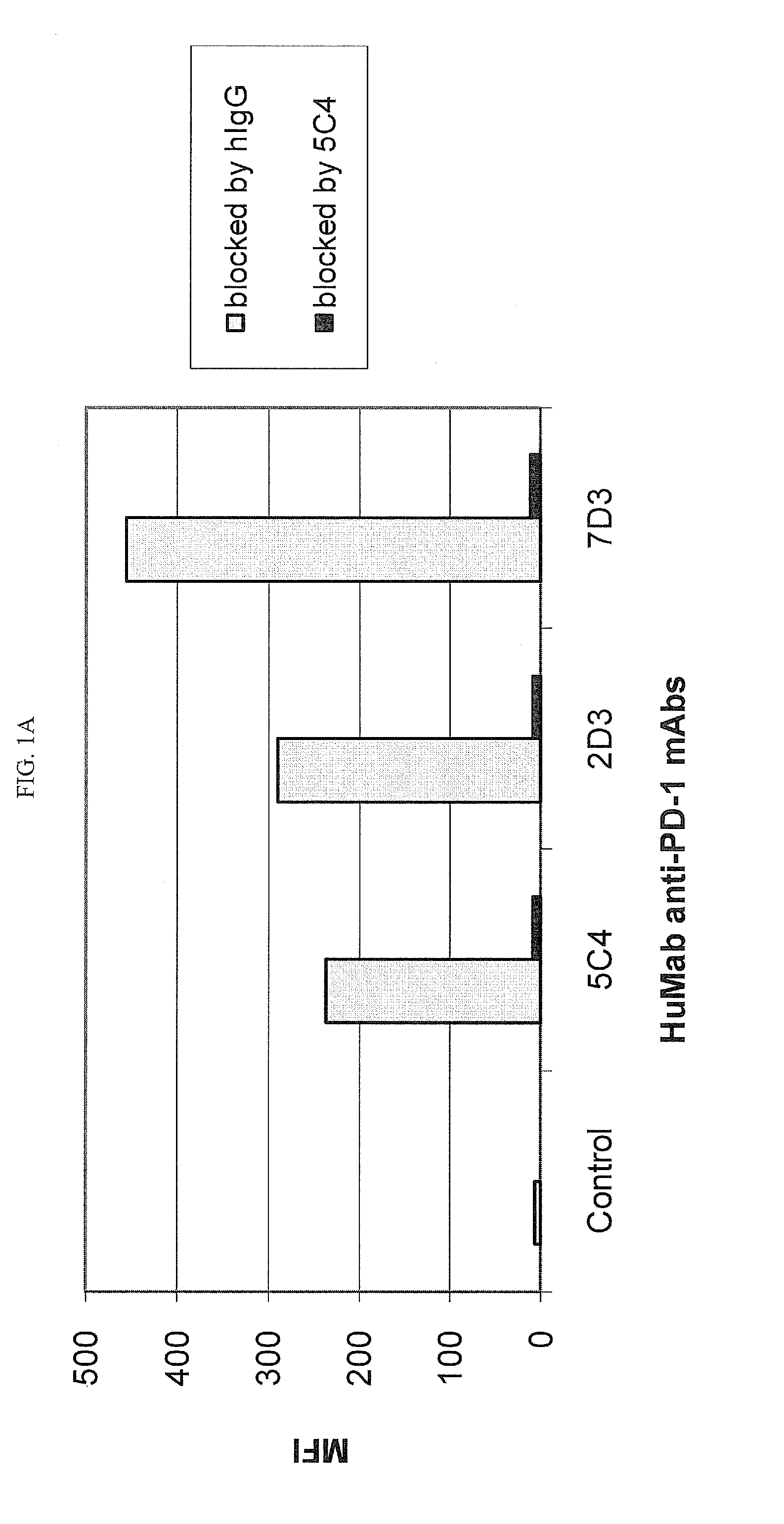
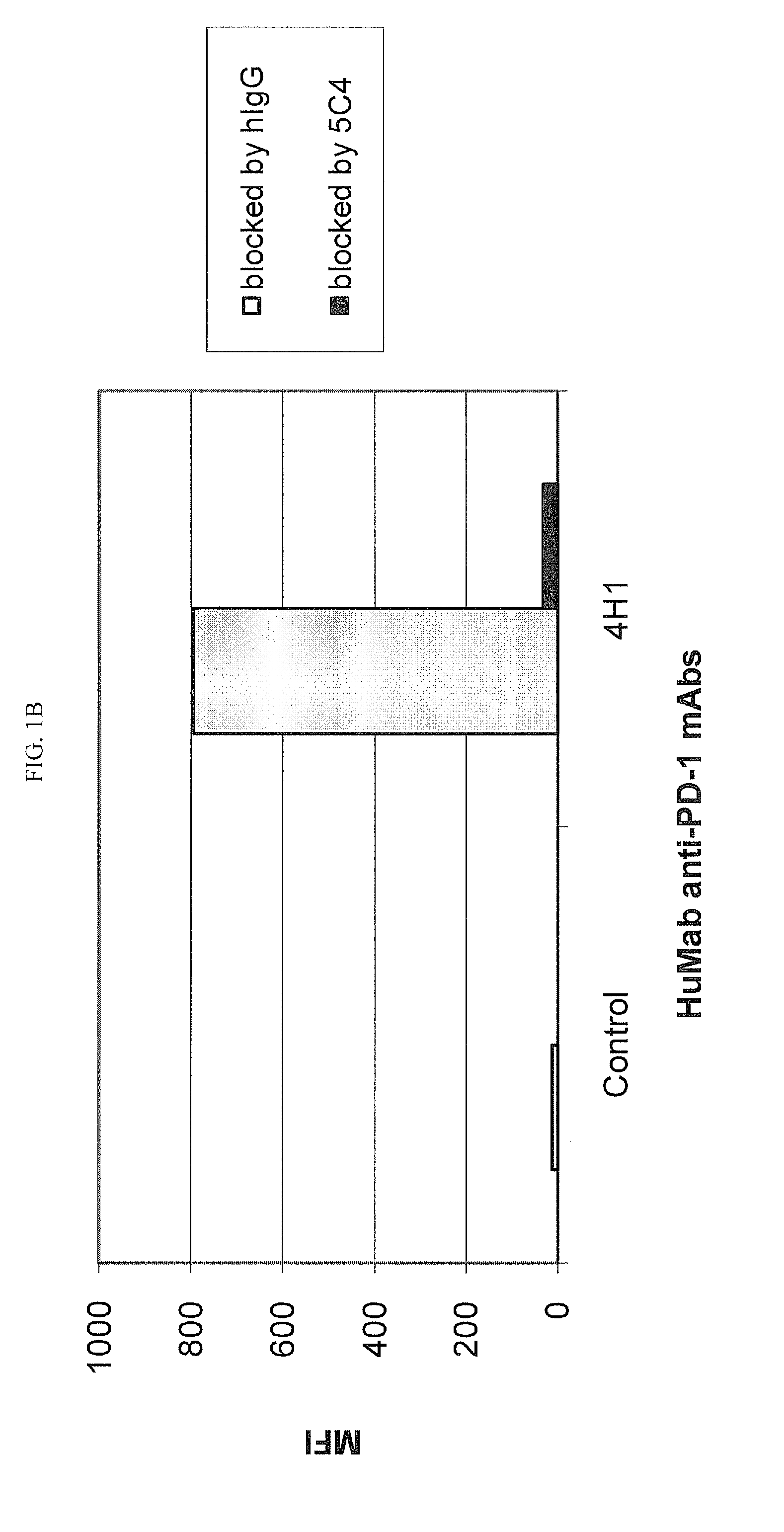
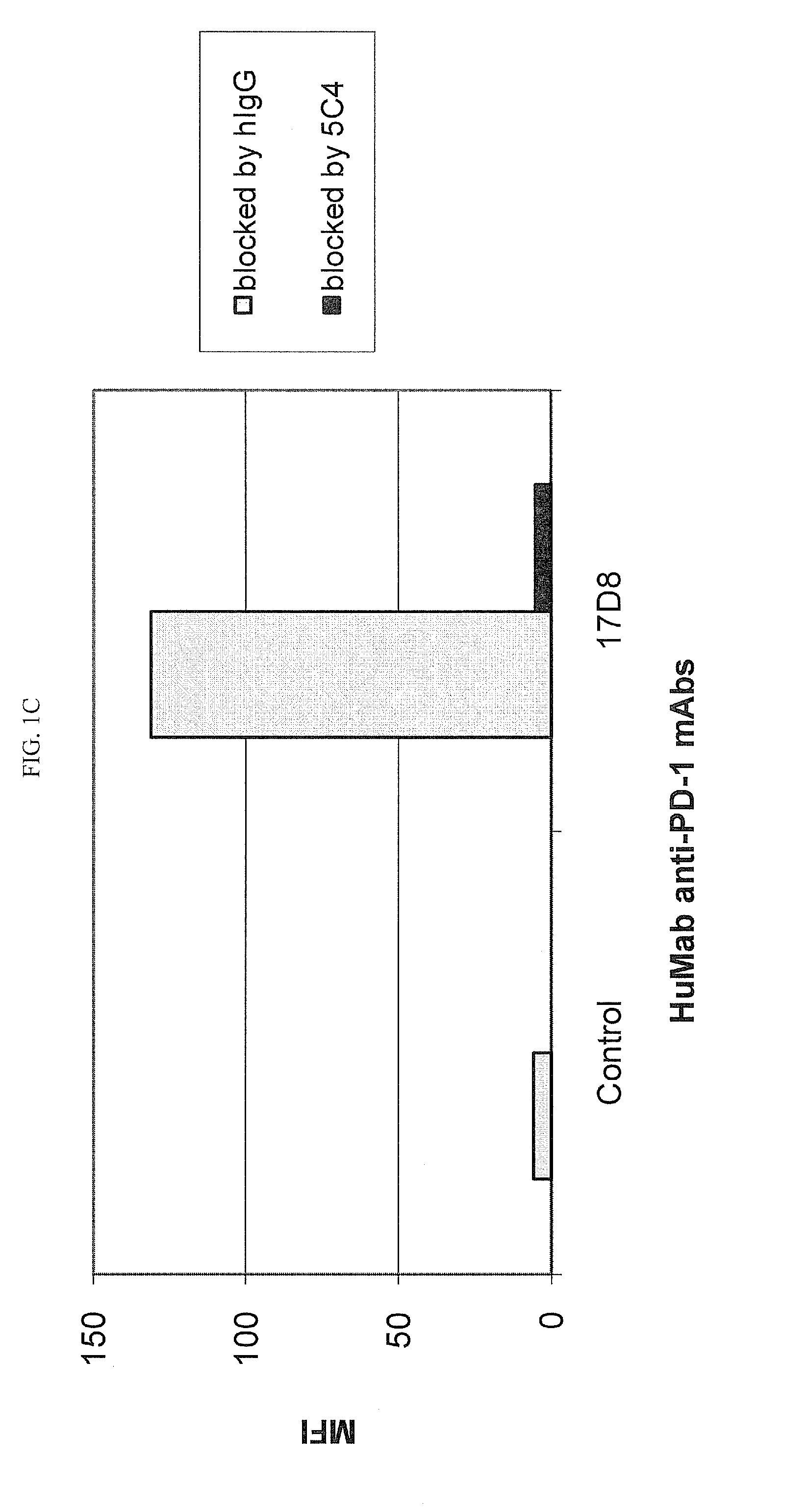
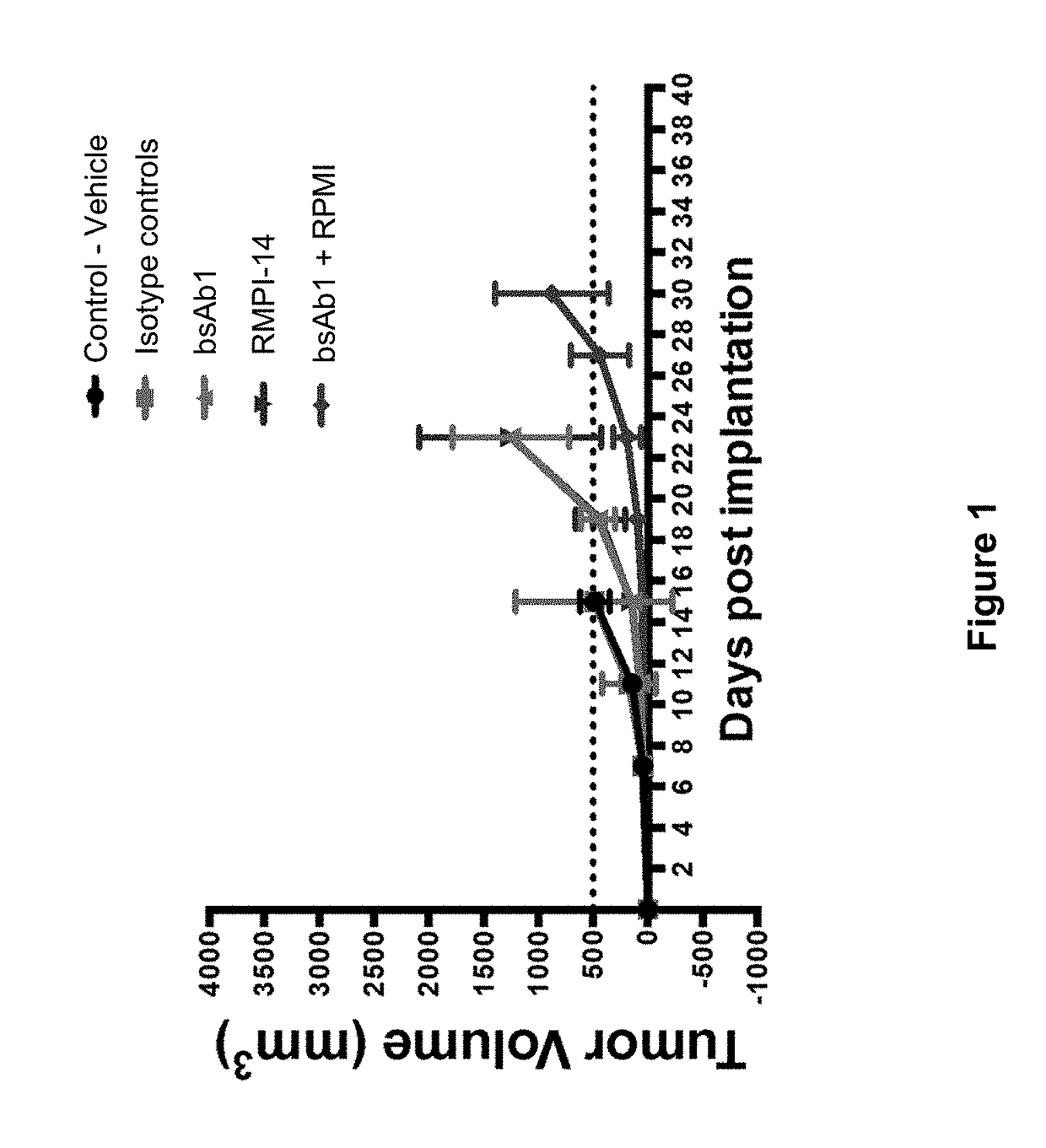
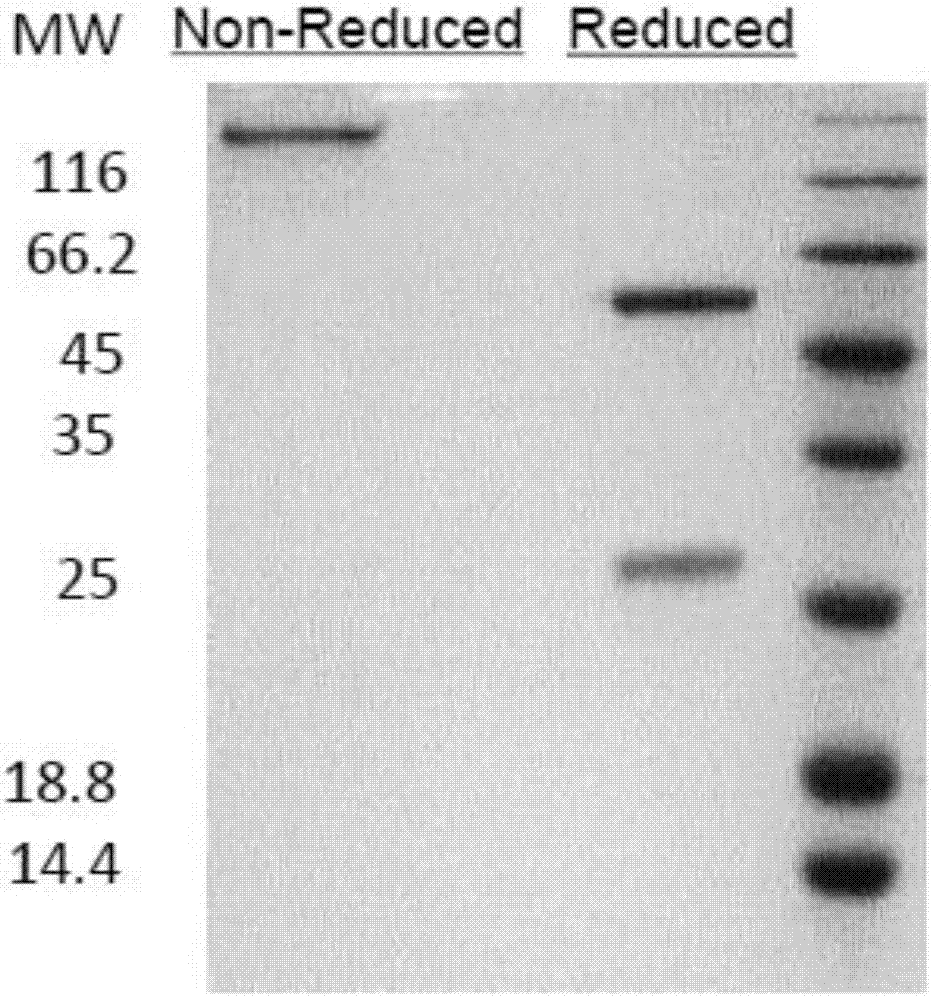
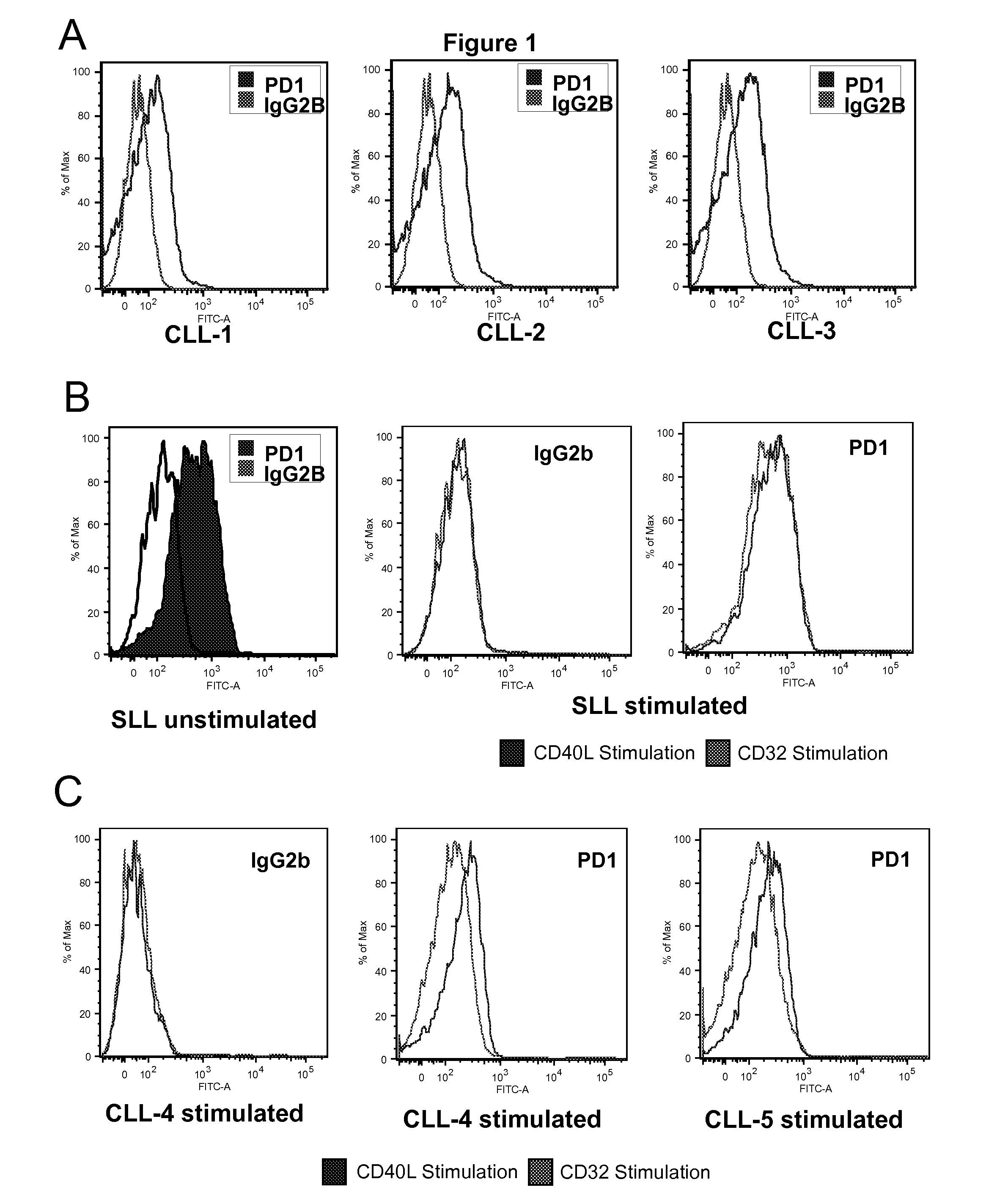
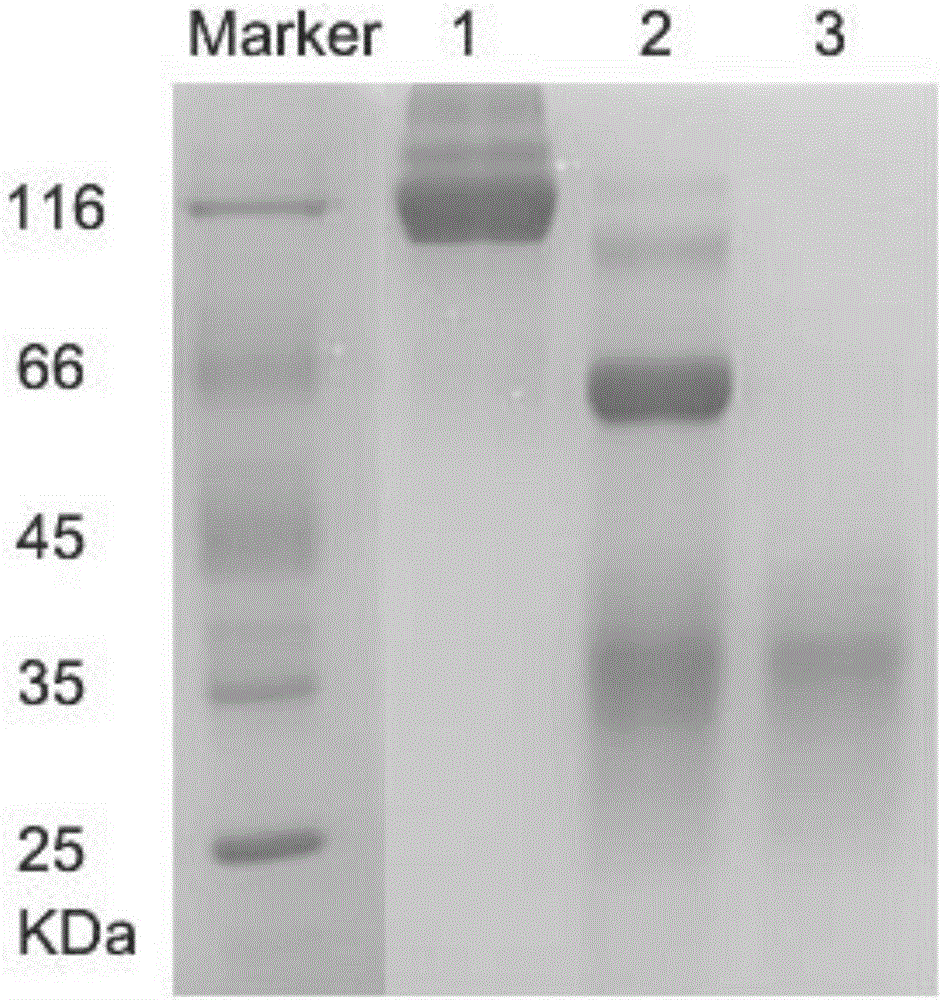
