The active ingredient(s) of the composition is released over a long time, up to 6 months.
Owner:AMSA INC
Edoxaban sustained release tablet and preparation method thereof
InactiveCN103919746AAnticoagulant therapy is stableReduce adverse reactionsPharmaceutical delivery mechanismPharmaceutical non-active ingredientsSulfonateCellulose
The invention provides a preparation method of an edoxaban sustained release matrix tablet. The specific prescription of the edoxaban sustained release tablet consists of the following components in percentage by weight: 14-21 percent of edoxaban p-toluene sulfonate hydrate, 0-36 percent of hydroxypropyl methylcellulose, 0-28 percent of carbomer, 10-28 percent of lactose, 29-38 percent of diluent, 0-3 percent of povidone and 0.6-4 percent of lubricant.
Owner:SHANDONG INST OF PHARMA IND
Medicinal composition containing ibuprofen sodium salt
InactiveCN102389423AOrganic active ingredientsNervous disorderSustained Release TabletBULK ACTIVE INGREDIENT
The invention relates to a medicinal composition containing ibuprofen sodium salt, which is prepared by taking ibuprofen sodium salt and other one or a plurality of medicinal components as active ingredients, and combing the active ingredients with pharmaceutically appropriate auxiliary materials. The medicinal composition can be made into oral preparations, including conventional tablets, dispersible tablets, sustained release tablets, capsules, sustained release capsules, soft capsules, particles, dry suspension, suspension and the like, and used for symptomatic treatment of being antipyretic, antiphlogistic, analgesic and anti-allergic, and improving sleeping.
