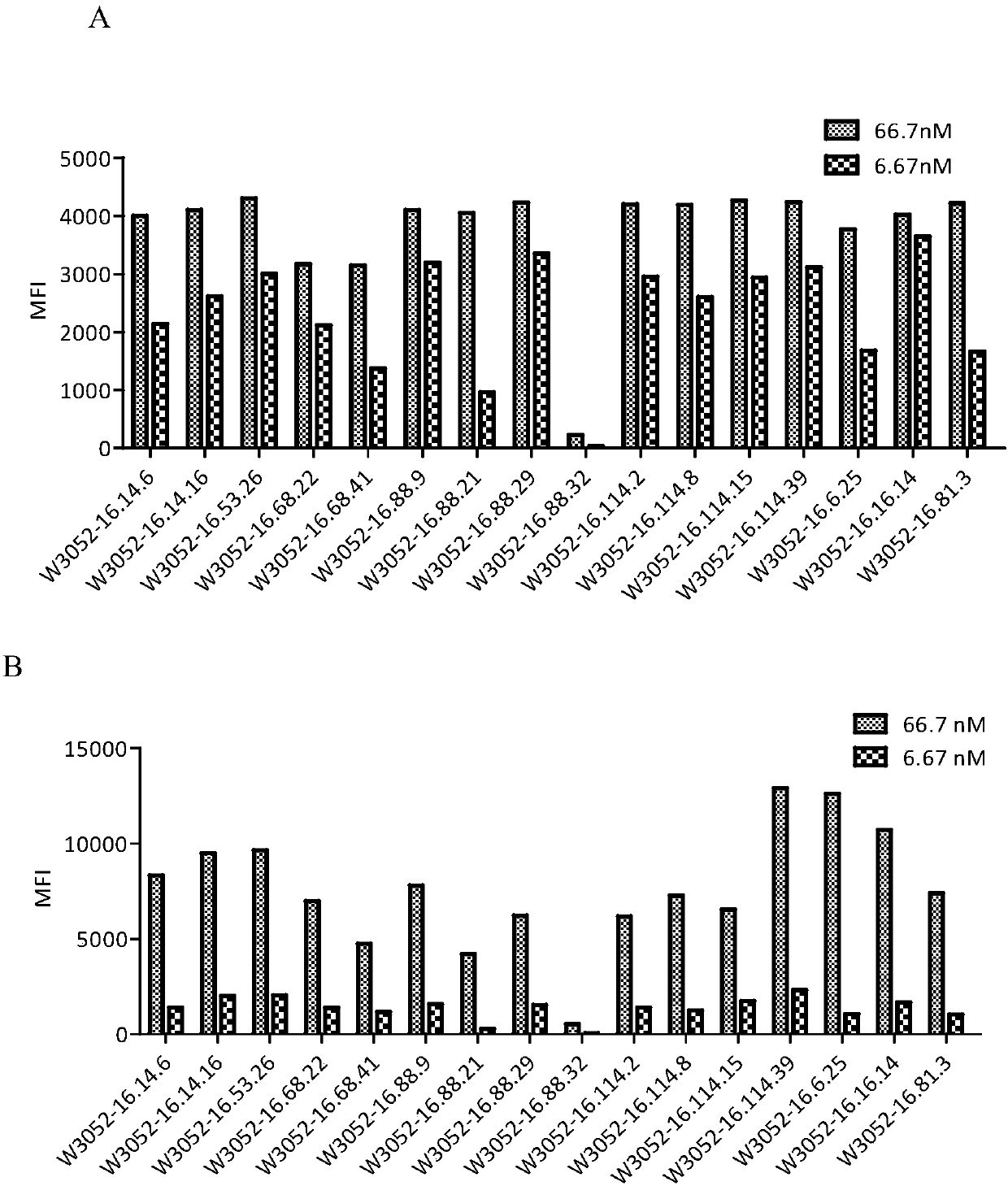
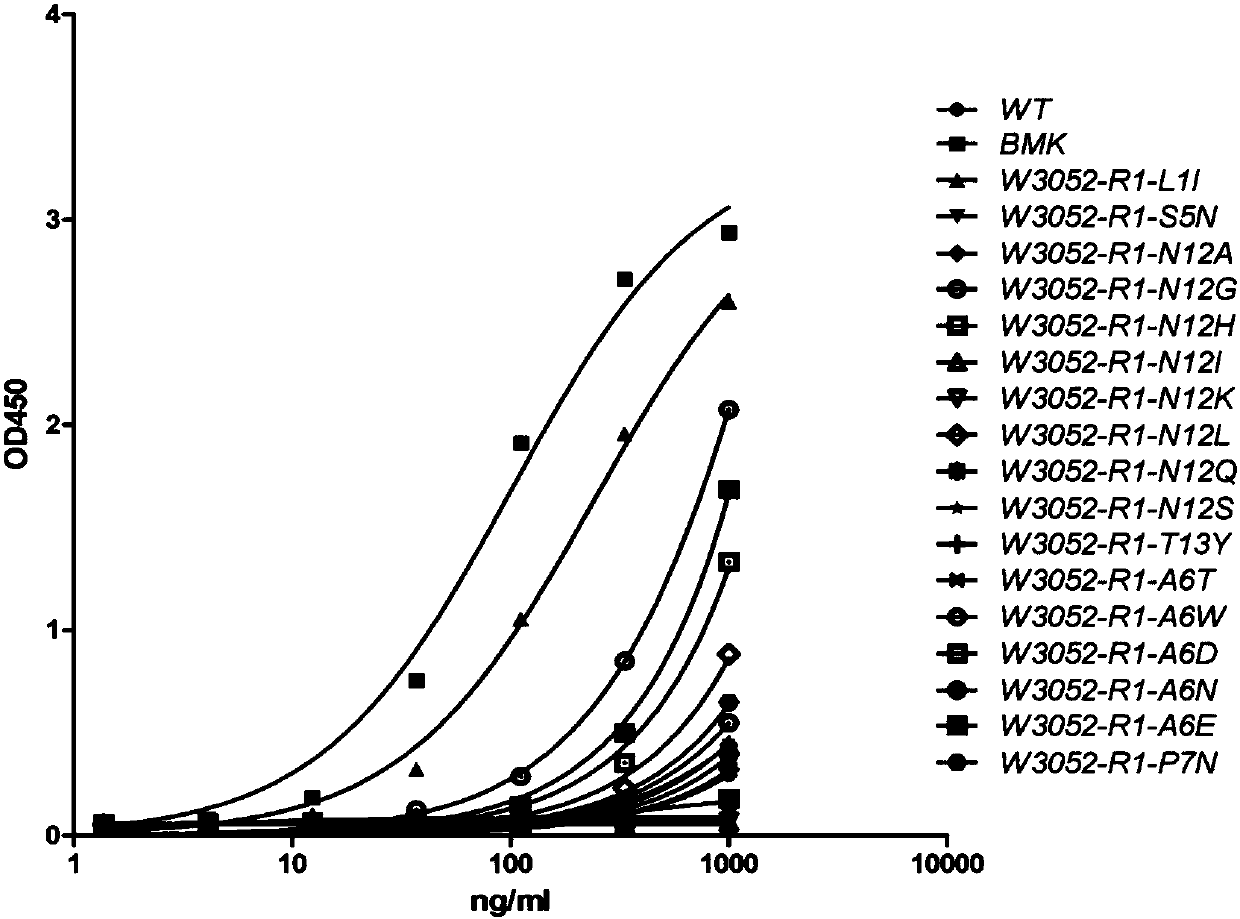
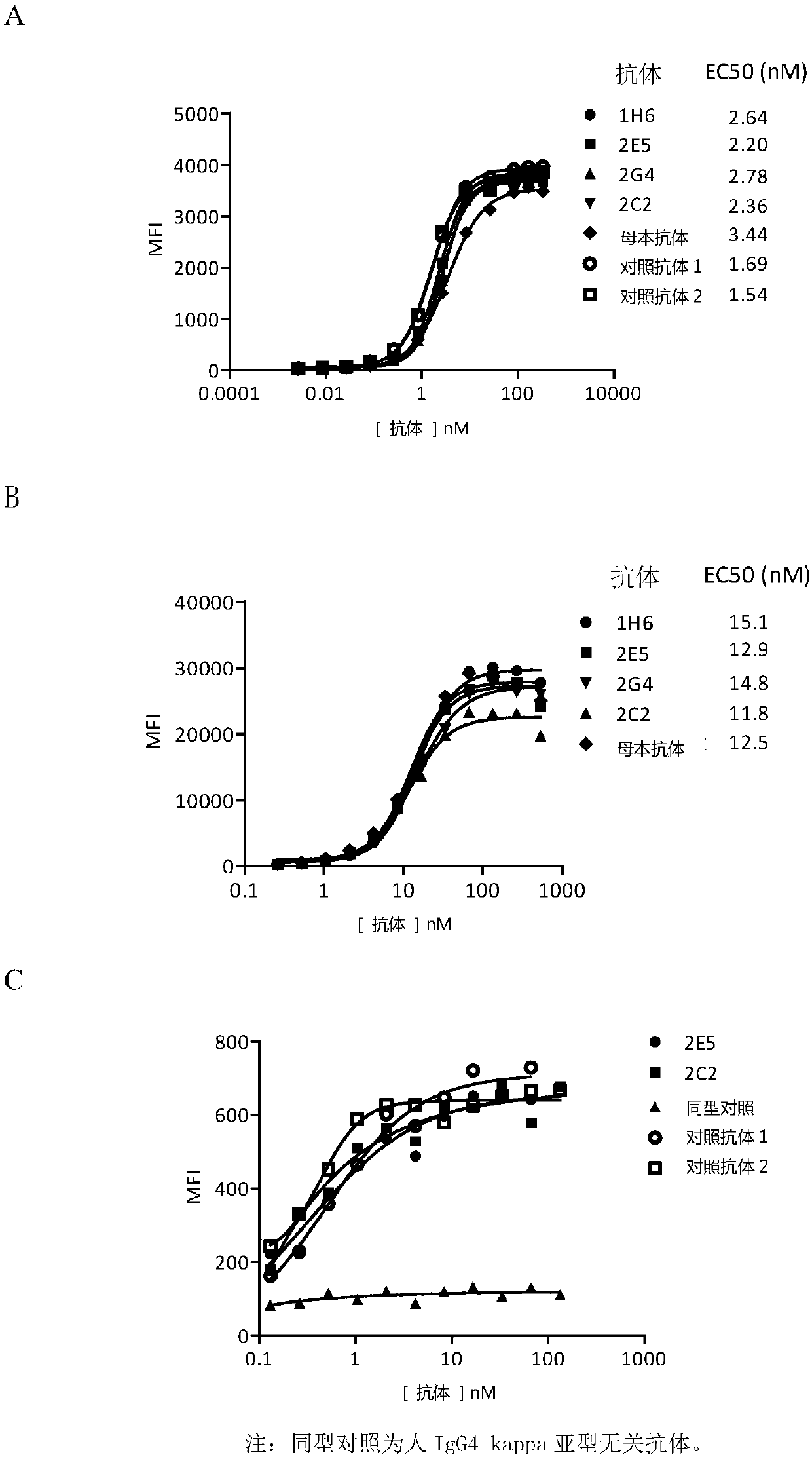
Novel monoclonal antibody against PD-1
A PD-1 and antibody technology, applied in the direction of antibodies, carriers, anti-tumor drugs, etc., can solve problems such as limiting the application of antibody drugs and reducing the therapeutic effect of antibody drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] Example 1 Preparation of experimental materials
[0245] 1. Antigen preparation
[0246] Synthesize the DNA encoding the full length or extracellular region of PD-1 and PD-L1, and insert them into the expression vector pcDNA3.3. After extracting a large amount of plasmid DNA, sequencing to verify the sequence of the inserted DNA fragment. The fusion protein PD-1 extracellular region and PD-L1 extracellular region contain different tags, including human Fc, murine Fc and His tags, etc., by transfecting PD-1 extracellular region gene sequence into CHO-S or HEK293 Expressed in cells. Five days after the transient transfection of the cells, the cell culture supernatant was collected, and the fusion protein was purified and quantified for immunization and screening.