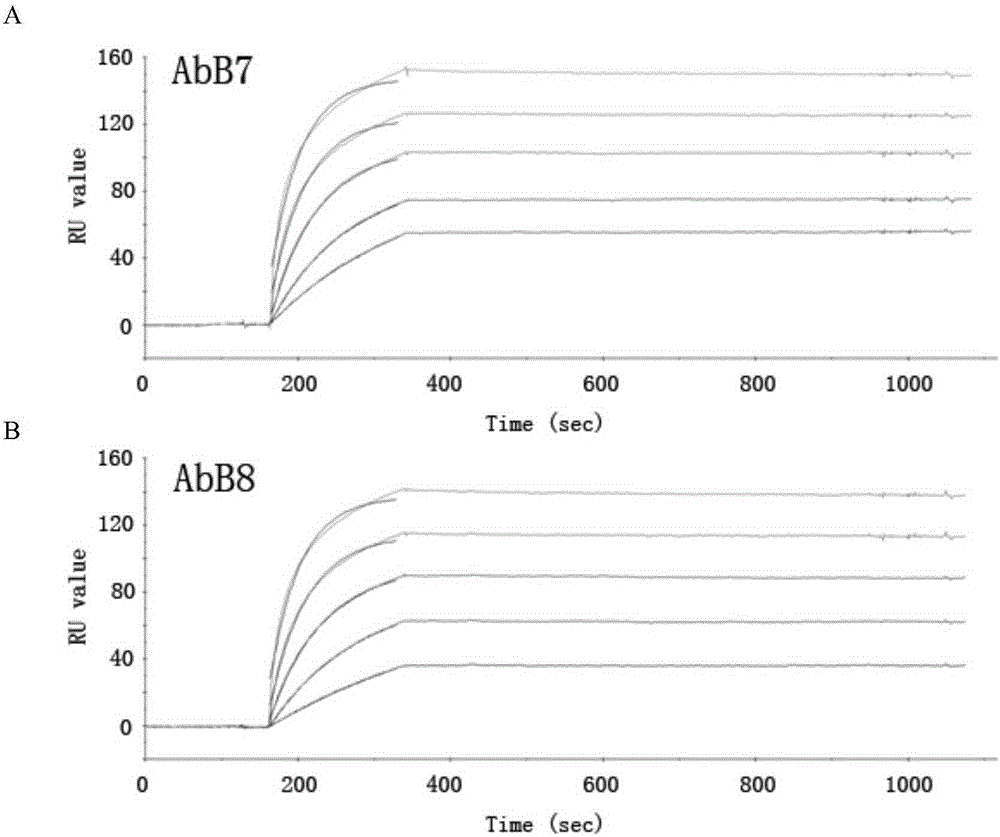
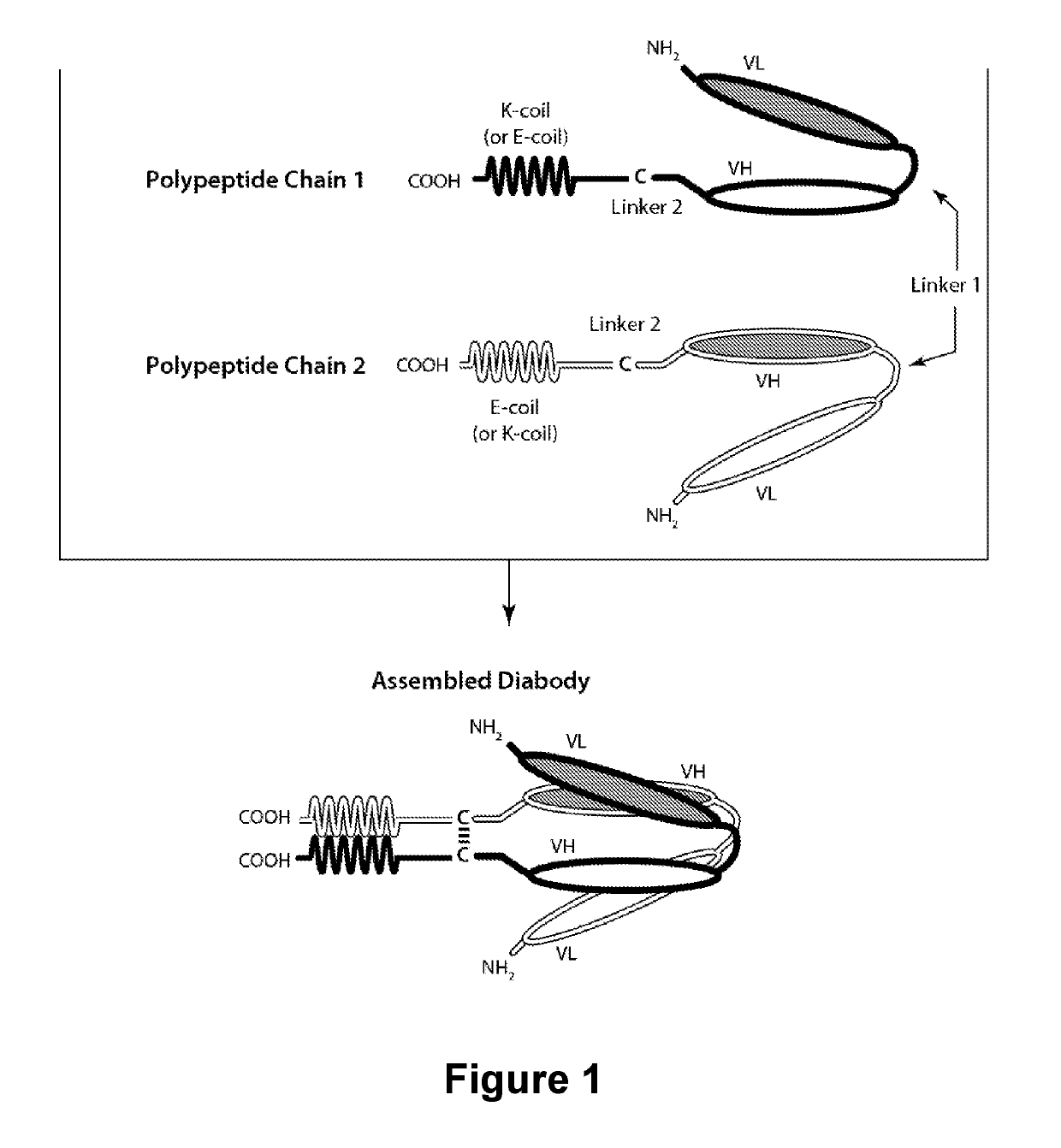
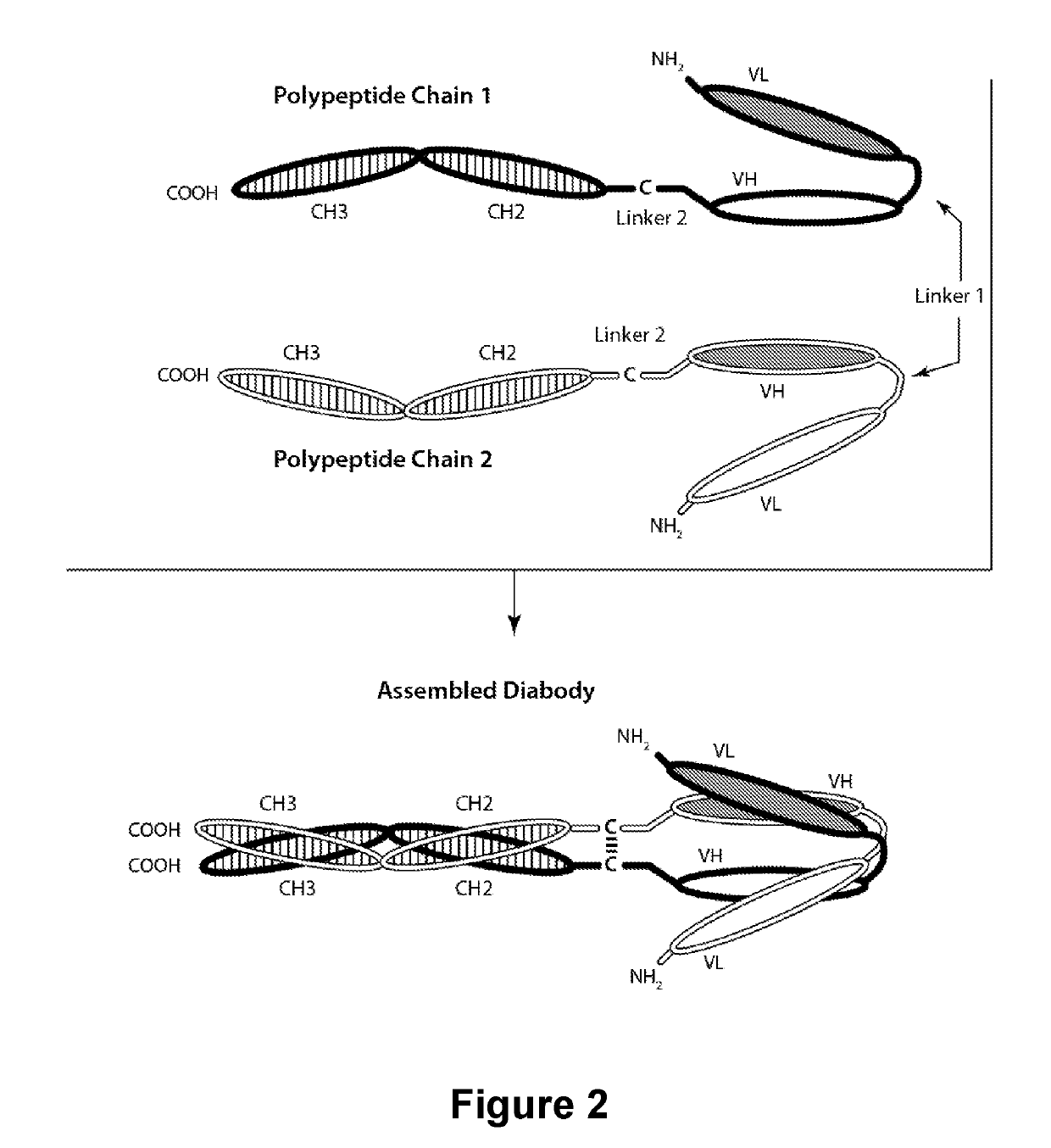
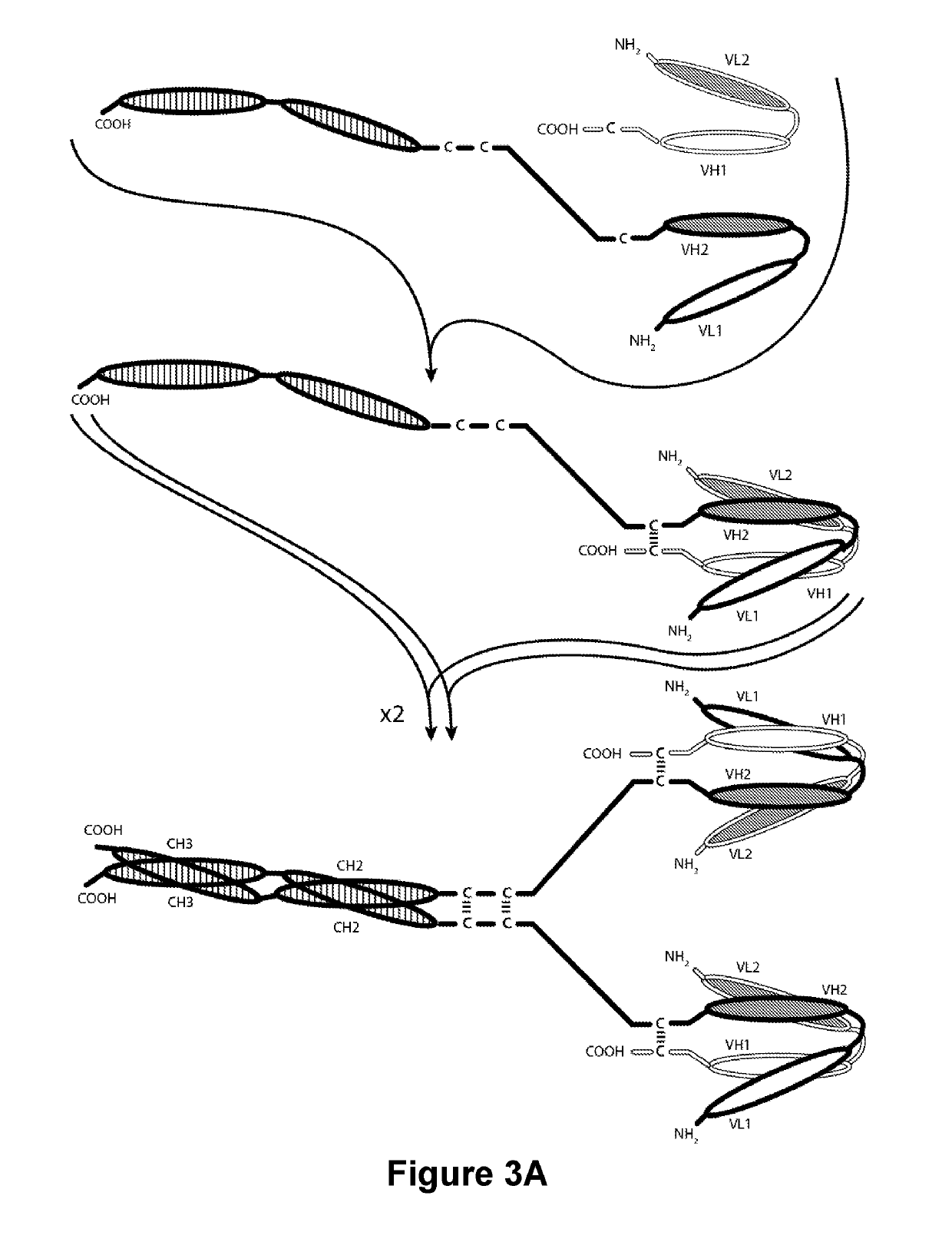
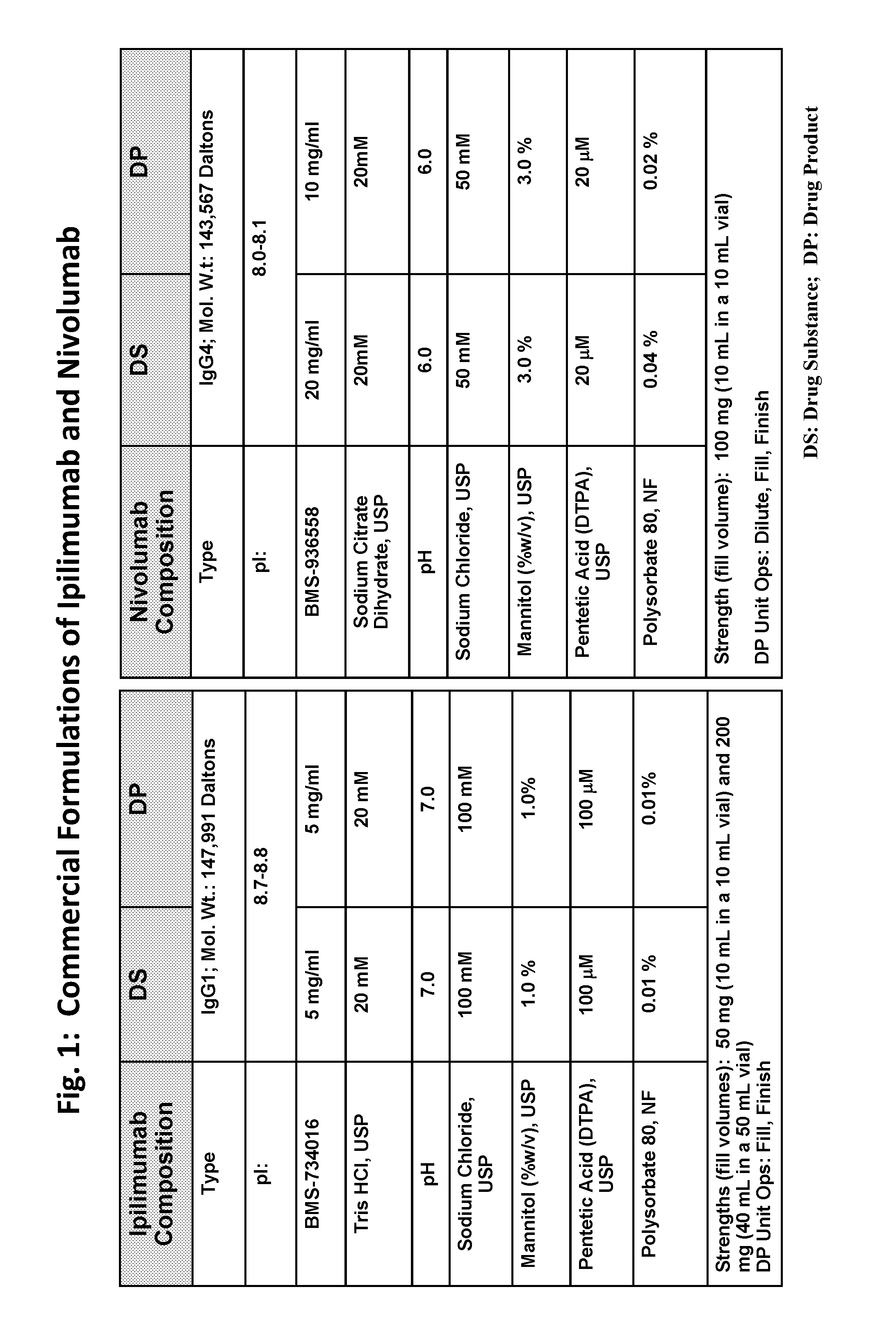
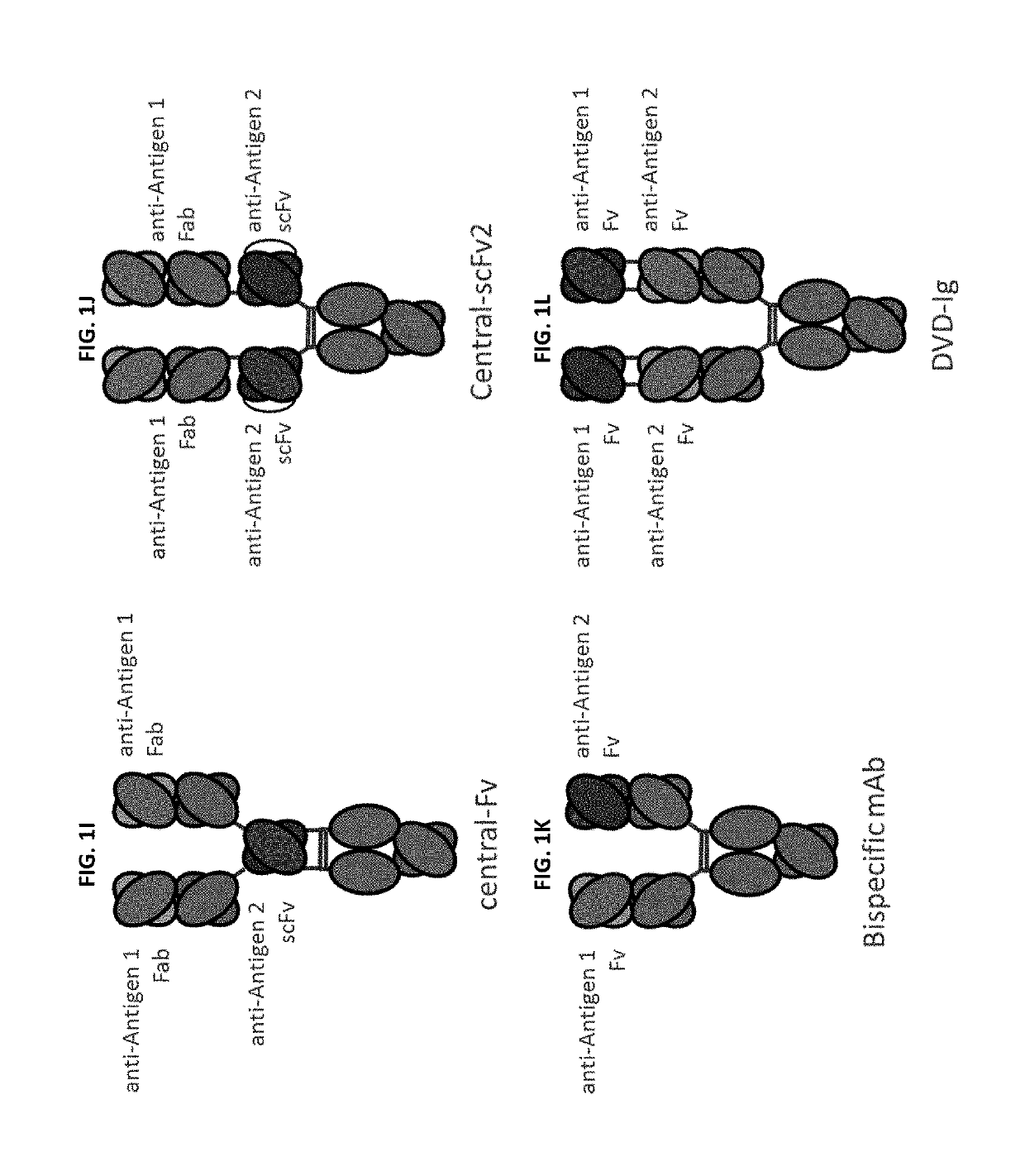
