Double-layer sustained release tablets for compensating iron and preparation thereof
A slow-release tablet and double-layer technology, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve problems such as low utilization rate, lack of iron, and slow onset of action The effect is good, the preparation method is simple, and the effect of improving nutritional status
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
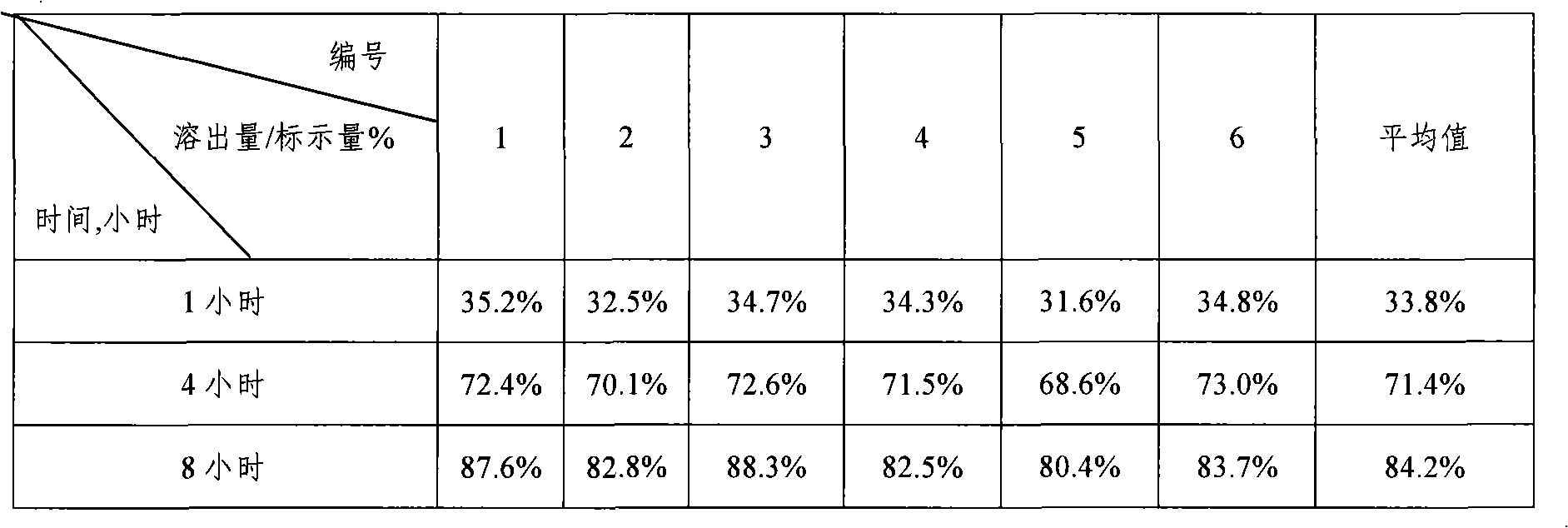
Embodiment 1
[0035] Prepare iron supplement sustained-release tablets in the following steps:
[0036] (1) Pulverize the required raw and auxiliary materials, pass through an 80-mesh sieve, and set aside;
[0037] (2) Sustained-release layer preparation: take by weighing 800g of iron glycinate, 320g of ferrous gluconate, 272g of hypromellose (K15M), 200g of lactose, mix well, pass through a 60 mesh sieve, add an appropriate amount of 20% dilute ethanol solution, Knead the soft material, pass through a 20-mesh sieve to obtain wet granules, dry in an oven at 50-60°C for 3-4 hours, pass through a 18-mesh sieve for granulation, and weigh to obtain the sustained-release layer granules;
[0038] Preparation of quick-release layer: Weigh 300g of angelica powder, 200g of astragalus powder, vitamin B 6 1g, filler: white granulated sugar powder 500g, disintegrant: sodium carboxymethyl starch 240g, binder: low viscosity hypromellose 240g dissolved in an appropriate amount of 20% dilute ethanol solu...
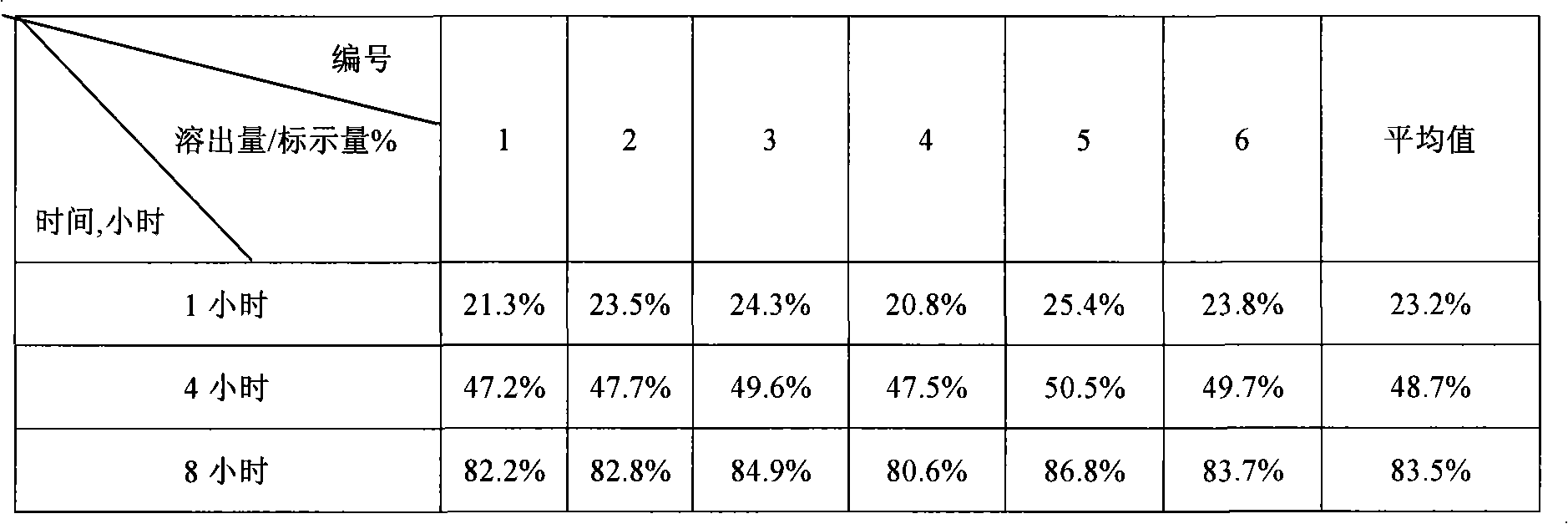
Embodiment 2
[0047] Prepare iron supplement sustained-release tablets in the following steps:
[0048] (1) Pulverize the required raw and auxiliary materials, pass through an 80-mesh sieve, and set aside;