Owner:FUKANGREN BIO PHARMA
Water-soluble drug sustained-release tablet and preparation method thereof
InactiveCN101288652ASimple preparation processSimple processPill deliveryPharmaceutical non-active ingredientsProlonged-release tabletWater soluble drug
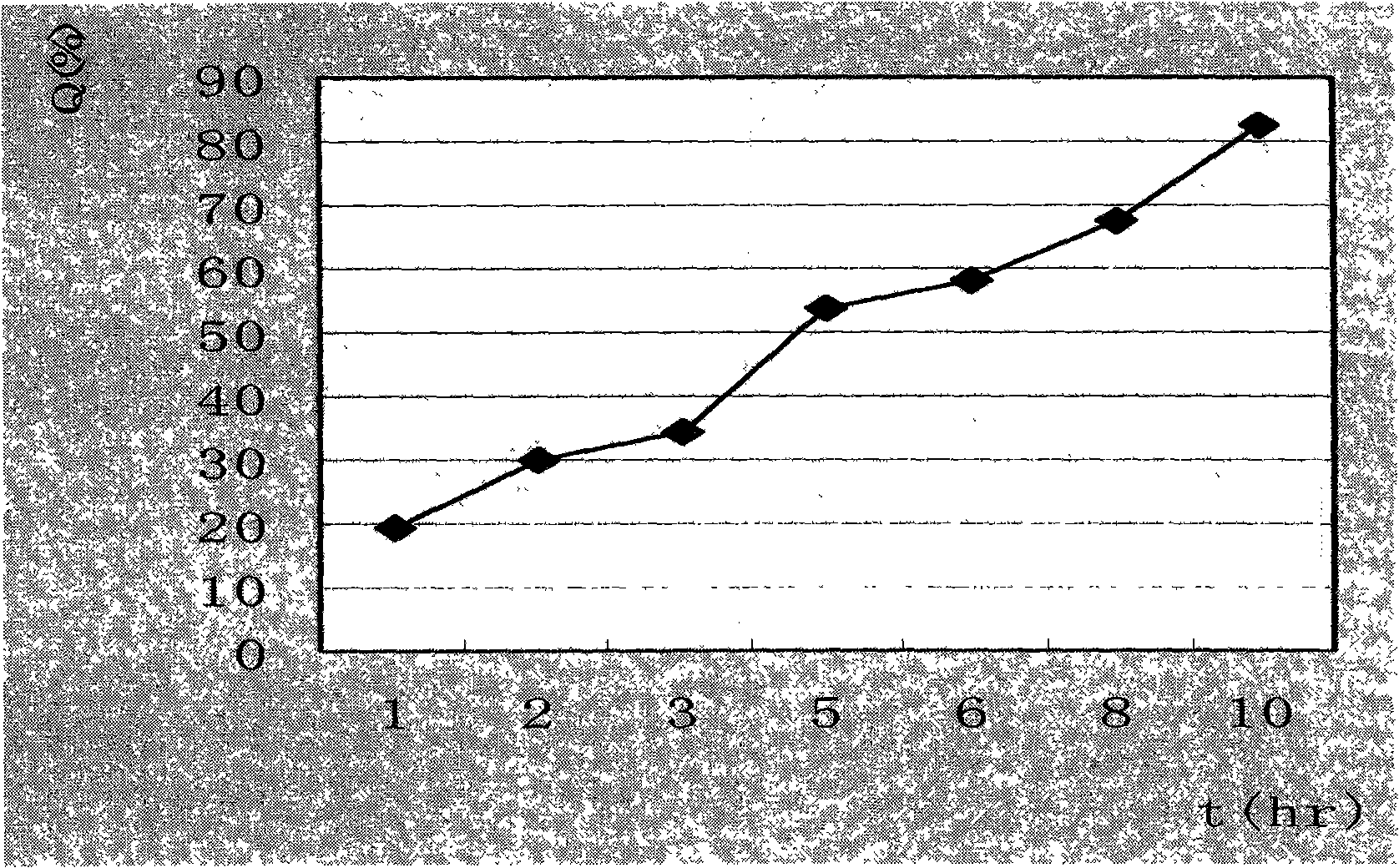
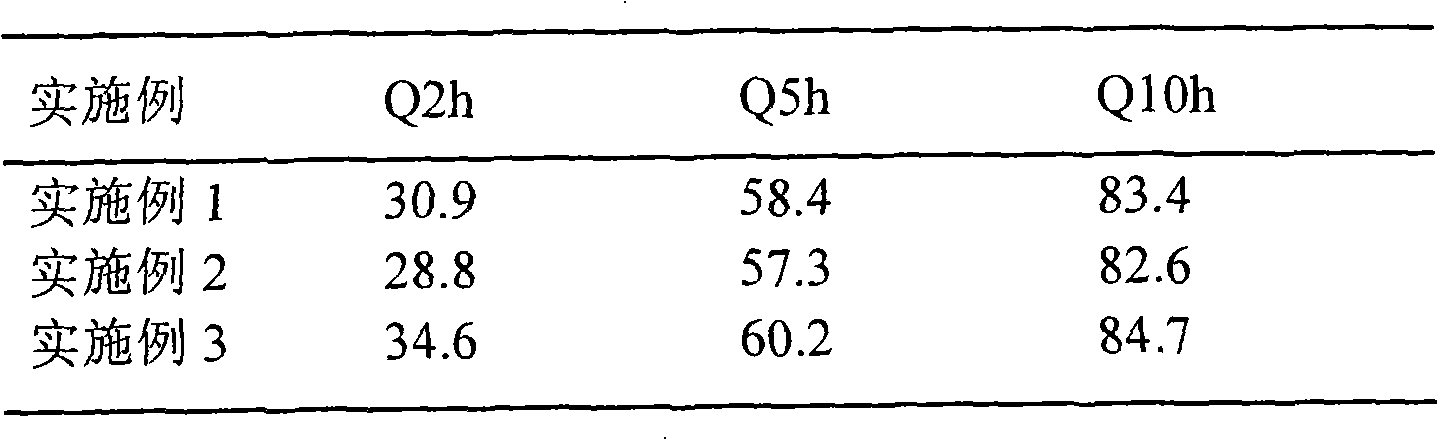
The invention relates to a water-soluble drug sustained-release tablet and a preparation method thereof, the technology is characterized in that: the components and the weight percentages are as follows: 1 to 20 percent of water-soluble drug, 20 to 70 percent of hydrophilic gel, 30 to 50 of percent waxy material and the rest of filler, lubricant and other excipients that are commonly used for ordinary tablets. The method takes the hydrophilic gel sustained-release material and the waxy sustained-release material as a mixed matrix, the water-soluble drug is added to change the features of fast dissolution and short half life of the drug release, thus achieving the purpose of slow and smooth release. The drug of the invention has good stability, the needed equipment is simple, the manufacturing process is simple and easy, the operatability is strong, the in vitro dissolution test shows that the release rate is 25 to 35 percent in 2 hours, 55 to 65 percent in 5 hours and more than 80 percent in 10 hours, thus having good sustained-release effect. In addition, the sustained-release tablet is less affected by the gastrointestinal tract environment, the absorption is stable, and the individual difference is small, thus being the ideal sustained-release tablet.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Nifedipine sustained release tablet