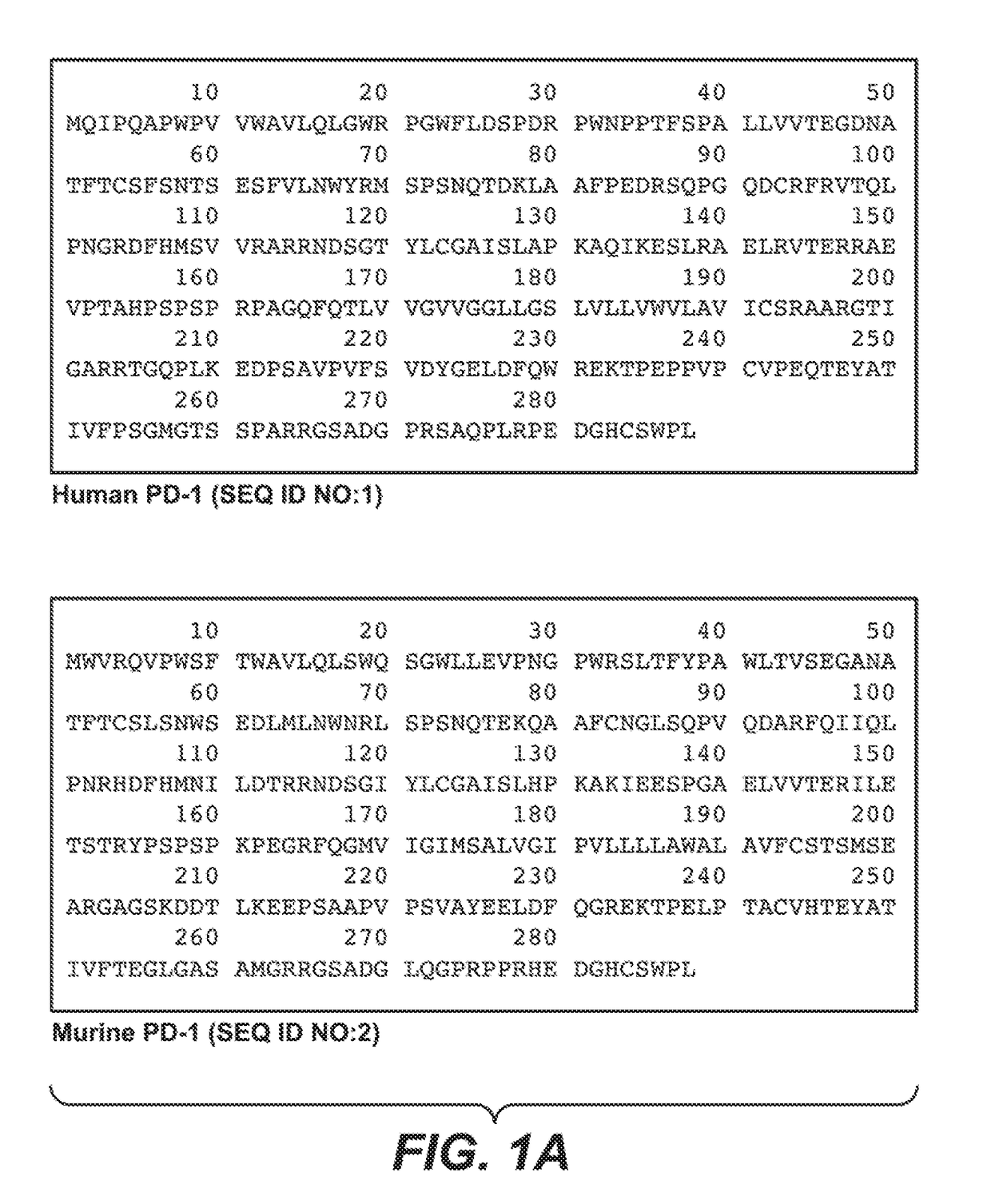
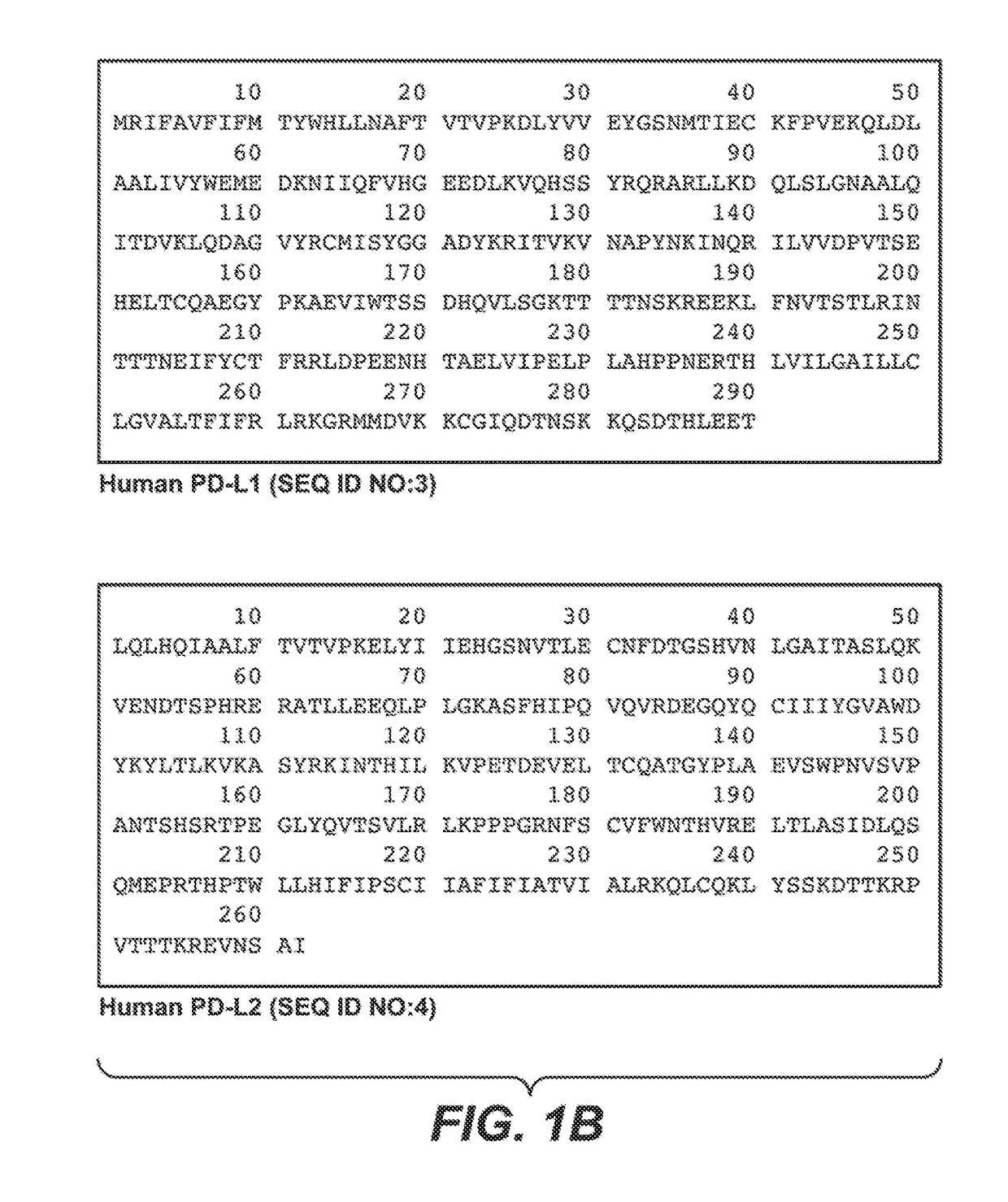
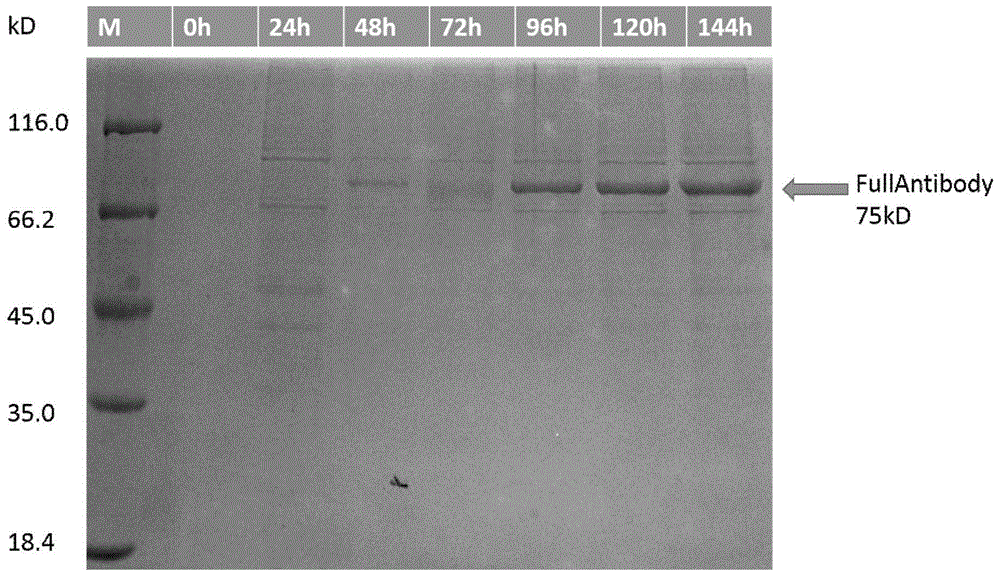
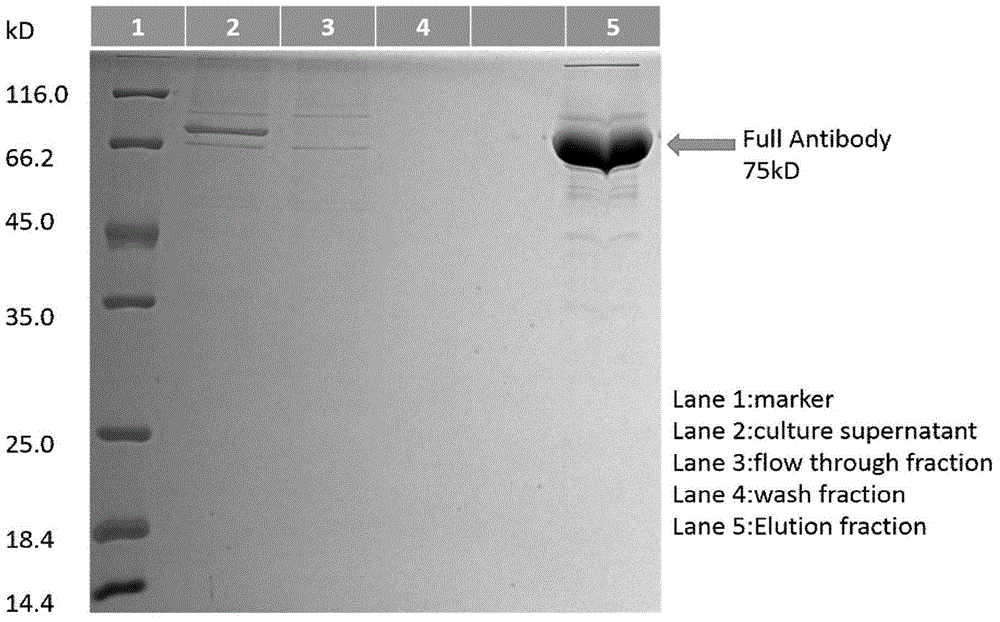
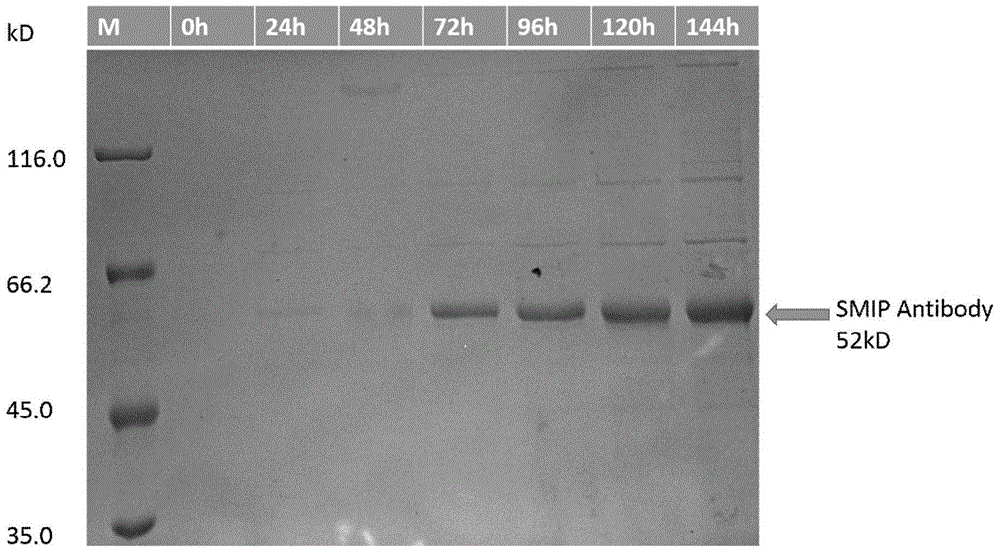
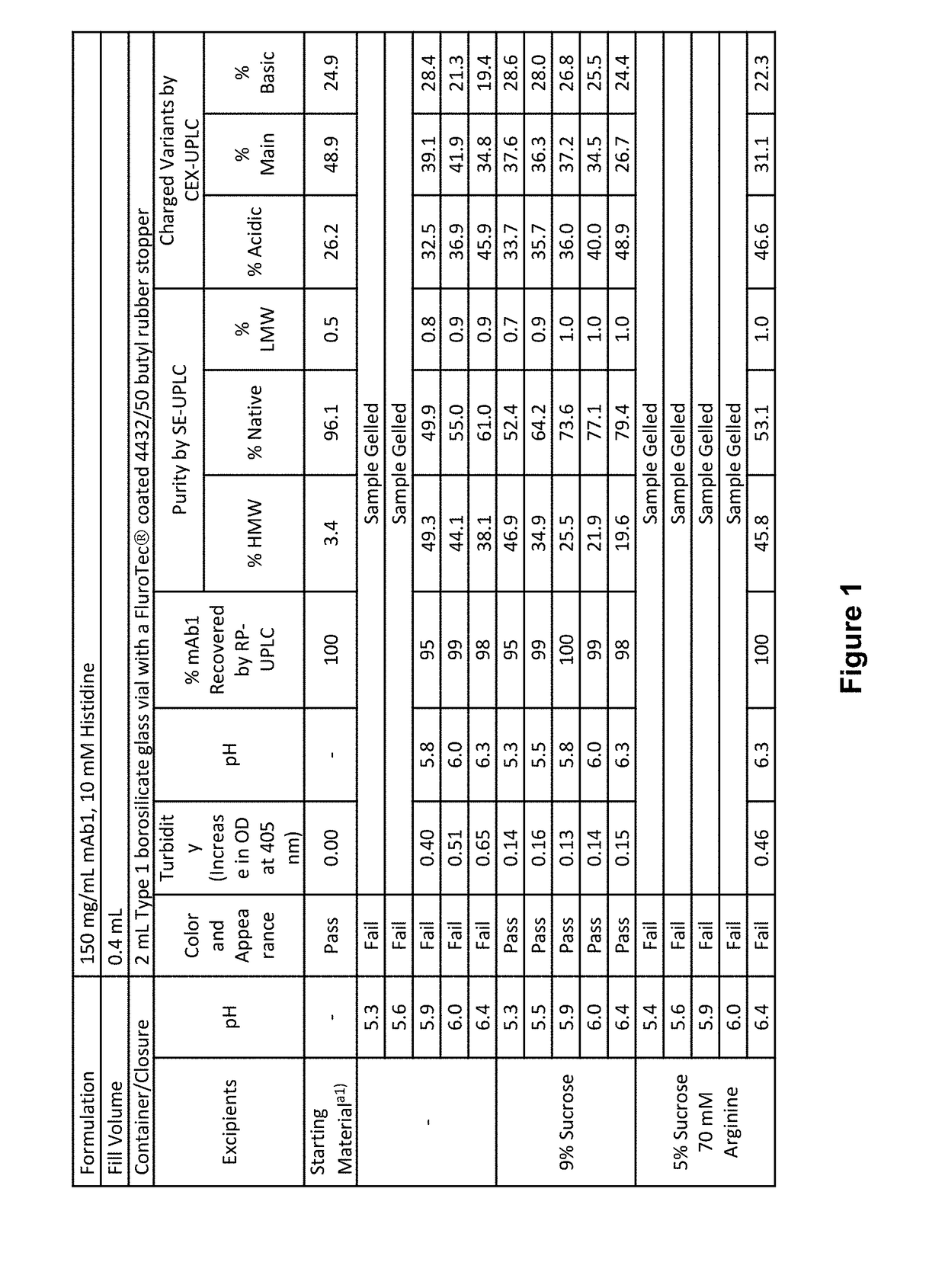
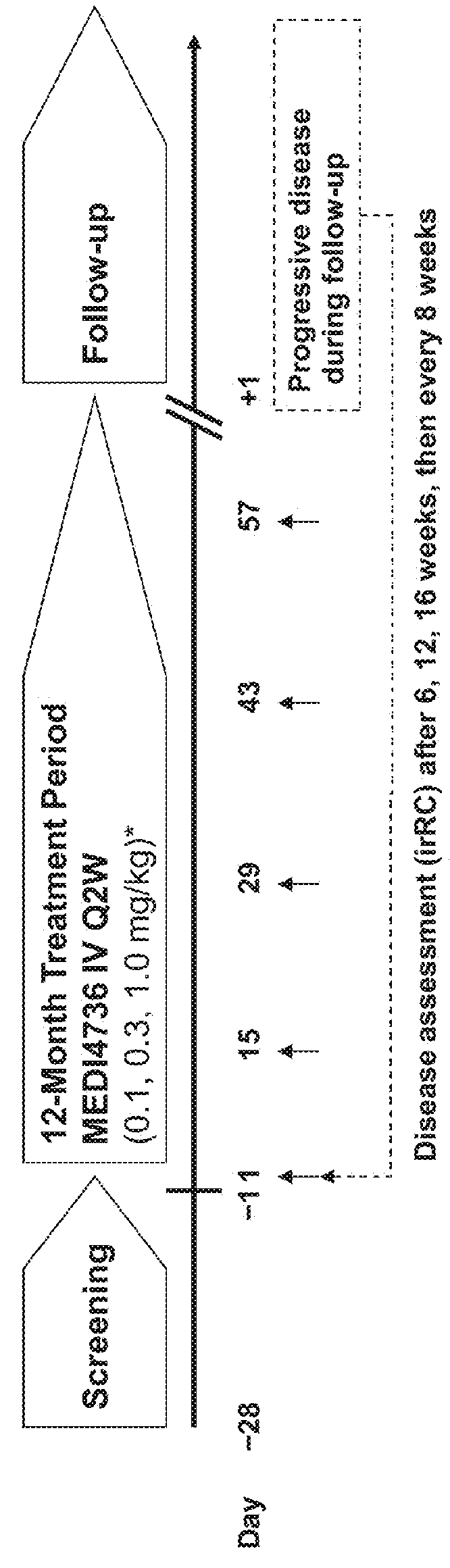
