Edoxaban sustained release tablet and preparation method thereof
A technology for edoxaban and sustained-release tablets, which is applied in the field of sustained-release matrix tablets containing active ingredient edoxaban and its preparation, and can solve the problems of reduced therapeutic effect, increased adverse drug reactions, and excessive fluctuations in blood drug concentration And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
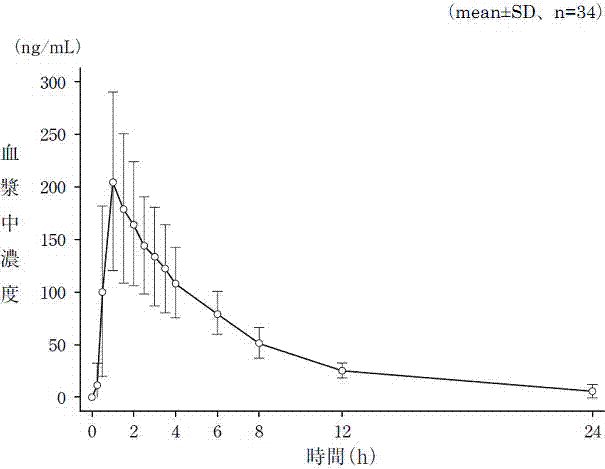
Image
Examples
Embodiment 1
[0047] Prescription %
[0048] Edoxaban p-toluenesulfonate hydrate 20.2g 20.2%
[0049] HPMC K100M 20g 20.0%
[0050] Carbomer 974P 10g 10.0%
[0051] Lactose 17g 17.0%
[0052] Starch 30g 30.0%
[0053] Talc 2g 2.0%
[0054] Magnesium Stearate 1g 1.0%.
[0055]Preparation method: 1. Pass the raw material through a 120-mesh sieve; 2. Mix the raw material, HPMC K100M, and Carbomer 974P evenly, granulate with about 90% ethanol, and then dry; 3. Sieve and granulate, add the prescribed amount of Talc and magnesium stearate, mix well and compress.
[0056] In the release test, the release rate of the sample was measured according to the second appendix XC of the 2010 edition of the Chinese Pharmacopoeia, and the first method of the second appendix XD of the Chinese Pharmacopoeia of the 2010 edition was used. Take 900ml of buffer salt with pH 6.8 as the solvent, rotate at 50 rpm, take samples at 1, 4 and 8 hours, and measure the release degree of ...
Embodiment 2
[0062] Prescription %
[0063] Edoxaban p-toluenesulfonate hydrate 40.4g 20.2%
[0064] HPMC K100M 36g 18.0%
[0065] Carbomer 974P 12g 6.0%
[0066] Lactose 34g 17.0%
[0067] Starch 72g 36.0%
[0068] Talc 4g 2.0%
[0069] Magnesium Stearate 2g 1.0%
[0070] Preparation method: 1. Pass the raw material through a 120-mesh sieve; 2. Mix the raw material, HPMC K100M, and Carbomer 974P evenly, granulate with about 90% ethanol, and then dry; 3. Sieve and granulate, add the prescribed amount of Talc and magnesium stearate, mix well and compress.
[0071] In the release test, the release rate of the sample was measured according to the second appendix XC of the 2010 edition of the Chinese Pharmacopoeia, and the first method of the second appendix XD of the Chinese Pharmacopoeia of the 2010 edition was used. Take 900ml of buffer salt with pH 6.8 as the solvent, rotate at 50 rpm, take samples at 1, 4 and 8 hours, and measure the release degree of the sample. The results are as...
Embodiment 3
[0077] Prescription %
[0078] Edoxaban p-toluenesulfonate hydrate 20.2g 20.2%
[0079] HPMC K100M 35g 35.0%
[0080] Lactose 11g 11.0%
[0081] Starch 30g 30.0%
[0082] PVP K30 3g 3.0%
[0083] Magnesium Stearate 1g 1.0%
[0084] Preparation method: 1. Pass the raw material through a 120-mesh sieve; 2. Mix the raw material and HPMC K100M uniformly, granulate with about 90% ethanol solution of PVP K30, and then dry; 3. Sieve and granulate, and add the prescribed amount of stearic acid. Magnesium, mix well and tablet.
[0085] In the release test, the release rate of the sample was measured according to the second appendix XC of the 2010 edition of the Chinese Pharmacopoeia, and the first method of the second appendix XD of the Chinese Pharmacopoeia of the 2010 edition was used. Take 900ml of buffer salt with pH 6.8 as the solvent, rotate at 50 rpm, take samples at 1, 4 and 8 hours, and measure the release degree of the sample. The results are as follows:
[0086] Time ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com