InactiveCN101966164AOrganic active ingredientsPharmaceutical delivery mechanismProlonged-release tabletMagnesium stearate
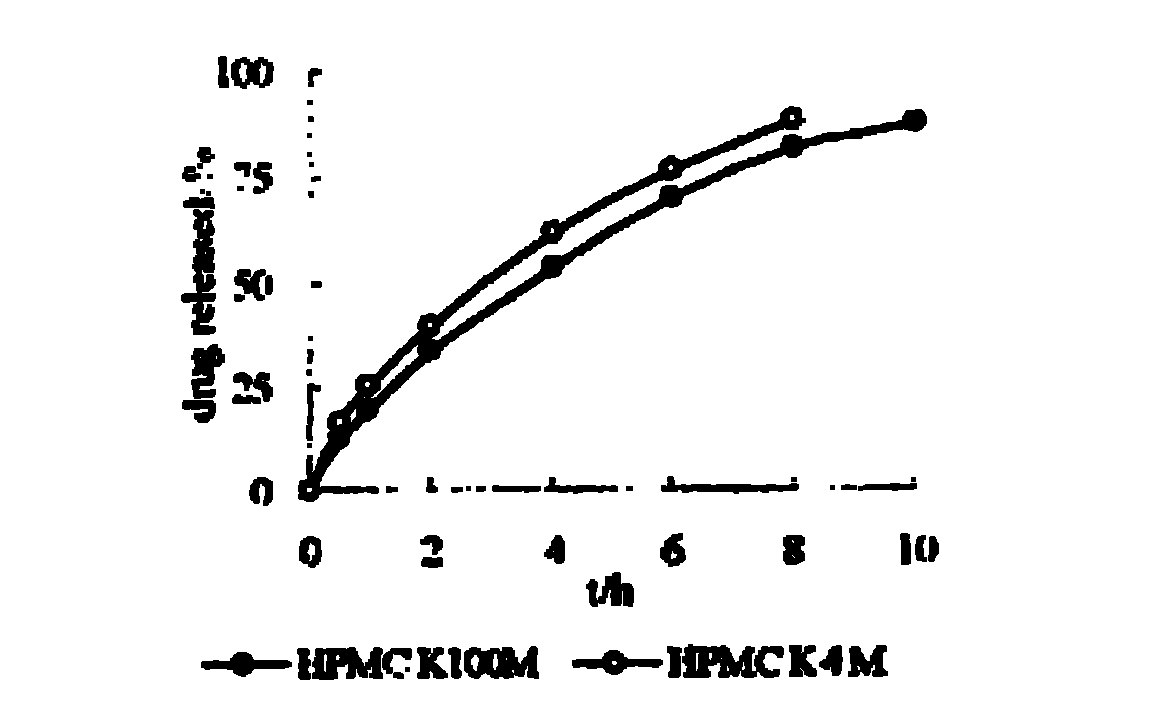
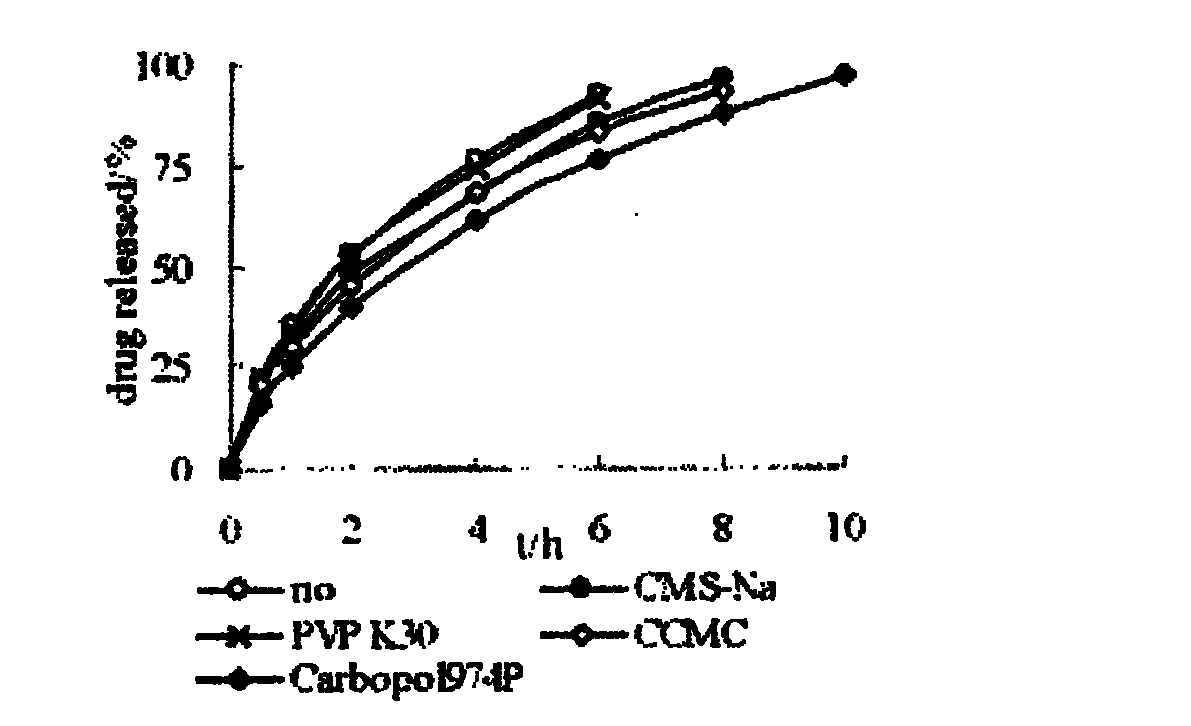
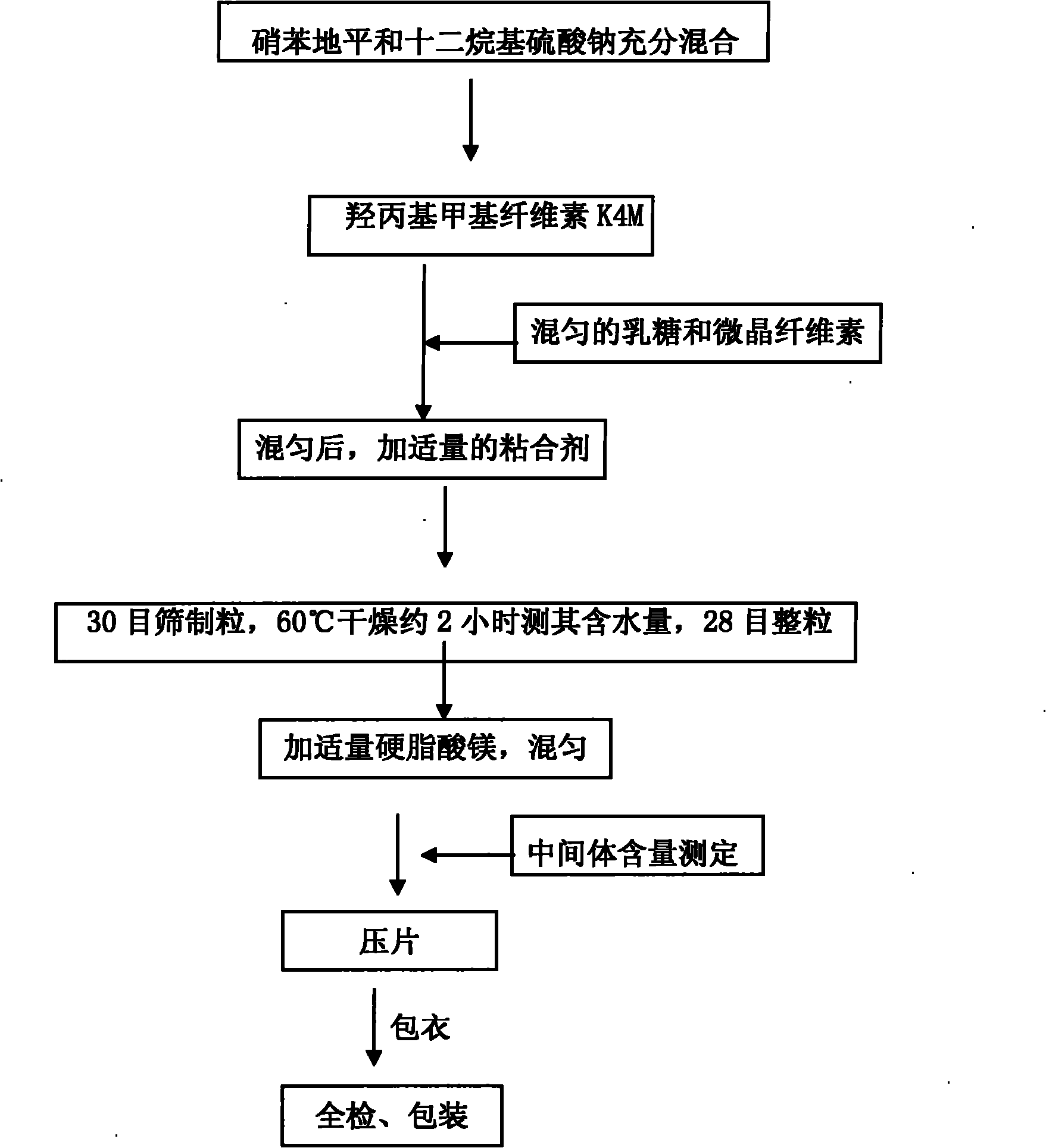
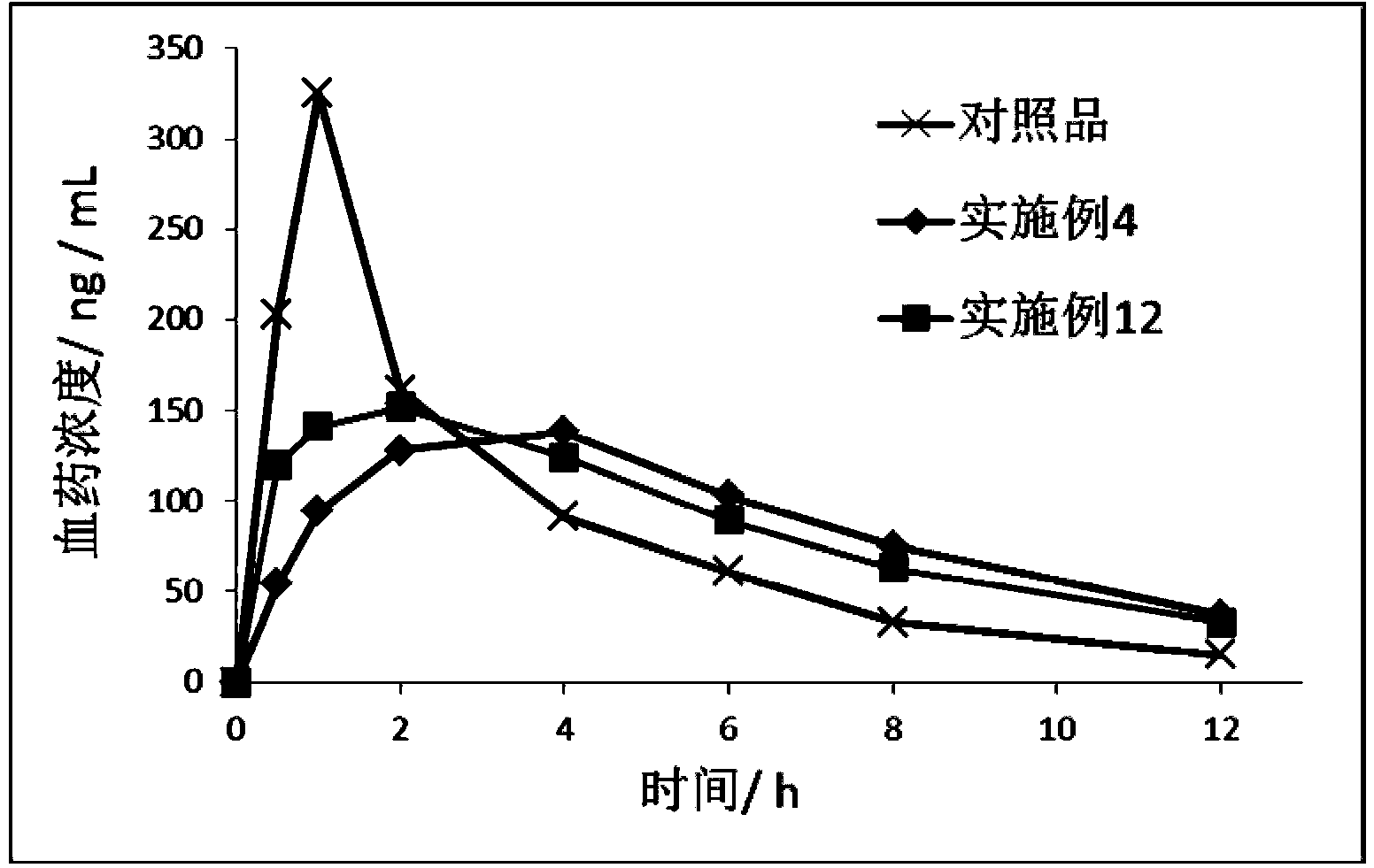
The invention relates to a nifedipine sustained release tablet and a preparation method thereof. The nifedipine sustained release tablet is characterized by comprising a tablet core and film coating liquid, wherein the tablet core comprises the following components in percentage by mass: 10 to 12 percent of nifedipine (Nif), 6.5 to 7.5 percent of hydroxypropyl methyl cellulose K4M, 45 to 47 percent of lactose, 34 to 35 percent of microcrystalline cellulose, 0.48 to 0.55 percent of lauryl sodium sulfate, a proper amount of 95 percent ethanol and a proper amount of magnesium stearate; and the film coating liquid comprises the following component in percentage by mass: 5.85 to 6.55 percent of Opadry II and 92.86 to 93.82 percent of distilled water. The preparation method comprises the following step of: mixing the nifedipine and polymer by utilizing the imported polymer and dispersion technology to form a hydrophilic matrix tablet so as to fulfill the aim of sustained release. Compared with an ordinary tablet, the sustained release tablet can continuously act for 12 hours relaxatively, is released in a sustained way and has less and slighter adverse reaction.
