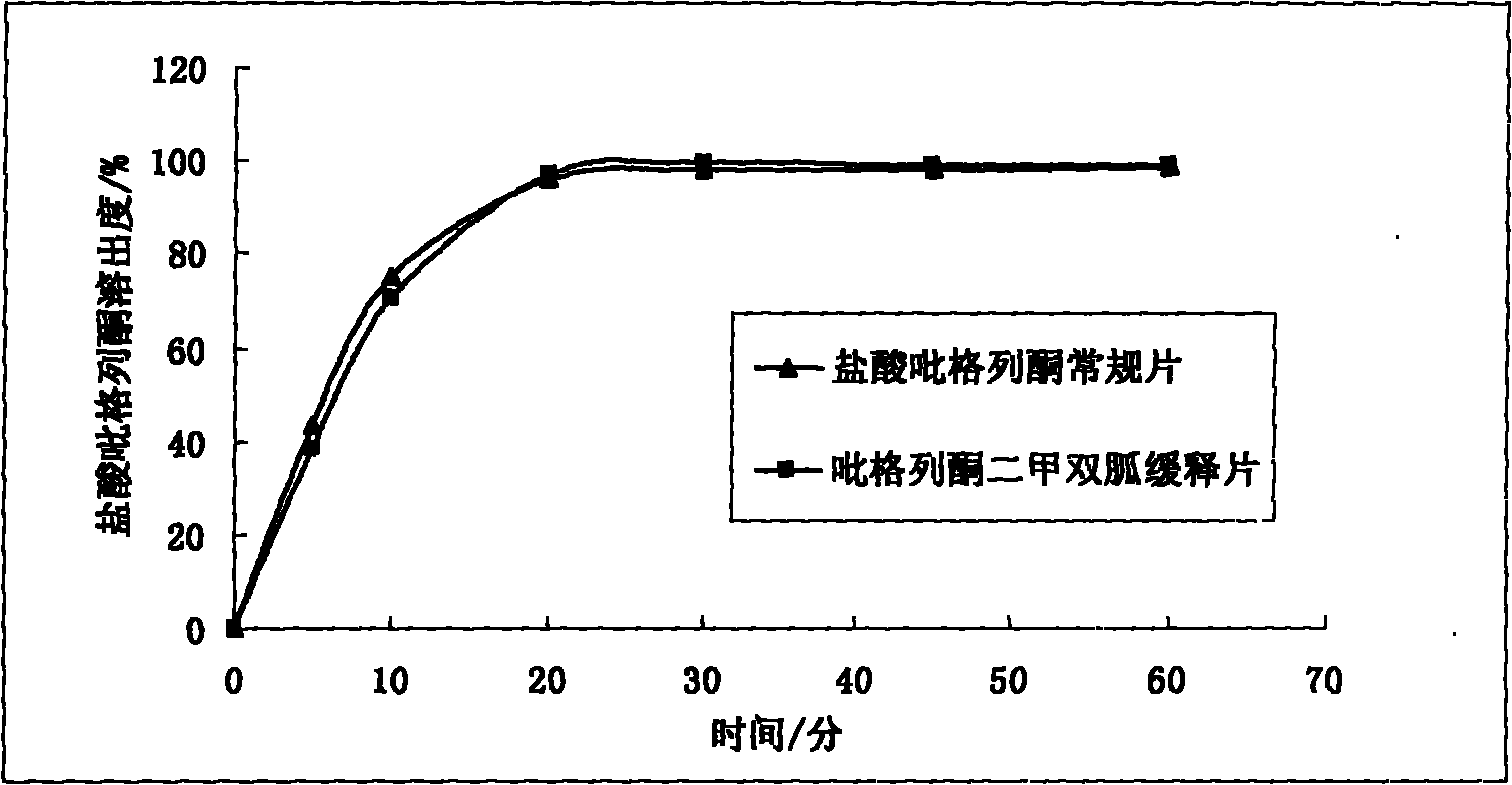
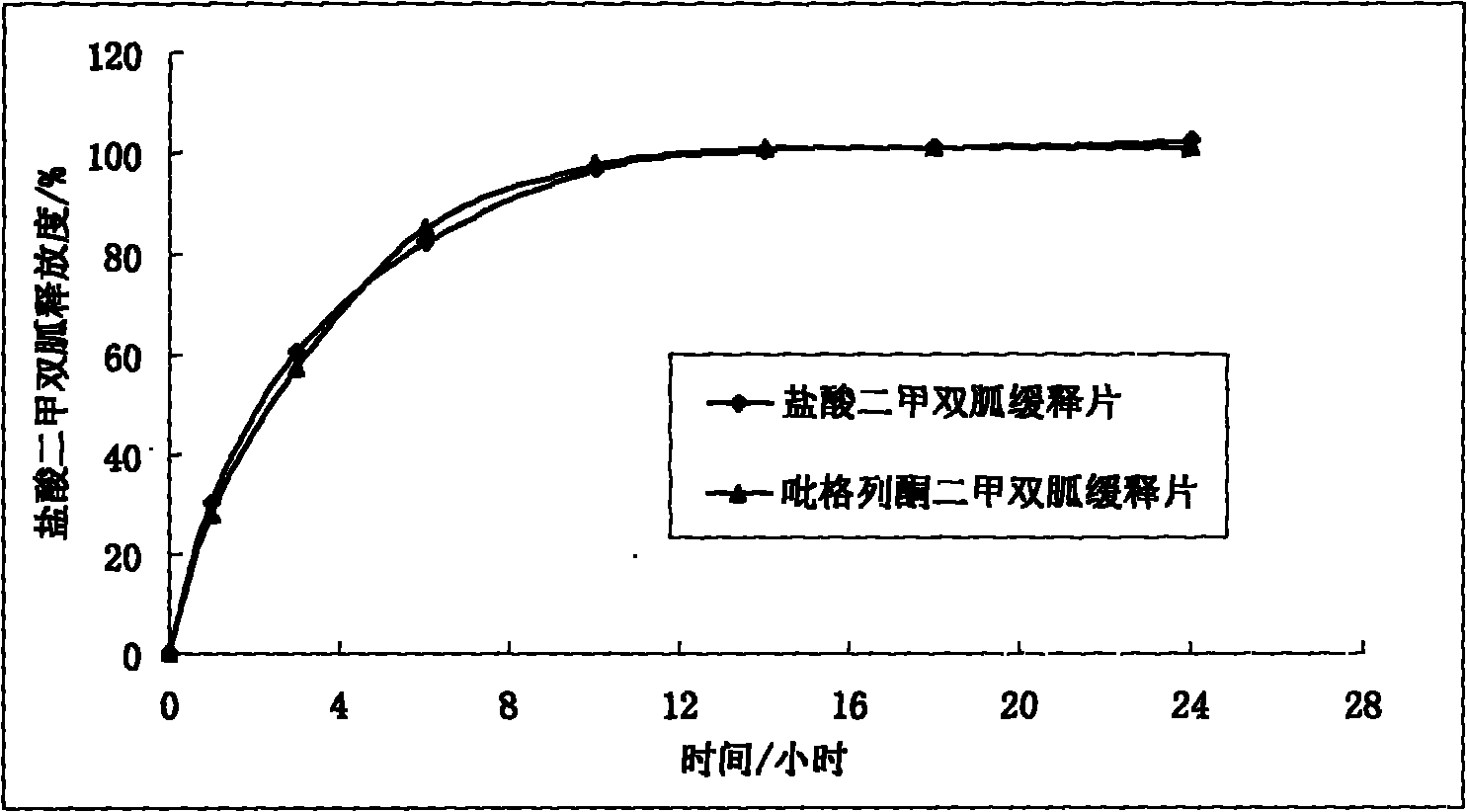
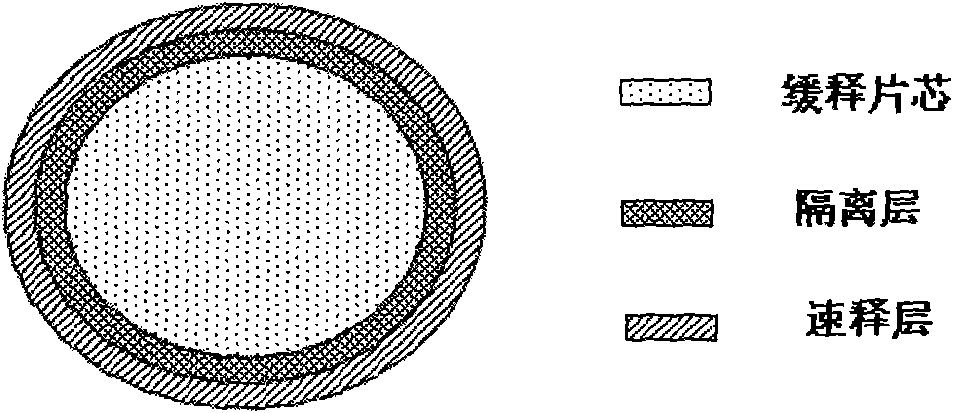Multicomponent sustained-release preparation and preparation method thereof
A slow-release preparation, multi-component technology, applied in the field of medicine, can solve the problems of the initial release of the inner layer of the drug and the inability of the outer layer of the drug to achieve full dissolution, and achieve the effects of large dose, short half-life and long half-life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Compound Sustained Release Tablets Containing Metformin Hydrochloride 500mg Pioglitazone Hydrochloride 15mg
[0027] (1) Preparation of Metformin Hydrochloride Sustained Release Tablet Core
[0028] Get metformin hydrochloride 500g, hydroxypropyl methylcellulose (METHOCEL K100MCR) 200g, sodium carboxymethylcellulose 75g, microcrystalline cellulose 20g, mix well, add 5% PVP ethanol solution to make granules, add after drying 0.5% magnesium stearate, compressed into tablets, each containing 500mg of metformin hydrochloride. Put the above-mentioned tablet in a coating pan, and coat with the following coating solution, and the weight of the coating is increased to about 2-4%.