[0247] 2. Establishment of stable cell line
[0248] In order to obtain antibody screening verification tools, PD-1 and PD-L1 transfected cell lines were prepared. Briefly, the pcDNA3.3 vector expression plasmid ...
Embodiment 2
[0249] Example 2 Production of antibody hybridomas
[0250] 1. Immunity
[0251] Female SD rats aged 6 to 8 weeks were sensitized with 10 μg of human PD-1 extracellular domain protein and 10 μg of mouse PD-1 extracellular domain protein (in TiterMax) via plantar injection, and then used in each week. The human PD-1 extracellular domain protein or mouse PD-1 extracellular domain protein in the aluminum phosphate gel adjuvant is immunized once through the sole of the foot until it is suitable for fusion. During the immunization period, the serum titer of anti-PD-1 antibodies was detected by ELISA or FACS every two weeks.
[0252] 2. Cell Fusion
[0253] When the antibody titer reaches a sufficiently high level, the rats are given the final adjuvant-free immunogen (human PD-1 extracellular domain protein and mouse PD-1 extracellular domain protein) to stimulate (with an equal volume of phosphate buffer Solution (PBS) instead of adjuvant). The SP2 / 0 cells were resuscitated one week b...
Embodiment 3
[0266] Example 3 Sequencing of antibody hybridoma cells, humanized construction of antibodies and affinity maturation
[0267] 1. Hybridoma antibody sequencing
[0268] Trizol reagent was used to isolate RNA from monoclonal hybridoma cells. The VH and VL segments of the PD-1 chimeric antibody are amplified by the following method: First, reverse transcription of RNA into cDNA by reverse transcriptase.
[0269] Reaction system (20μL)
[0270]
[0271] Reaction conditions
[0272]
first step
Second step
third step
the fourth step
Temperature(℃)
25
37
85
4
time
10 minutes
120 minutes
5
∞
[0273] The obtained cDNA was used as a template, and the following PCR amplification was performed using specific primers for the gene of interest. The PCR reaction operation is as follows:
[0274]
[0275] Reaction conditions:
[0276]
[0277]
[0278] The resulting PCR reaction product (10 μL) was ligated to the pMD18-T vector. 10μL ligation product was transformed into Top10 competent cel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com