Owner:ANHUI YONSENT PHARMA
Sustained-Release Tablet and Process for Preparing the Same
A method for producing a sustained-release tablet having improved stability and content uniformity is provided. The method involves first preparing a core tablet by granulating, drying, milling, blending, and compressing a mixture of active and inactive ingredients. Four coating layers are applied to the core tablet: an inner layer, an enteric coating layer, an active layer, and an outer layer. The active ingredient may be a tetracycline, such as doxycycline. A sustained-release tablet prepared according to the method is also described.
Owner:GALDERMA SA
Immunomodulator slow-release preparation and preparation method thereof
ActiveCN103610658AProlong the action timeUniform and constant action timeOrganic active ingredientsPill deliveryBlood concentrationProlonged-release tablet
The invention discloses an immunomodulator slow-release preparation and a preparation method thereof. A lenalidomide slow-release tablet is composed of a slow-release layer and an optional quick-release layer, wherein the slow-release layer contains active ingredients of lenalidomide and a slow-release framework material simultaneously; the quick-release layer does not contain the slow-release framework material. The lenalidomide slow-release tablet disclosed by the invention is capable of slowly and uniformly releasing medicines by virtue of the slow-release framework material, so as to reduce the release speed, delay the time to peak, prolong the action time of lenalidomide, and provide a uniform and constant blood concentration. Moreover, The lenalidomide slow-release tablet disclosed by the invention is simple in prescription and excellent in quality stability; the preparation process is simple to operate, free from special treatment and production equipment, low in production cost, and beneficial to batch-enlarged industrial production for the product; the preparation method is high in yield, the granulation and crushing procedures are simple and practicable to operate, the intermediate material is good in stability, flowability, compressibility and content uniformity, and completely meets the requirements of tabletting, and the surface of the prepared tablet is smooth and beautiful.
Owner:AC PHARMA CO LTD
Cinnamic aldehyde series preparation and its preparation process
InactiveCN1634000ALow priceReduce manufacturing costAntibacterial agentsPharmaceutical delivery mechanismDiseaseDrug content
The invention provides a preparation for treating viral myocarditis, wherein the medicinal reactivity of cinnamic aldehyde for antiviral, immunity adjustment, cardiac rhythm stabilization and blood vessel amplificatus, the cinnamic aldehyde can be prepared into cinnamic aldehyde slow release tablet, cinnamic aldehyde slow release capsule and cinnamic aldehyde injection for treating viral myocarditis. The invention has the advantages of stabilized medicinal content, high biological availability, small amount of administration, high curative effect, and low toxicity.
Owner:中国人民解放军第四军医大学药物研究所
Tacrolimus sustained release tablets and preparation method thereof
InactiveCN101361722AImprove medication complianceReduce the number of dosesOrganic active ingredientsPharmaceutical delivery mechanismLiver and kidneySide effect
The invention relates to a sustained release tablet dosage, in particular to a Tacrolimus sustained release tablet used for liver and kidney transplantation patients and a preparation method thereof, belonging to the field of medicine technology. The invention is characterized in that Tacrolimus and adhesive are taken and dissolved in ethanol to prepare Tacrolimus solution; sustained release materials, thinner and flow agent are taken, mixed and dispersed uniformly and then added into the Tacrolimus solution to prepare wet particles, which are dried, ground and added and mixed with lubricant; finally, the Tacrolimus sustained release tablet is prepared after tabletting and drying. The sustained release tablet has the unique advantages of: reducing administration times, improving the administration adaptability of patients; after administration, having stable blood concentration, small toxic and side effects and improving the drug effect and safety; reducing the total dosage of drugs so as to achieve the greatest curative effect by the lowest dosage; being capable of being realized on most of tablet production lines and easily accepted by manufacturing units and manufacturers due to relatively simple preparation process; and having relatively low manufacturing cost and broad clinical application prospect.
Owner:贾祥波 +1
Aspirin sustained release tablet and preparing method thereof
The invention relates to the field of pharmaceutical preparation, in particular to a sustained-release aspirin tablet, and is characterized by consisting of aspirin with 75 portions, ethyl cellulose with 10 to 25 portions, polyacrylic resin with 7.5 to 10 portions, starch with 4 to 10 portions, hydroxypropyl methyl cellulose with 1 to 6 portions, tartaric acid with 0.32 to 0.64 portions, talcum powder with 2.7 to 3.6 portions. The invention also discloses a pharmaceutical preparation method.
Owner:CHANGZHOU PHARMA FACTORY
Maleic acid trimebutine slow release tablet comprising quick release part and preparing method thereof
ActiveCN1927185AActive ingredients are well absorbedPromote absorptionDigestive systemEster active ingredientsFumaropimaric acidTrimebutine
This invention concerns a the maleic acid trimebutine sustained-releasing tablets with rapid releasing part and preparation of pharmaceutical pharmaceuticals, including double slice and tablet mixed and suppressed by particles with different releasing characteristics, it is found in the invention that after the maleic acid trimebutine and fumaric acid being mixed and granulated, the stability and compressibility of the maleic acid trimebutine is improved, and its citation moist is lowered, and it can be used for preparation of the praeparatum of the present invention, the present invention includes the rapid releasing part and sustained-releasing part.
Owner:KAIKAII YUANSHENG PHARMA CO LTD
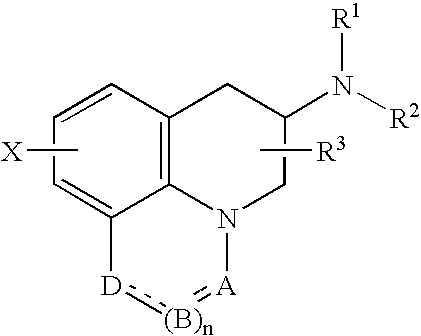
Sustained-release tablet composition comprising a dopamine receptor agonist
InactiveUS20050020589A1Simple methodBiocideAnimal repellantsSustained Release TabletCompound (substance)
A sustained-release pharmaceutical composition in a form of an orally deliverable tablet comprises as active pharmaceutical agent a compound of formula or a pharmaceutically acceptable salt thereof, wherein R1, R2 and R3 are the same or different and are H, C1-6 alkyl (optionally phenyl substituted), C3-5 alkenyl or alkynyl or C3-10 cycloalkyl, or where R3 is as above and R1 and R2 are cyclized with the attached N atom to form pyrrolidinyl, piperidinyl, morpholinyl, 4-methylpiperazinyl or imidazolyl groups; X is H, F, Cl, Br, I, OH, C1-6 alkyl or alkoxy, CN, carboxamide, carboxyl or (C1-6 alkyl)carbonyl; A is CH, CH2, CHF, CHCl, CHBr, CHI, CHCH3, C═O, C═S, CSCH3, C═NH, CNH2, CNHCH3, CNHCOOCH3, CNHCN, SO2 or N; B is CH, CH2, CHF, CHCl, CHBr, CHI, C═O, N, NH or NCH3, and n is 0 or 1; and D is CH, CH2, CHF, CHCl, CHBr, CHI, C═O, O, N, NH or NCH3. The agent is. dispersed in a matrix comprising a hydrophilic polymer and a starch having a tensile strength of at least about 0.15 kN cm−2 at a solid fraction representative of the tablet. The composition exhibits sustained-release properties effective for treatment of Parkinson's disease. The tablet is optionally coated. Tablets of the invention have improved resistance to attrition or erosion during manufacture, packaging and handling.