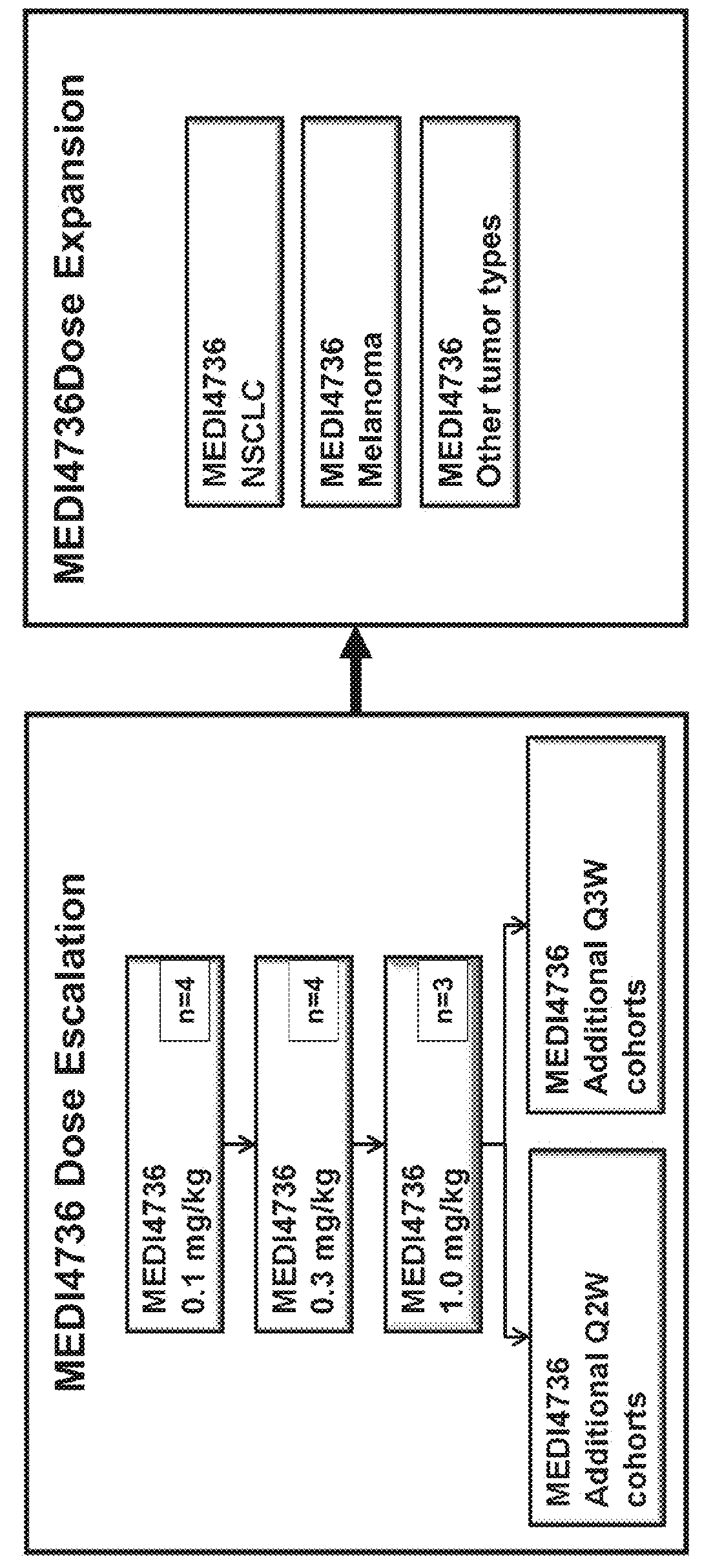
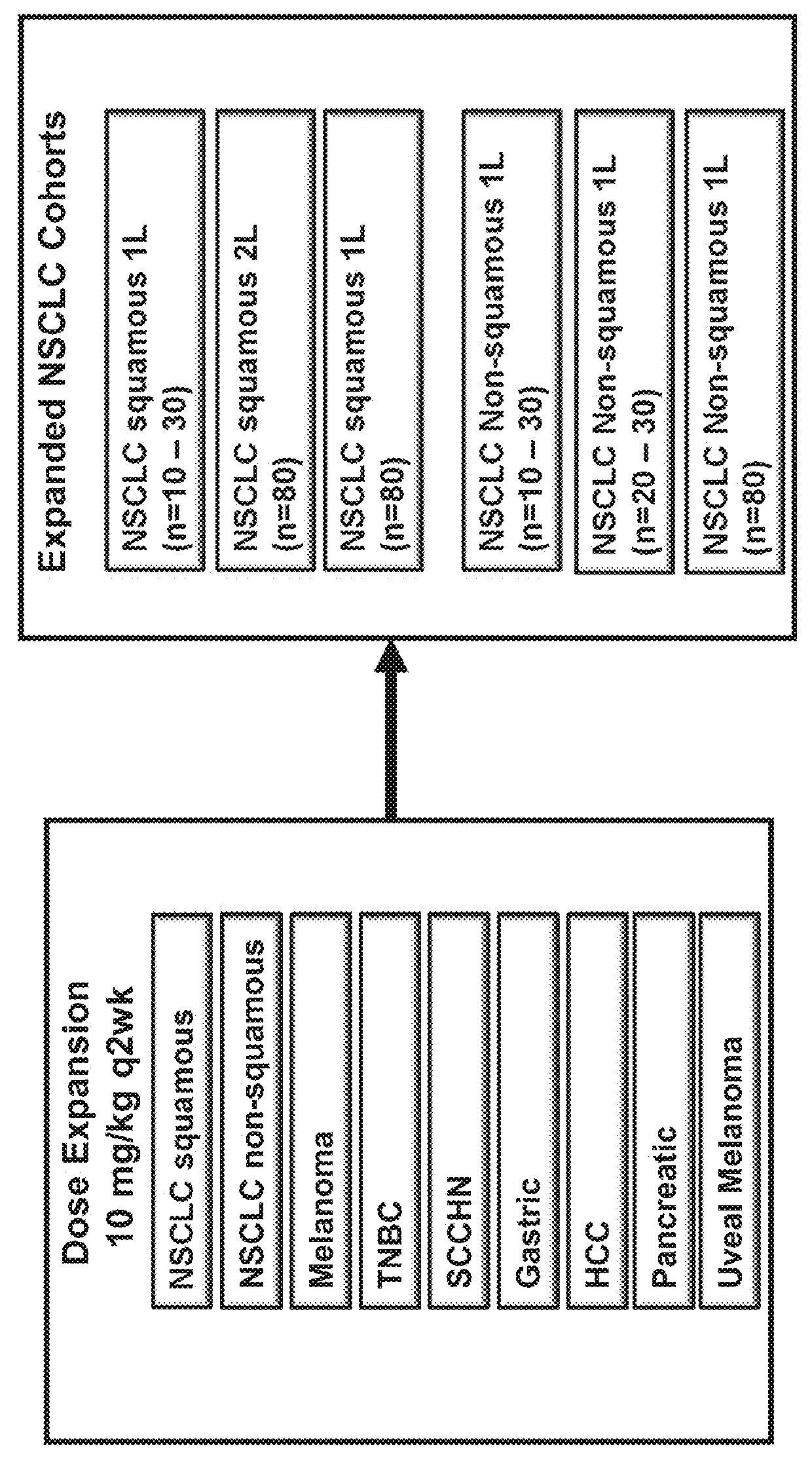
Patents
Literature
98 results about "Anti pd 1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anti-PD-1 monoclonal antibodies. Other names: anti-programmed cell death-1 (PD-1) monoclonal antibodies, programmed death-ligand 1 (PD-L1) blocking antibodies. Programmed cell death protein 1 (PD-1) is an inhibitory receptor that is expressed on some tumor cells and causes down regulation of the immune system by reducing T-cell activity.