[0029] Eudragit RS 100 13g
[0030] Eudragit S 100 0.3g
[0031] Triethyl citrate 3.0g
[0033] Ethanol 400ml
[0034] (2) Preparation of isolation layer
[0035] Take the above-mentioned metformin hydrochloride sustained-release tablet core, put it in a coating pan, ...
Embodiment 2
[0054] Compound Sustained Release Tablets Containing Metformin Hydrochloride 500mg Glimepiride 1mg
[0055] (1) Preparation of Metformin Hydrochloride Sustained Release Tablet Core
[0056] The preparation method is the same as in Example 1.
[0057] (2) Preparation of isolation layer
[0058] Take the above-mentioned metformin hydrochloride sustained-release tablet core, put it in a coating pan, and coat it with the following coating solution, and the weight of the coating will increase to about 1.5-2%.
[0059] Copovidone 12g
[0060] Micronized silica gel 0.4g
[0061] Macrogol 400 1.0g
[0062] Ethanol 200ml
[0063] (3) Glimepiride coated drug
[0064] Take by weighing hydroxypropyl methylcellulose E5, copovidone, polyethylene glycol 400, polysorbate 80 according to the prescription quantity, add appropriate amount of absolute ethanol to dissolve, take glimepiride and micropowder silica gel according to the prescription quantity, Add an appropriate amount of absolu...
Embodiment 3
[0075] Compound Sustained Release Tablets Containing Niacin 500mg Simvastatin 5mg
[0076] (1) Preparation of niacin sustained-release tablet core
[0077] Take niacin 500g, hydroxypropyl methylcellulose (METHOCEL K15MCR) 180g, mix well, add 5% PVP alcohol solution to make granules, add 0.5% magnesium stearate after drying, press into tablets, each tablet contains Niacin 500mg.
[0078] (2) Preparation of isolation layer
[0079] Take the above-mentioned niacin sustained-release tablet core, put it in a coating pan, and coat it with the following coating solution, and the weight of the coating will increase to about 1.5% to 2%.
[0080] Hydroxypropyl Methyl Cellulose E5 11.0g
[0081] Differential Silica Gel 0.6g
[0082] Triethyl citrate 1.1g
[0083] Ethanol 200ml
[0084] (3) Simvastatin coated drug
[0085] Take hydroxypropyl methylcellulose E5, copovidone, and triethyl citrate according to the prescription quantity, add appropriate amount of absolute ethanol to dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com