Owner:PFIZER INC
Lisinopril controlled-release tablet and preparation method thereof
InactiveCN103006612AThe effect of delayed releaseAchieve the effect of releaseDipeptide ingredientsPharmaceutical delivery mechanismFoaming agentWater insoluble
The invention relates to a lisinopril controlled-release tablet and a preparation method thereof, belonging to the field of pharmaceutic preparation technology and solving the problem that existing lisinpopril controlled-release tablet cannot delay release and release mildly. The tablet consists of core of the tablet containing lisinopril and a coating wrapped outside the core of the tablet, the core of the tablet containing the lisinopril comprises the following components by weight percent: 5.0%-20% of lisinopril, 25%-55% of framework material, 20%-50% of filler, and1.0%-3.0% of lubricant, the coating comprises the following components in weight percent: 60%-90% of water insoluble coating material and 10%-40% of pore-foaming agent. The method comprises the preparation of core of the tablet containing lisinpopril, the preparation of coating liquor, and the preparation of the tablet by wrapping the coating liquor outside the core of table so as to form the coating. The lisinopril controlled-release tablet can delay release and release mildly, and the method is simple and beneficial to industrialization.
Owner:石雷 +1
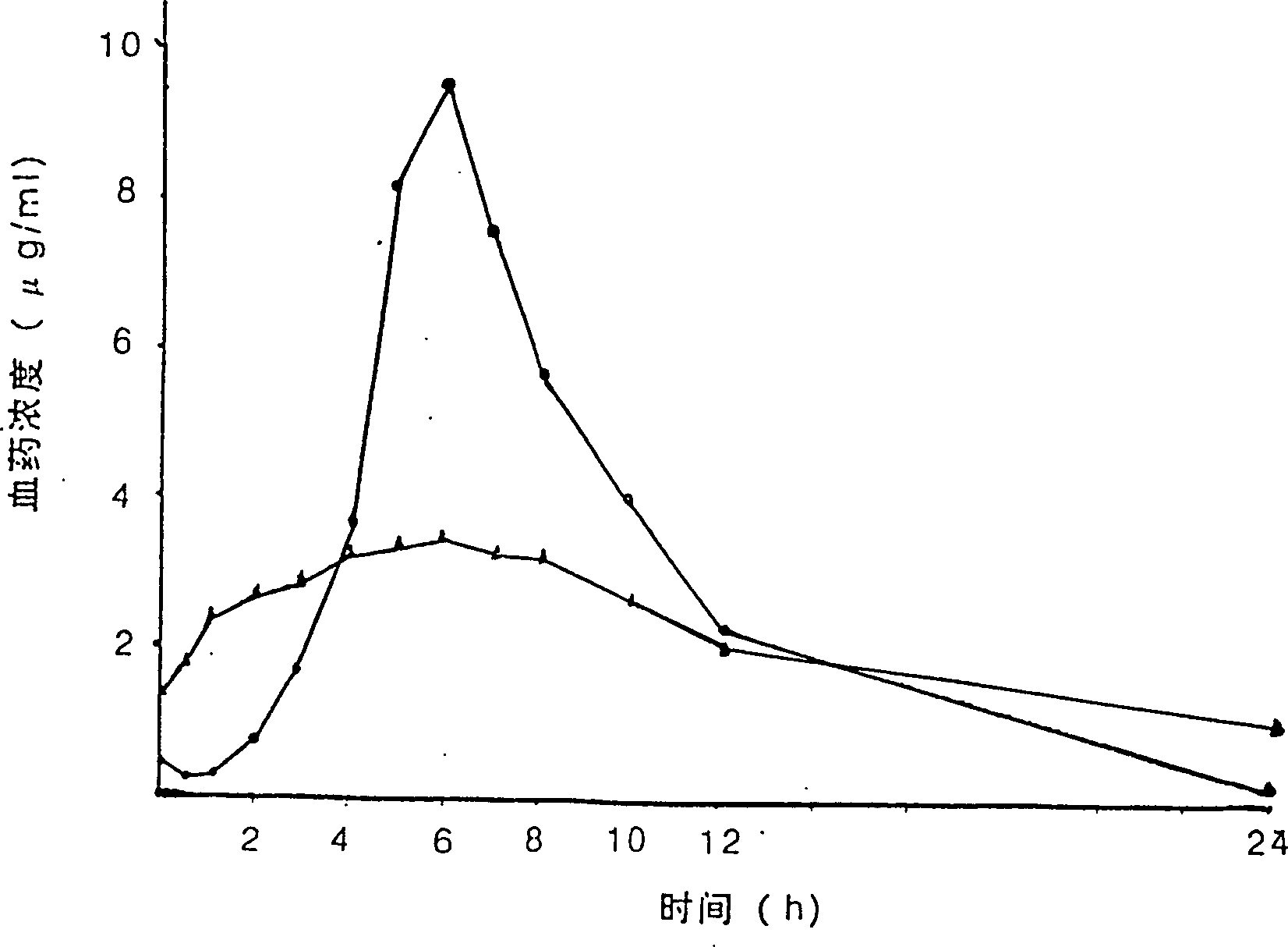
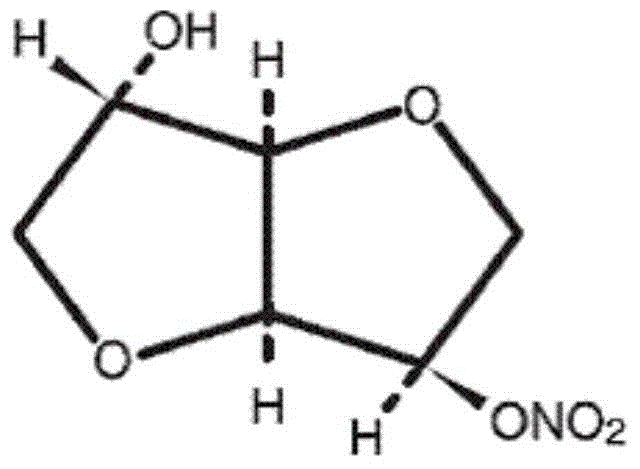
Isosorbide mononitrate sustained-release tablet and preparation method thereof
InactiveCN102416005AResidue reductionLower dosePharmaceutical delivery mechanismCardiovascular disorderProlonged-release tabletImmediate release
The invention relates to an isosorbide mononitrate sustained-release tablet and a preparation method thereof, belonging to the technical field of medicinal preparations. The sustained-release tablet comprises a coating of isosorbide mononitrate drug loaded microballoons which account for 48% to 55% of the total weight of the tablet and a quick-release layer which accounts for 45% to 52% and comprises isosorbide mononitrate, a binder, a filler and a lubricant. According to the invention, isosorbide mononitrate is divided into two parts, i.e., the part of quick release and the part of sustained release, which enables the tablet to take effect rapidly at an initial stage of administration, maintain an effective plasma concentration after the effective plasma concentration is reached and retain the effective plasma concentration at a low level so as to reduce residual quantity of the tablet at administration time of the tablet the next day, thereby effectively avoiding generation of drug resistance.
