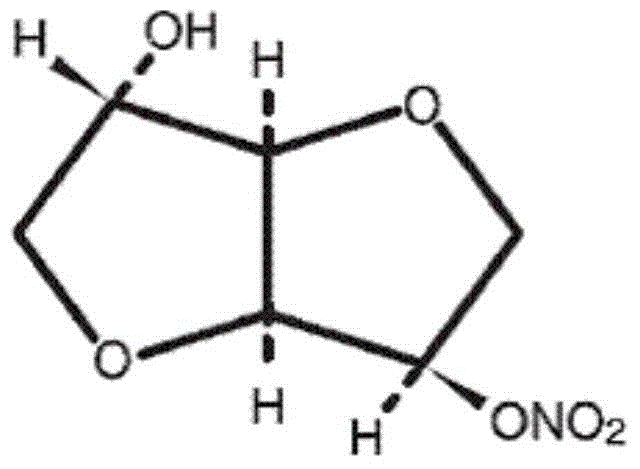
Isosorbide mononitrate sustained release tablets and preparation process thereof
A technology of isosorbide dinitrate and sustained-release tablets, which is applied in the directions of non-active ingredients medical preparations, cardiovascular system diseases, pharmaceutical formulations, etc. Inhomogeneous and other problems, to achieve the effect of good drug release stability, no membrane aging, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] Preparation Process:
[0033] ① Dissolve raw material isosorbide mononitrate in water, add mesoporous carbon (specific surface area is 1018m 2 / g, the pore volume is 0.51cm 3 / g), continuous stirring, ultrasound, after the drug enters the mesoporous carbon channel to reach equilibrium, spray drying to remove the solvent, and obtain the raw material-mesoporous carbon composite;
[0034] ②Put the raw material-mesoporous carbon composite into the fluidized bed, preheat the material to a temperature of about 34°C, and use the acetone-water (volume ratio 8:2) solution of ethyl cellulose and hydroxyethyl cellulose (solid content 5%) bottom spray coating to prepare sustained-release coated pellets, and the weight gain of the coating is 14.6% (the coating film accounts for the weight percentage of the raw material-mesoporous carbon composite);
[0035] ③Mix the sustained-release coated pellets with lactose, pregelatinized starch, crospovidone, and magnesium steara...
Embodiment 2
[0037]
[0038] Preparation Process:
[0039] ① Dissolve raw material isosorbide mononitrate in water, add mesoporous carbon (specific surface area is 1058m 2 / g, the pore volume is 0.86cm 3 / g), continuous stirring, ultrasound, after the drug enters the mesoporous carbon channel to reach equilibrium, spray drying to remove the solvent, and obtain the raw material-mesoporous carbon composite;
[0040] ②Put the raw material-mesoporous carbon composite into the fluidized bed, preheat the material to a temperature of about 34°C, and use the acetone-water (volume ratio 8:2) solution of ethyl cellulose and hydroxyethyl cellulose (solid content 5%) bottom spray coating to prepare sustained-release coated pellets, and the weight gain of the coating is 12% (the coating film accounts for the weight percentage of the raw material-mesoporous carbon composite);
[0041] ③Mix the sustained-release coated pellets with microcrystalline cellulose, sodium carboxymethyl starch, and magnesiu...
Embodiment 3
[0043]
[0044]
[0045] Preparation Process:
[0046] ① Dissolve raw material isosorbide mononitrate in water, add mesoporous carbon (specific surface area is 1018m 2 / g, the pore volume is 0.51cm 3 / g), continuous stirring, ultrasound, after the drug enters the mesoporous carbon channel to reach equilibrium, spray drying to remove the solvent, and obtain the raw material-mesoporous carbon composite;
[0047] ②Put the raw material-mesoporous carbon composite into the fluidized bed, preheat the material to a temperature of about 34°C, and use the acetone-water (volume ratio 8:2) solution of ethyl cellulose and hydroxyethyl cellulose (solid content 5%) bottom spray coating to prepare sustained-release coated pellets, and the coating weight increased to 7.5% (the coating film accounts for the weight percentage of the raw material-mesoporous carbon composite);
[0048] ③Mix the sustained-release coated pellets with microcrystalline cellulose, starch, croscarmellose sodium...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com