Anti-PD-L1 antibodies and uses therefor
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
Human Anti-pd-1, pd-l1, and pd-l2 antibodies and uses therefor
ActiveUS20110271358A1Reduced antigen binding affinityLess immunogenicAntibacterial agentsAntipyreticTransplant rejectionAutoimmune disease
The present invention is based, in part, on the identification of novel human anti-PD-1, PD-L1, and PD-L2 antibodies. Accordingly, the invention relates to compositions and methods for diagnosing, prognosing, and treating conditions that would benefit from modulating PD-1, PD-L1, and / or PD-L2 activity (e.g., persistent infectious diseases, autoimmune diseases, asthma, transplant rejection, inflammatory disorders and tumors) using the novel human anti-PD-1, PD-L1, and PD-L2 antibodies described herein.
Owner:DANA FARBER CANCER INST INC +2
Cancer immunotherapy by disrupting pd-1/pd-l1 signaling
ActiveUS20130309250A1Reduces and suppresses signalingReliable responseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenTissue sample
The disclosure provides a method for immunotherapy of a subject afflicted with cancer, comprises administering to the subject a composition comprising a therapeutically effective amount of an antibody that inhibits signaling from the PD-1 / PD-L1 signaling pathway. This disclosure also provides a method for immunotherapy of a subject afflicted with cancer comprising selecting a subject that is a suitable candidate for immunotherapy based on an assessment that the proportion of cells in a test tissue sample from the subject that express PD-L1 on the cell surface exceeds a predetermined threshold level, and administering a therapeutically effective amount of an anti-PD-1 antibody to the selected subject. The invention additionally provides rabbit mAbs that bind specifically to a cell surface-expressed PD-L1 antigen in a FFPE tissue sample, and an automated IHC method for assessing cell surface expression in FFPE tissues using the provided anti-PD-L1 Abs.
Owner:BRISTOL MYERS SQUIBB CO
Anti-pd-1 antibody and use thereof
This invention provides antibodies or functional fragments thereof that bind to PD-1 with high affinity. The invention provides nucleic acid molecules encoding the antibodies or the fragments thereof according to the present invention, expression vectors and host cells for expressing the antibodies or the functional fragments thereof according to the present invention, as well as methods for producing the antibodies or the functional fragments thereof according to the present invention. The present invention also provides immunoconjugates and pharmaceutical compositions comprising the antibodies or the functional fragments thereof according to the present invention. The present invention additionally provides methods for treating a plurality of diseases (comprising cancers, infectious diseases and inflammatory diseases) by using the antibodies or the functional fragments thereof disclosed herein.
Owner:JUNMENG BIOSCIENCES CO LTD +1
Antibodies that bind PD-L1 and uses thereof
ActiveUS9212224B2Reduces and suppresses signalingReliable responseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenTissue sample
Owner:BRISTOL MYERS SQUIBB CO
Combination of Anti-PD-1 Antibodies and Anti-CD20/Anti-CD3 Antibodies to Treat Cancer
InactiveUS20170174779A1Growth inhibitionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenCD20
The present invention provides methods for treating, reducing the severity, or inhibiting the growth of cancer (e.g., a B-cell cancer such as Hodgkin's lymphoma or acute lymphoblastic leukemia). The methods of the present invention comprise administering to a subject in need thereof a therapeutically effective amount of an antibody or antigen-binding fragment thereof that specifically binds to programmed death 1 (PD-1) receptor in combination with a therapeutically effective amount of a bispecific antibody that specifically binds to CD20 and CD3.
Owner:REGENERON PHARM INC
Anti-CTLA4-anti-PD-1 bifunctional antibody, pharmaceutical composition and use thereof
ActiveCN106967172ABinding blockRelief of immunosuppressionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsMolecular ImmunologyT lymphocyte
Belonging to the field of tumor treatment and molecular immunology, the invention relates to an anti-CTLA4-anti-PD-1 bifunctional antibody, a pharmaceutical composition and use thereof. Specifically, the anti-CTLA4-anti-PD-1 bifunctional antibody comprises: a PD-1 (programmed death-1) targeted first protein functional region, and a CTLA4 (cytotoxic T lymphocyte sociated antigen 4) targeted second protein functional region. The bifunctional antibody provided by the invention can well bind to CTLA4 and PD-1 specifically, specifically remove the immunosuppression of CTLA4 and PD-1 on the body, and activate T lymphocytes, thus having good application prospect.
Owner:AKESO PHARMA INC
Method for Treating and Diagnosing Hematologic Malignancies
ActiveUS20110177088A1Avoid overactivationStrong cytotoxicityAnimal cellsPeptide/protein ingredientsPD-L1Oncology
The invention relates to methods for treating and diagnosing hematologic malignancies, Chronic lymphocytic leukemia and Small Lymphocytic Lymphoma in particular, using PD-1 ligands (PD-L1, PD-L2 or anti-PD-1 antibodies).
Owner:OLIVE DANIEL +2
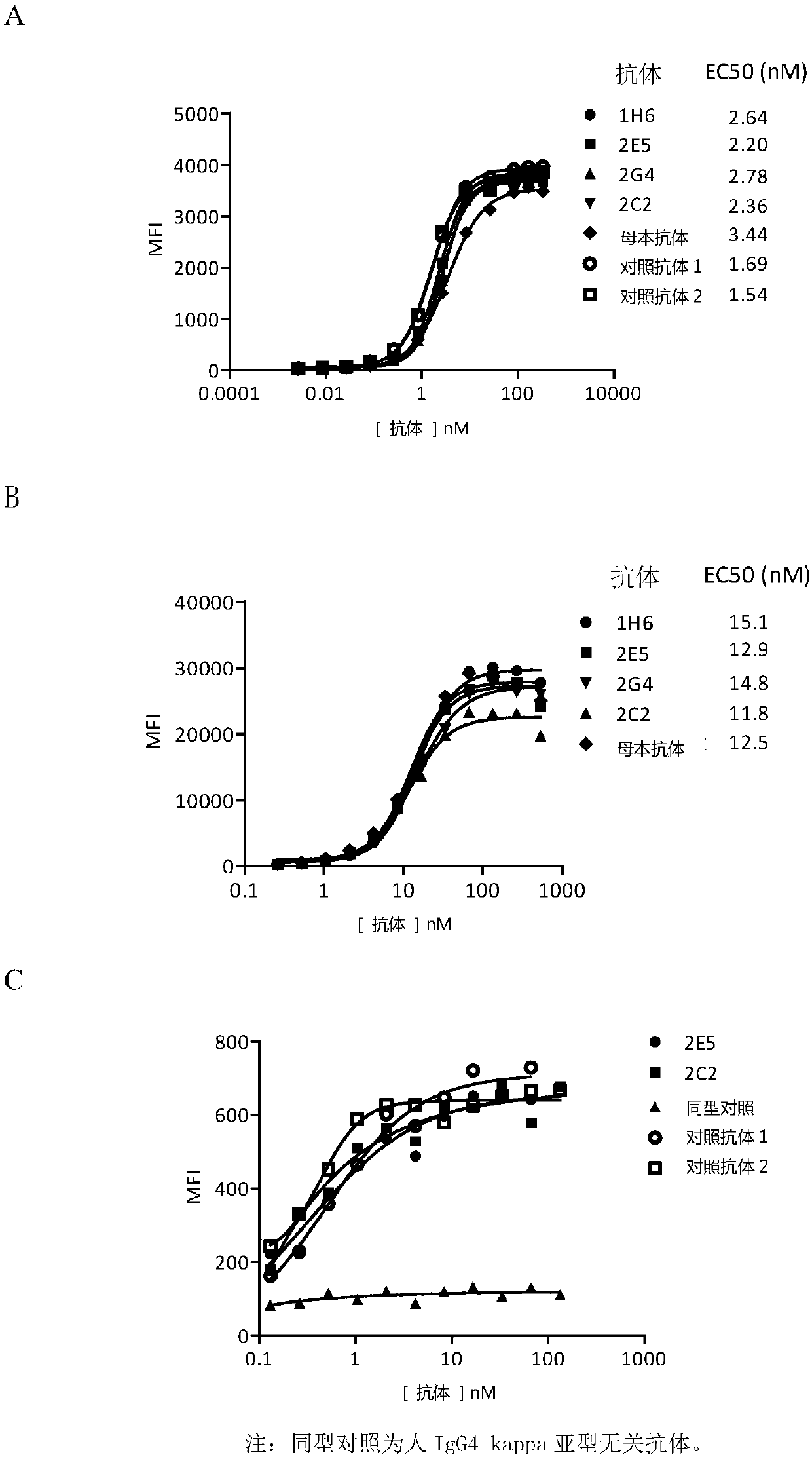
Anti-PD-1 humanized monoclonal antibody and application thereof
ActiveCN105175544AHigh affinityPlay effectivelyImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMolecular ImmunologyNucleotide
The invention discloses an anti-PD-1 humanized monoclonal antibody and application thereof, belonging to the technical field of molecular immunology. The anti-PD-1 humanized monoclonal antibody provided by the invention contains a light chain and a heavy chain, wherein the amino acid sequence of the light chain is as shown in SEQ ID NO.2, and the amino acid sequence of the heavy chain is as shown in SEQ ID NO.4 or SEQ ID NO.6. Meanwhile, the invention also provides a nucleotide sequence coding the light chain and heavy chain. The anti-PD-1 humanized monoclonal antibody provided by the invention has very high affinity with human PD-1 protein, and can interdict PD-1 / PD-L1 combination so as to promote T cell proliferation and IFN-gamma secretion.