Owner:NANJING CHENGONG PHARM CO LTD
Multicomponent sustained-release preparation and preparation method thereof
InactiveCN102058557AShort half-lifeExtended half-lifeMetabolism disorderSulfonylurea active ingredientsSustained Release TabletIsolation layer
The invention provides a multicomponent sustained-release preparation and a preparation method thereof. An isolation layer is arranged between a sustained-release part and a quick release part in the sustained-release preparation to effectively isolate the interaction of a sustained-release tablet core and an external quick release coat, the external quick release part is quickly released completely, the isolation layer is quickly dissolved when meeting an aqueous medium, and the release of the sustained-release tablet core is not influenced. The technology can be applied to compound sustained-release preparations as required.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH +1
Tegasevod maleate oral preparation and its preparation process-for curing intestinal irritability syndrome
InactiveCN1443535AQuality improvementQuality is easy to controlOrganic active ingredientsDigestive systemEffervescent tabletOral medicine
The present invention relates to an oral medicine preparation for curing intestinal irritability syndrome using constipatino as main sympton-tijaseluo maleate. It is made up by using tijaseluo maleate as active component and adding proper auxiliary material through a certain preparation process, and can be made into various oral dosage forms of tablet, oral disintegrant table, effervescent tablet, capsule, suspension gel and powder preparation, etc.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Compound vitamin B6 sustained release tablets and preparation process thereof
InactiveCN101756916AActive ingredients are well absorbedPromote absorptionOrganic active ingredientsDigestive systemMedicineWood splinter
The invention provides a preparation method of double-layer sustained release tablets containing folic acid, calcium carbonate, vitamin B12 and vitamin B6, and the combination is a double-layered tablet, wherein, vitamin B6 is a slow release layer and the folic acid is a quick release layer. In the method, vitamin B6 is prepared into the slow release layer and the folic acid and the like are prepared into the quick release layer, and then the two layers are pressed into the double-layered tablets. The method solves the common problem of splinters owing to long-time placement of the double-layered tablets; and the prepared compound vitamin B6 sustained release tablets has good appearance, tight combination between an upper layer and a lower layer, wherein, the swelling rate and the swelling degree of the two layers are consistent under the influence of the external humiture, which can always maintain good appearance and release speed. The compound vitamin B6 sustained release tablets have simple preparation method, available raw materials, applicability to large-scale industrial production and good application prospect.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Epalrestat slow-release tablet and preparation method thereof
InactiveCN102440976ASimple processSimple and fast operationOrganic active ingredientsMetabolism disorderCelluloseOral medication
The invention provides an epalrestat slow-release tablet and a preparation method thereof. The slow-release tablet is prepared from the following components in parts by weight: 1-40 parts of epalrestat, 5-60 parts of slow-release material, 0-90 parts of filler, 0.1-2 parts of lubricant and right amount of adhesive. The slow-release material comprises hydroxypropyl methylcellulose or a mixture of hydroxypropyl methylcellulose and ethyl cellulose. The preparation method comprises the following steps: evenly mixing the epalrestat, slow-release material and filler according to the prescription amounts, adding the adhesive to obtain a soft material, granulating the soft material, drying, finishing, adding the lubricant, and tabletting. Compared with the conventional oral epalrestat common preparation, the epalrestat slow-release tablet provided by the invention can smoothly and slowly release the medicines after being orally dosed, and can maintain therapeutic action for a longer time.