[0049] (2) Sustained-release layer preparation: Weigh 800g of heme iron, 1200g of methylcellulose (4000cPa.s), 160g of granulated sugar, mix well, pass through a 60-mesh sieve, and mix the adhesive (low-viscosity hypromellose ) 200g dissolved in an appropriate amount of 20% dilute ethanol solution, added to the powder, kneaded to make soft material, passed through a 20-mesh sieve to obtain wet granules, dried in an oven at 50-60°C for 3-4 hours, passed through a 18-mesh sieve for granulation , weighed to obtain the slow-release layer granular material;
[0050] Quick-release layer preparation: Weigh 200g of Panax notoginseng powder, 160g of Codonopsis powder, filler (white granulated sugar powder 132g), disintegrating agent (sodium carboxymethyl starch 30g), binder (low viscosity hypro...
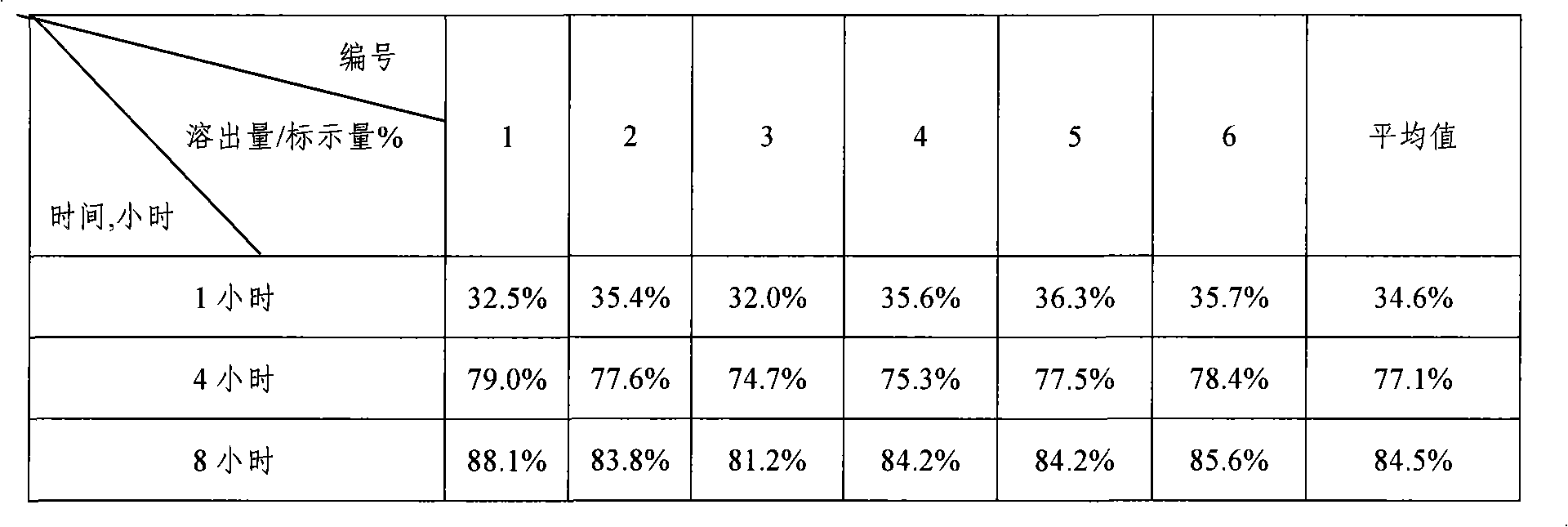
Embodiment 3
[0058] Prepare iron supplement sustained-release tablets in the following steps:
[0059] (1) Pulverize the required raw and auxiliary materials, pass through an 80-mesh sieve, and set aside;
[0060] (2) Sustained-release layer preparation: Weigh 120g of sodium ferric edetate, 60g of methylcellulose (4000cPa.s), 300g of starch, mix well, pass through a 60-mesh sieve, and mix 120g of binder (dextrin) Dissolve in an appropriate amount of 20% dilute ethanol solution, add to the powder, knead to make soft material, pass through a 20-mesh sieve to obtain wet granules, dry in an oven at 50-60°C for 3-4 hours, pass through a 18-mesh sieve for granulation, weigh Heavy, get slow-release layer granules;
[0061] Preparation of quick-release layer: Weigh 720g of red date powder, 720g of wolfberry powder, vitamin B 6 10g, folic acid 2g, disintegrant (microcrystalline cellulose) 360g, binder (starch) 480g dissolved in an appropriate amount of 20% dilute ethanol solution, added to the po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com