Owner:ANHUI RUBIOX VISION BIOTECH
PD-1-Binding Molecules and Methods of Use Thereof
ActiveUS20190127467A1Low affinityIncreased serum half-lifeAntibacterial agentsNervous disorderEpitopeCheck point
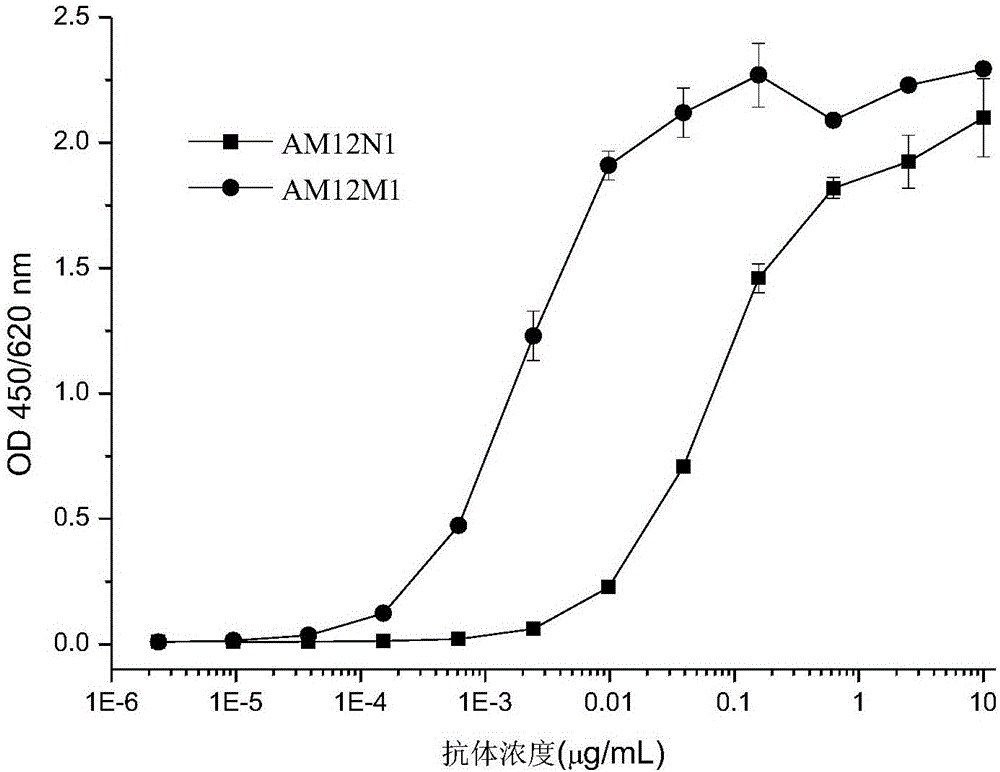
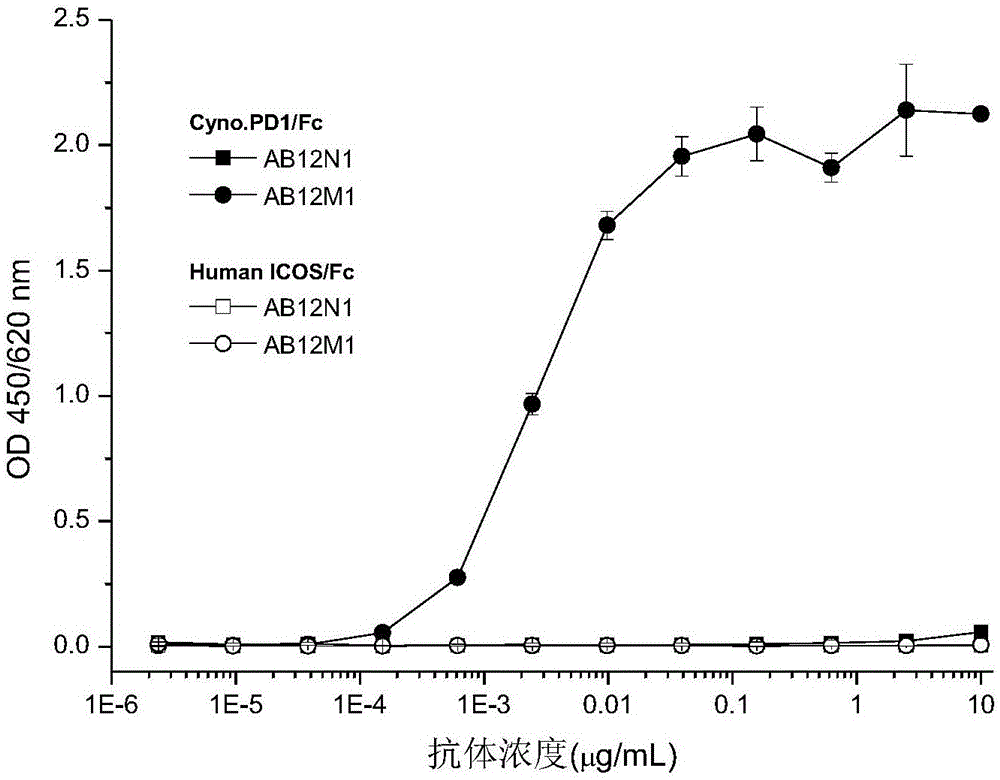
The present invention is directed to selected anti-PD-1 antibodies capable of binding to both cynomolgus monkey PD-1 and to human PD-1: PD-1 mAb 1, PD-1 mAb 2, PD-1 mAb 3, PD-1 mAb 4, PD-1 mAb 5, PD-1 mAb 6, PD-1 mAb 7, PD-1 mAb 8, PD-1 mAb 9, PD-1 mAb 10, PD-1 mAb 11, PD-1 mAb 12, PD-1 mAb 13, PD-1 mAb 14, or PD-1 mAb 15, and to humanized and chimeric versions of such antibodies. The invention additionally pertains to PD-1-binding molecules that comprise PD-1 binding fragments of such anti-PD-1 antibodies, immunocongugates, and to bispecific molecules, including diabodies, BiTEs, bispecific antibodies, etc., that comprise (i) such PD-1-binding fragments, and (ii) a domain capable of binding an epitope of a molecule involved in regulating an immune check point present on the surface of an immune cells. The present invention also pertains to methods of using molecules that bind PD-1 for stimulating immune responses, as well as methods of detecting PD-1.
Owner:MACROGENICS INC
Compositions comprising a combination of an Anti-pd-1 antibody and another antibody
InactiveUS20160304607A1Change is minimalSenses disorderNervous disorderAnticarcinogenProgrammed death
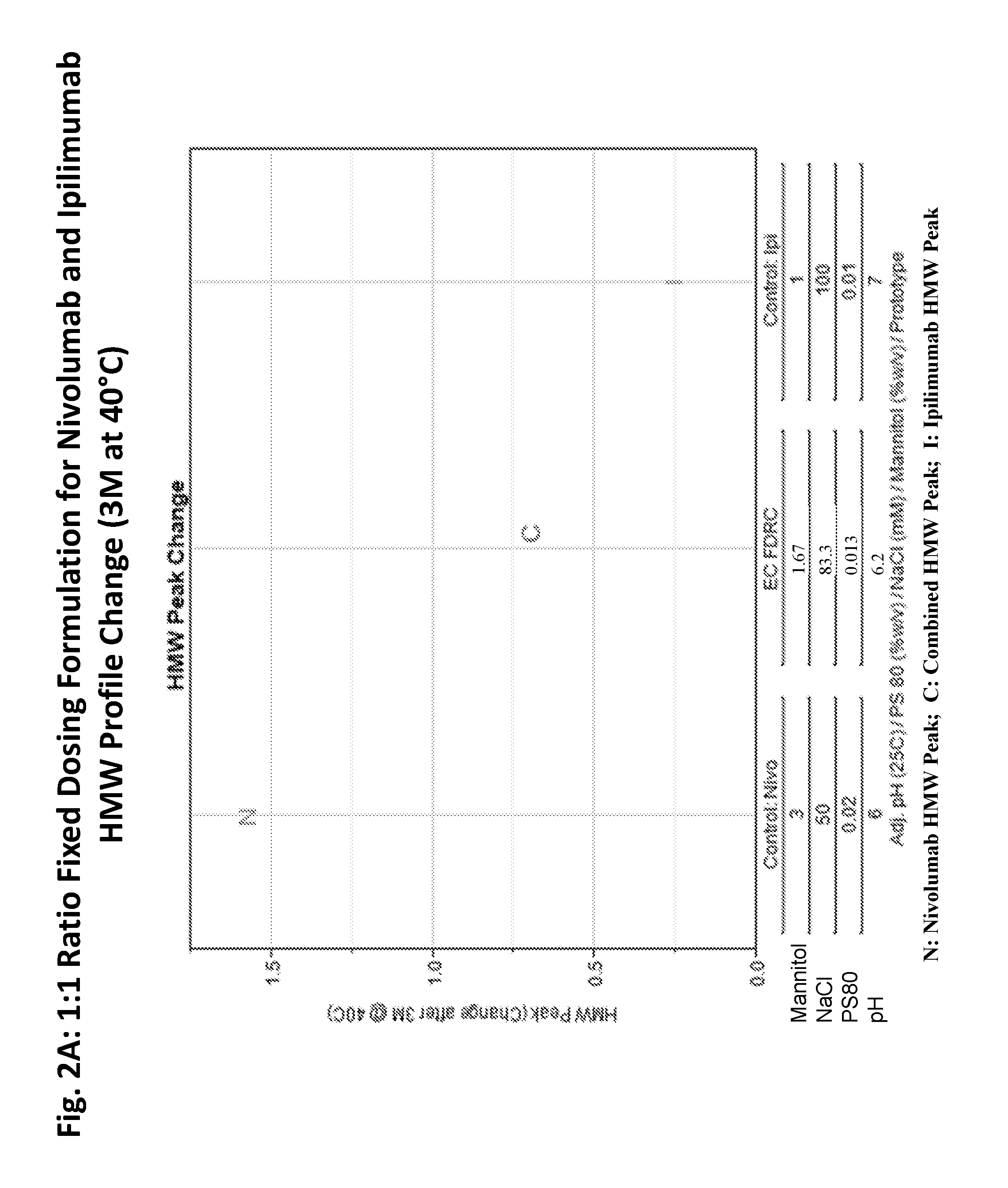
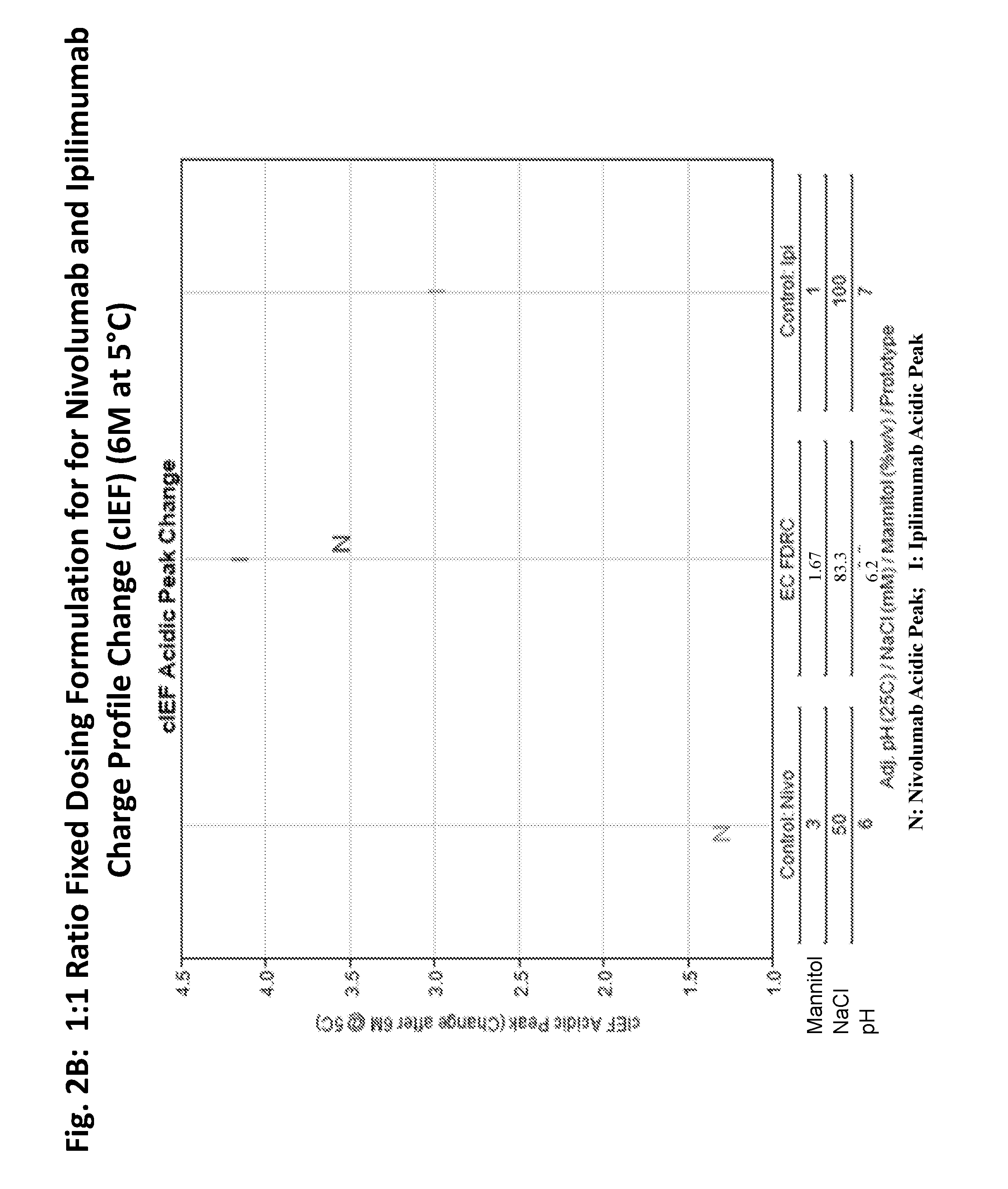
This provides pharmaceutical compositions that comprise a combination of an anti-cancer agent which is an first antibody and a second antibody. In some embodiments, the first antibody is an anti-Programmed Death-1 (PD-1) antibody. In certain embodiments, the composition is a fixed dose formulation. In certain embodiments, the composition is administered as a flat-dose. The disclosure also provides a kit for treating a subject afflicted with a disease, the kit comprising a dosage of any composition disclosed herein and instructions for using the composition in any of the disclosed methods for treating a disease.
Owner:BRISTOL MYERS SQUIBB CO
Anti-PD-1 (Programmed Death-1) antibody and application thereof
ActiveCN106519034AHigh binding affinityIncrease productionAntibacterial agentsAntipyreticInfective disorderAntibody
The invention provides an antibody specifically combined with PD-1 (Programmed Death-1) with high affinity, and also provides a nucleic acid molecule for coding the antibody, an expression vector and a host cell for expressing the antibody, and a production method of the antibody. In addition, the invention also provides an immune conjugate and a medicinal composition containing the antibody, and application of the antibody to the preparation of a medicine for treating multiple diseases (including a cancer, an infectious disease and an inflammatory disease).
Owner:LUNAN PHARMA GROUP CORPORATION
Treatment of lung cancer using a combination of an Anti-pd-1 antibody and another Anti-cancer agent
InactiveUS20170158776A1Durable clinical responseReliable responseHeavy metal active ingredientsImmunoglobulins against growth factorsAnticarcinogenTyrosine-kinase inhibitor
This disclosure provides a method for treating a subject afflicted with a lung cancer, which method comprises administering to the subject therapeutically effective amounts of: (a) an anti-cancer agent which is an antibody or an antigen-binding portion thereof that specifically binds to a Programmed Death-1 (PD-1) receptor and inhibits PD-1 activity; and (b) another anti-cancer agent. The other anti-cancer agent can be a platinum-based doublet chemotherapy, an EGFR-targeted tyrosine kinase inhibitor, bevacizumab, an anti-Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) antibody, or any other therapy used to treat lung cancer in the art or disclosed herein.