Owner:NANJING HAILING TRADITIONAL CHINESE MEDICINE RES CO LTD +1
Preparation method and application of nitrogen-doped carbon nano tube composite L-cysteine modified glassy carbon electrode
InactiveCN106290519AHigh catalytic activityIncreased current responseMaterial analysis by electric/magnetic meansDifferential pulse voltammetrySodium sulfate
The invention discloses a preparation method and application of a nitrogen-doped carbon nano tube composite L-cysteine modified glassy carbon electrode. The preparation method mainly comprises the following steps: preparing an electrodeposit nitrogen-doped carbon nano tube and an electropolymerization L-cysteine modifed glassy carbon electrode; and performing sensitive quantitative analysis determination on theine and caffeine by use of a differential pulse voltammetry method. Experimental results indicate that in a 0.01mol / L sulfuric acid-sodium sulfate buffer solution with the pH value of 1.70, the modified electrode has obvious catalysis and sensitivity increasing effects on theine and caffeine; under optimum conditions, the differential pulse voltammetry method is adopted for measurement, the concentrations of theine and caffeine are respectively within a range of 0.1-70.0 microns and a range of 0.4-140.0 microns and have good linear relationship with a peak current, and the detection limits respectively reach 0.04 and 0.20 microns. The method is used for measuring contents of theine and caffeine in a theine slow-release tablets and a Coke sample, and the results are satisfactory.
Owner:SHANGHAI UNIV
Felodipine sustained-release tablet and preparation technology thereof
ActiveCN104758266ARapid drug releaseStable drug releaseOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSustained Release Tablet
The invention discloses a felodipine sustained-release tablet and a preparation technology thereof. According to the preparation technology of the felodipine sustained-release tablet, fast-release solid dispersions, sustained-release micro-pills and pharmaceutically acceptable accessories are mixed and the mixture is compressed to obtain the preparation. The fast-release solid dispersions comprise felodipine, copovidone and polacrilin potassium; the sustained-release micro pills are obtained by coating the fast-release solid dispersions with the ethyl cellulose film with a pore-forming agent. The sustained-release tablet disclosed by the invention has the advantages of fast early-stage releasing speed and slow and stable later-stage releasing speed, so that the drug can be completely released and an effective blood drug concentration can be maintained for a long time; the felodipine sustained-release tablet is high in bioavailability, simple in preparation technology and applicable to industrial mass production.
Owner:CHANGZHOU NO 4 PHARMA FACTORY +1
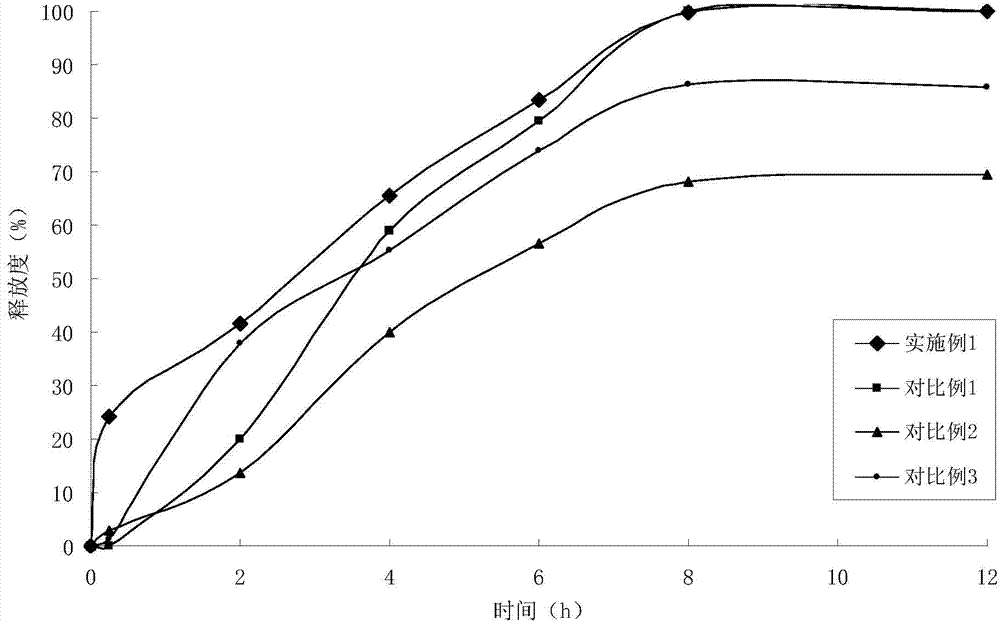
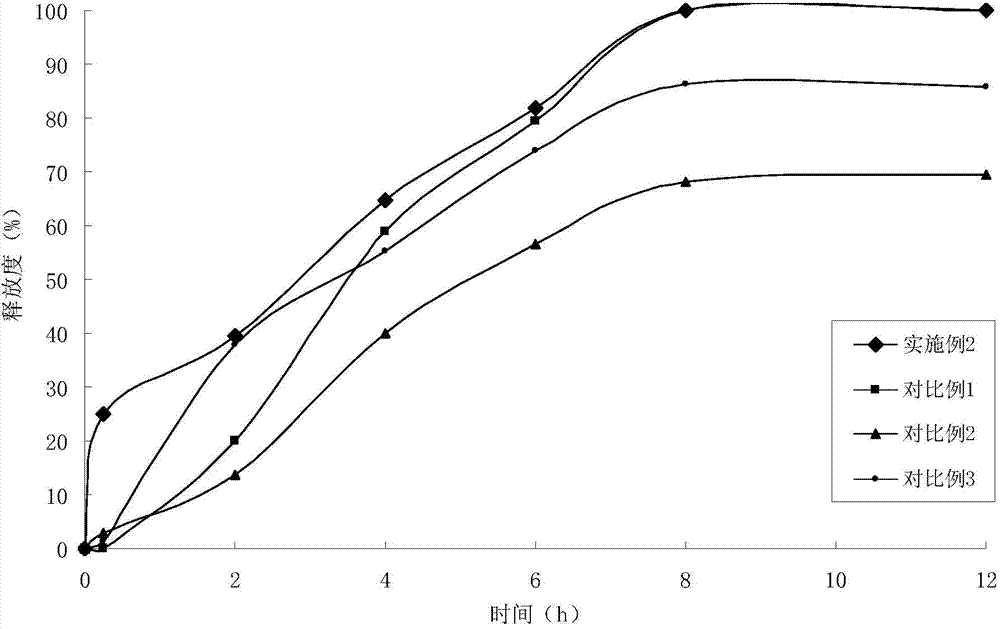
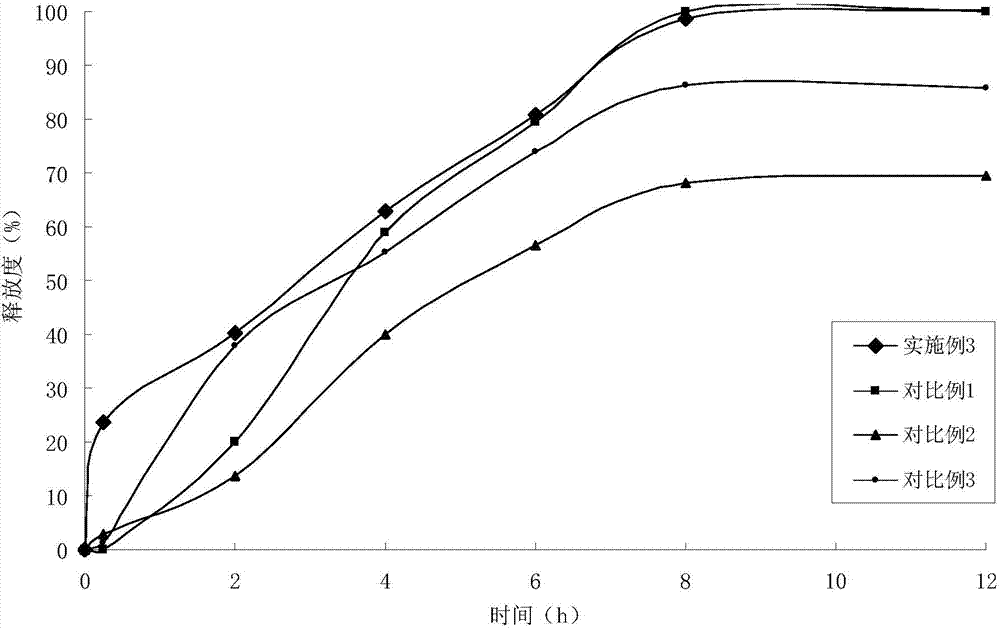
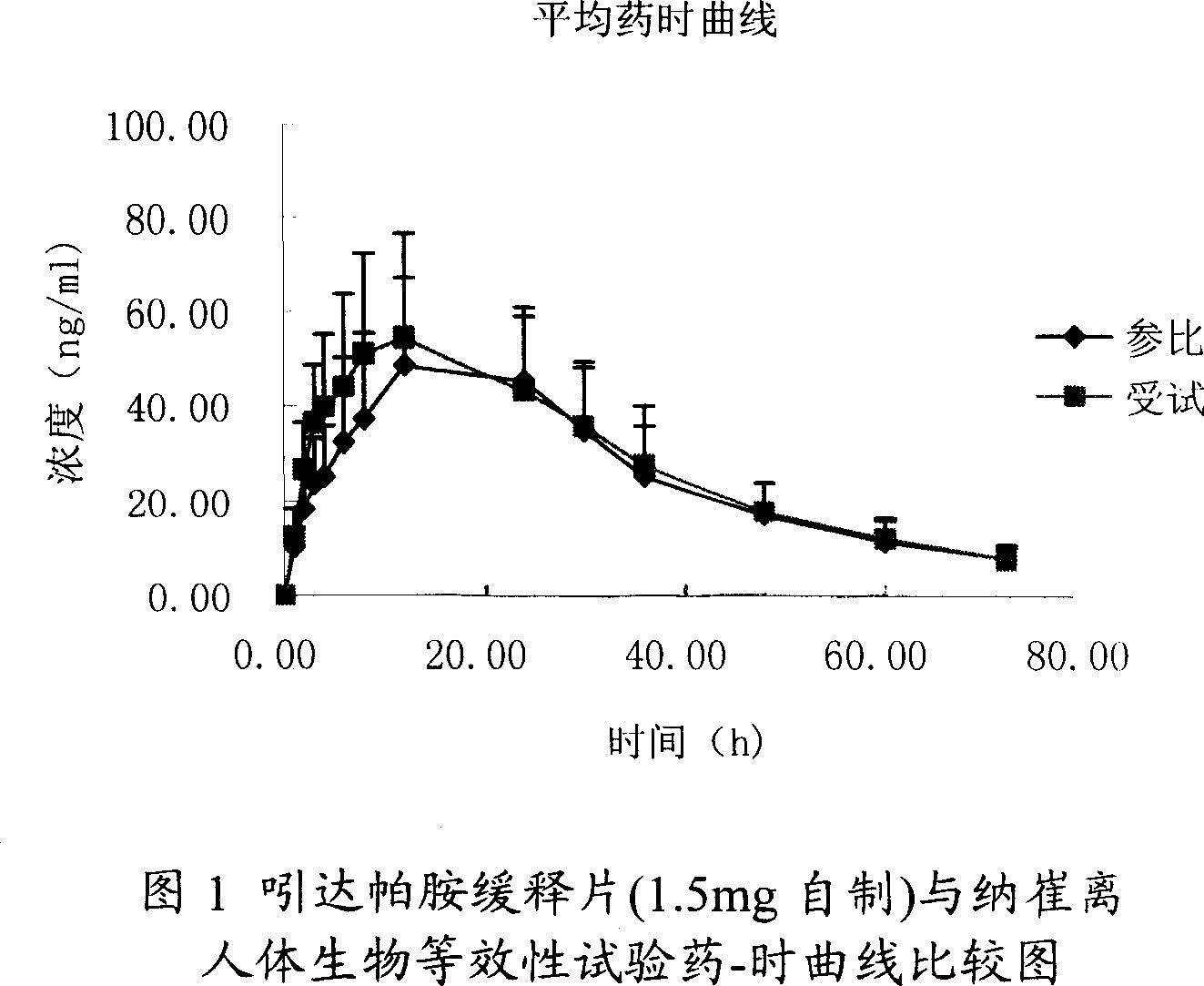
Indapamide slow release tablet and its preparing method
InactiveCN1943564AImprove complianceAvoid side effectsOrganic active ingredientsPill deliveryProlonged-release tabletPotassium
The invention relates to a indapamide retard tablet and its preparation process, said retard tablet comprising indapamide, bone material, filler and lubricant, in each said tablet containing controllable 0.5-1.5mg( each tablet containing 0.15 g. or 150g / 1000 tablet ).
Owner:KANGYA OF NINGXIA PHARMA
Divalproex sodium sustained-release tablet and preparation process thereof
The invention provides a method for preparing a divalproex sodium-containing sustained-release tablet for treating epilepsy. The sustained-release tablet contains divalproex sodium, a sustained-release material, a binder and a lubricant. The preparation method comprises the following steps of: mixing the divalproex sodium and the sustained-release material to obtain a mixture, adding the binder for wet granulation, drying, granulating, adding into other uniformly mixed auxiliary materials, and tabletting to obtain the divalproex sodium-containing sustained-release tablet. The sustained-release tablet can be sustainedly released for 24 hours, and is good in appearance, stable in performance and convenient to take.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA +1
Drug composition containing sitagliptin and melbine
ActiveCN107669683AQuick effectLong-term maintenance of blood sugar balanceOrganic active ingredientsMetabolism disorderSustained Release TabletSitagliptin
The invention discloses a drug composition containing sitagliptin and melbine. A melbine sustained release tablet core is coated with sitagliptin in a mixed suspension mode. The invention also discloses composition of a sitagliptin suspension. By carefully selecting preparation prescriptions, antioxidants are not used in the prescriptions, a production process is simple, and meanwhile and the technical advantages of uniform preparation content, consistent dissolution, good stability and the like are also achieved.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Coated sustained release tablets of a hygroscopic compound for once-a-day therapy
InactiveUS20030215509A1Moderate weight and volumeEasy to swallowBiocideAnimal repellantsProlonged-release tabletExcipient
The present invention provides coated sustained release tablets of a hygroscopic compound for once-a-day therapy, said tablets having a moderate weight and volume and comprising-(a) a core comprising the hygroscopic compound and pharmaceutically acceptable excipients, (b) a first coating layer comprising a polymer selected from the group consisting of water-insoluble polymers, pH dependent polymers or a mixture thereof, and (c) a second coating layer comprising a water-insoluble polymer.
Owner:SUN PHARMA INDS
Aspilrin slow release tablet
A slow-releasing aspirin table for treating acute ischemic apoplexy and cerebrovascular and cardiovascular diseases is prepared from aspirin and auxiliaries through proportionally mixing hydroxypropyl methylcellulose, polyacrylic resin II and aspirin, mixing with the solution of hydroxypropyl methylcellulose in alcohol to obtain soft material, granulating by nylon sieve, drying, and loading in capsules, or further mixing with talc powder and tabletting. Its advantages are stable concentration in blood and durable curative effect.
Owner:郑州市协和制药厂
Isosorbide mononitrate sustained release tablets and preparation process thereof
InactiveCN104644589AStable drug releaseGood drug release stabilityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsSustained Release TabletFoaming agent
The invention discloses isosorbide mononitrate sustained release tablets and a preparation process thereof. According to the preparation, the sustained release aim is achieved by adopting a method for combining mesoporous carbon and membrane controlled coating. The process comprises the following steps: preparing a mesoporous carbon-isosorbide mononitrate complex; by taking ethyl cellulose as a sustained release coating material and taking hydroxyethyl cellulose as a pore-foaming agent, performing bottom-spray coating by adopting a fluidized bed to obtain sustained release coated pills; and mixing the pills with other pharmaceutically acceptable auxiliary materials, and pressing into the tablets. The sustained release tablets disclosed by the invention have the advantages that the drug release is stable, membrane aging is avoided in the storage process, the drug release stability is high, the bioavailability is high, and the preparation process is simple.
Owner:王加峰
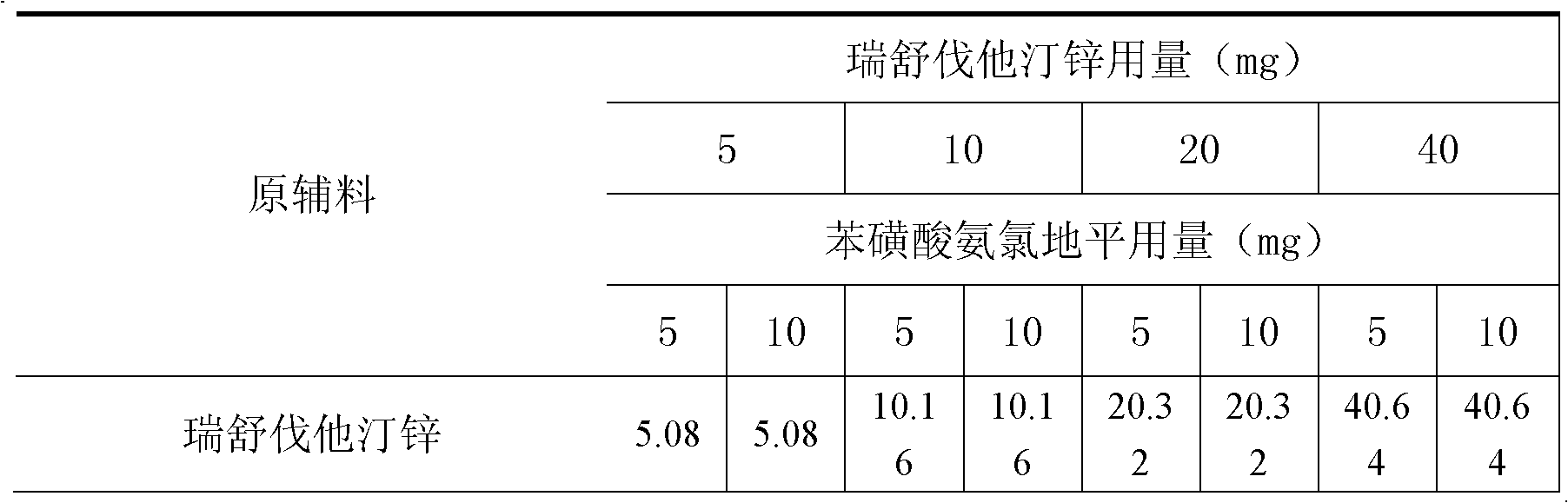
Pharmaceutical composition containing a statin in the form of a zinc salt
InactiveCN102294032AMetabolism disorderEster active ingredientsAdditive ingredientOrally disintegrating tablet
The present invention relates to a pharmaceutical composition containing a statin in the form of a zinc salt. It is an oral preparation made of zinc salts of statins and a calcium ion antagonist as the active pharmaceutical ingredients and pharmaceutically acceptable pharmaceutical excipients, including granules, ordinary tablets, chewable tablets, dispersible tablets, Orally disintegrating tablets, buccal tablets, capsules, dropping pills, sustained-release tablets, etc. As a new preparation for the treatment and prevention of various hypertension and hyperlipidemia, the preparation is widely used clinically.
Owner:FUKANGREN BIO PHARMA
Fluvoxamine maleate sustained release tablets and preparation method thereof
InactiveCN104352471ADefinite curative effectLittle side effectsNervous disorderPharmaceutical product form changeCompulsive disordersPlastic packaging
The invention provides fluvoxamine maleate sustained release tablets. The fluvoxamine maleate sustained release tablets are prepared from 50 parts of fluvoxamine maleate, 50-150 parts of sustained release skeleton material and 5-25 parts of lubricating agent by weight. A preparation method of the fluvoxamine maleate sustained release tablets comprises the steps of material preparing, blending, granulating, blending, tabletting and aluminium-plastic packaging. The fluvoxamine maleate sustained release tablets and the preparation method have the beneficial effects that the fluvoxamine maleate sustained release tablets are used for treating depression and associated symptoms as well as symptoms of obsessive-compulsive disorder and have definite curative effects and small side effects; the novel sustained release preparations are adopted; sustained release refers to reducing the medicine release rates of medicines from the dosage forms and reducing the absorption rates of the medicines into bodies, thus achieving more stable treatment effects; compared with oral liquids, the fluvoxamine maleate sustained release tablets have the advantages of good medicine stability, convenience in packaging, transportation and storage, and the like; the preparation method is simple and practicable and is suitable for industrial production.
Owner:HARBIN SHENGJI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com