Owner:BRISTOL MYERS SQUIBB CO
Treatment of hodgkin's lymphoma using an Anti-pd-1 antibody
InactiveUS20160347836A1Inhibitory activityMicrobiological testing/measurementBiological material analysisAnticarcinogenCTLA4 Protein
This disclosure provides methods for treating Hodgkin's lymphoma in a subject comprising administering to the subject an anti-Programmed Death-1 (PD-1) antibody. In some embodiments, this invention relates to methods for treating Hodgkin's lymphoma in a subject comprising administering to the subject a combination of an anti-cancer agent which is an anti-Programmed Death-1 (PD-1) antibody and another anti-cancer agent such as an anti-Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) antibody.
Owner:BRISTOL MYERS SQUIBB CO
Anticancer agent comprising Anti-pd-1 antibody or Anti-pd-l1 antibody
ActiveUS20120237522A1Recovery of iNKT cell responsivenessAntibody ingredientsImmunoglobulinsLymphatic SpreadAnticarcinogen
Provided is an anticancer agent which comprises an anti-PD-1 antibody or an anti-PD-L1 antibody as an active ingredient, functioning to reverse the unresponsiveness of iNKT cells in which anergy has been induced by administration with an iNKT cell ligand. The anti-PD-1 or anti-PD-L1 antibody blocks the PD-1 / PD-L1-mediated signaling pathway not only to prevent the iNKT cell ligand-induced iNKT cell anergy, but also to reverse the unresponsiveness of already anergic iNKT cells to produce cytokines. In addition, the anti-PD1 or anti-PD-L1 antibody ensures the potent anti-tumor activity of iNKT cells as demonstrated by a significant reduction in the number of metastatic nodules in B16F10 melanoma metastasis models in vivo. Collectively, the anticancer agent can be very useful in the treatment of cancer, particularly metastatic cancer.
Owner:SEOUL NAT UNIV R&DB FOUND
Bispecific and monospecific antibodies using novel Anti-pd-1 sequences
ActiveUS20190263909A1Hybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsMonospecific antibodyAnti pd 1
Owner:XENCOR INC
Anti-PD-1 antibodies and their uses
ActiveUS9914783B1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibodyNucleic acid
Owner:ABBVIE BIOTHERAPEUTICS
Anti-PD-1 (programmed cell death-1) humanized antibody
ActiveCN104479020AHigh expressionHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHeavy chainHumanized antibody
The invention relates to the technical field of biology, and in particular relates to an anti-PD-1 humanized antibody. The anti-PD-1 humanized antibody is characterized in that the amino acid sequence of a variable region of a heavy chain of the antibody is shown as SEQ ID NO: 2 or is a conserved variation sequence; the amino acid sequence of a variable region of a light chain of the antibody is shown as SEQ ID NO: 4 or is a conserved variation sequence. The anti-PD-1 humanized antibody shows relatively high expression quantity in mammalian cells, and is obvious in affinity with PD-1 and able to inhibit the combination of a ligand and a receptor.
Owner:SHANGHAI HENLIUS BIOTECH INC
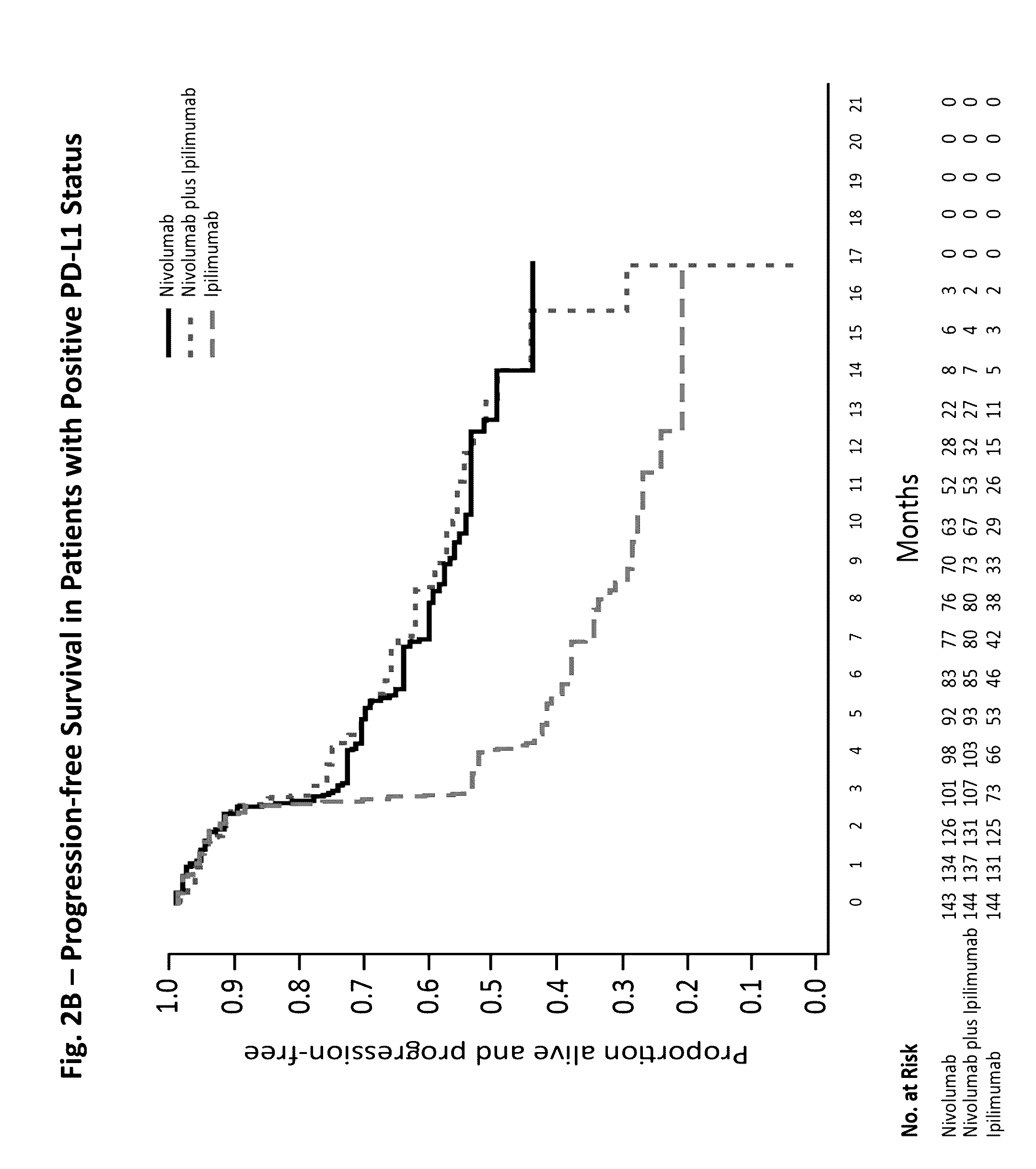
Treatment of PD-L1-Positive Melanoma Using an Anti-PD-1 Antibody
InactiveUS20160362489A1Shrink tumorBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsProgression-free survivalMelanoma
The invention provides a method of treating a melanoma comprising (i) identifying a patient having a PD-L1 positive melanoma and (ii) administering to the patient an anti-PD-1 antibody or an antigen-binding portion thereof (“an anti-PD-1 antibody monotherapy”). The methods of the invention can extend progression-free survival for over 12 months and / or reduces the tumor size at least about 10%, about 20%, about 30%, about 40%, or about 50% compared to the tumor size prior to the administration.
Owner:BRISTOL MYERS SQUIBB CO
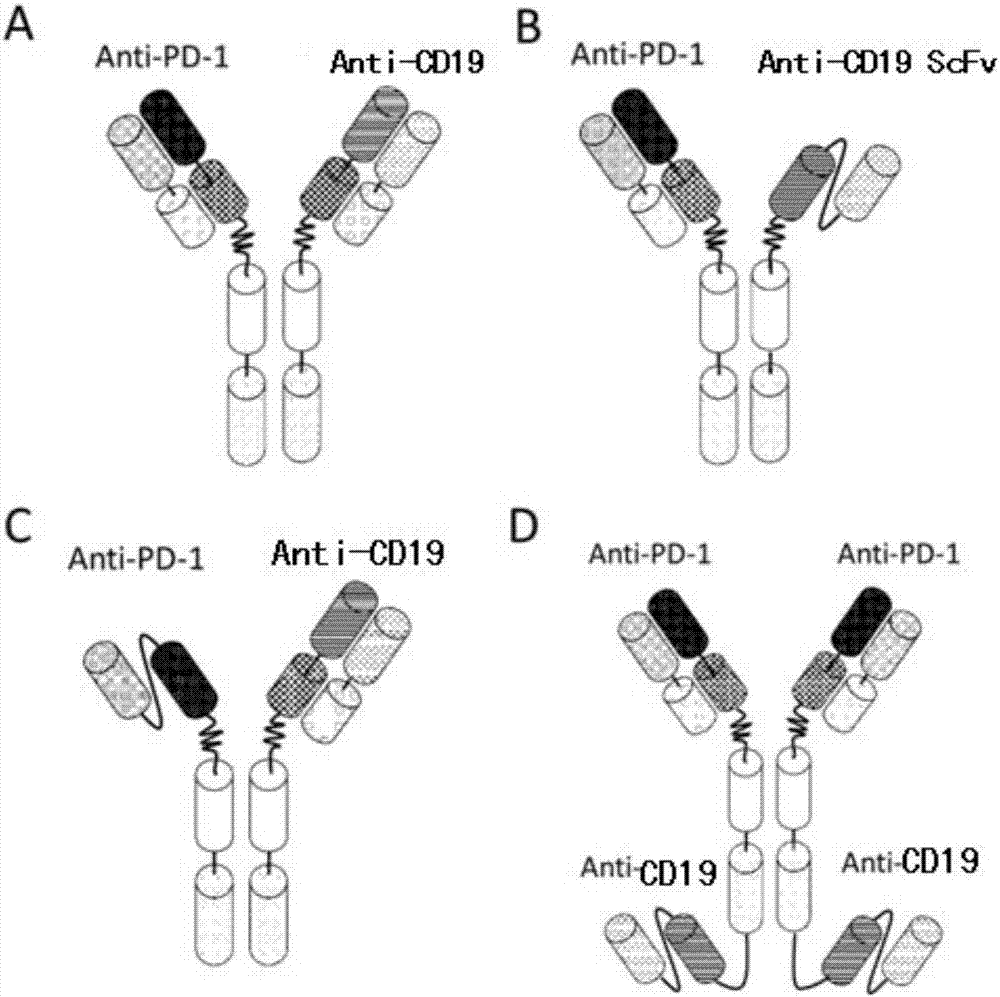
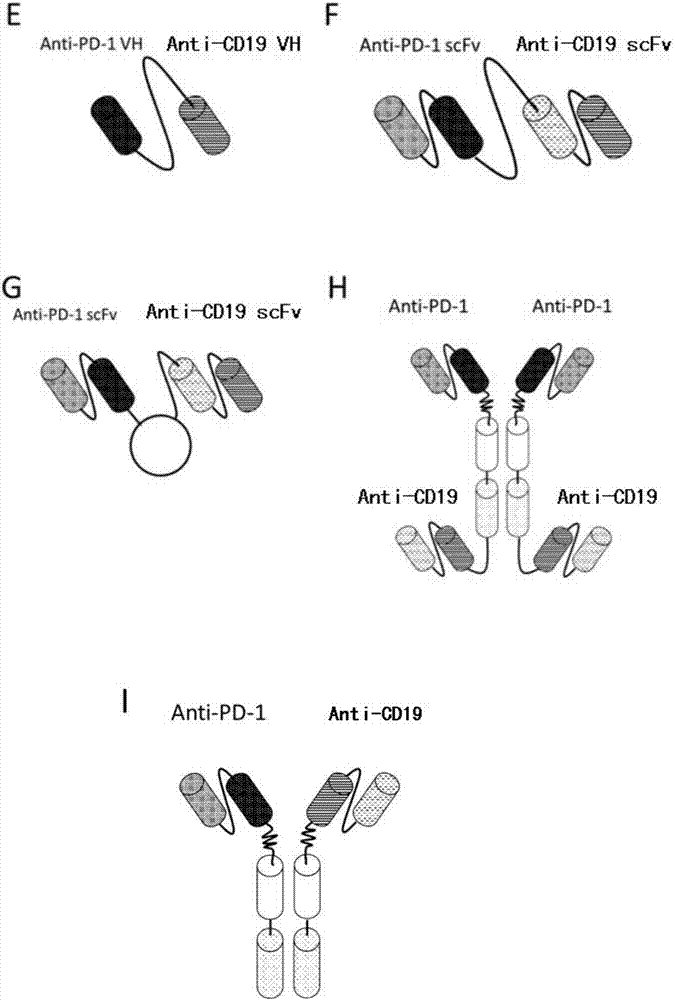
Anti-PD1 and anti-CD19 bispecific antibody and applications thereof
ActiveCN106939050AImprove biological activityGood biological stabilityHybrid immunoglobulinsAntibody ingredientsCell Surface AntigensTherapeutic effect
The present invention relates to anti-PD1 and anti-CD19 bispecific antibody and applications thereof, wherein the anti-PD1 and anti-CD19 bispecific antibody, or a variant, or a functional fragment thereof comprises: a domain capable of specifically recognizing and binding an immune cell surface antigen PD-1, wherein the domain comprises the heavy chain variable region (anti-PD-1 VH) of an anti-PD-1 specific antibody; and a domain capable of specifically recognizing and binding CD19, wherein the domain comprises the heavy chain variable region (anti-CD19 VH) of an anti-CD19 specific antibody. According to the present invention, the bispecific antibody has the whole human sequence, such that the occurrence of HAMA in clinical treatment application is effectively avoided, the occurrence of drug resistance can be effectively reduced, and the utilization efficiency and the treatment effect of the medicine can be improved; and the anti-PD-1 and anti-CD19 bispecific antibody can well mediate T cells to efficiently and specifically kill B-lymphocyte derived tumor cells compared to the existing anti-PD-1 specific antibody and the existing anti-CD19 specific antibody.
Owner:SHUNHAO CELL BIOTECHNOLOGY (TIANJIN) CO LTD
Pd-1 antibodies in combination with a cytokine-secreting cell and methods of use thereof
InactiveUS20100285013A1Good curative effectBiocidePeptide/protein ingredientsTumor responseSecreting cell
Owner:ER SQUIBB & SONS INC +1
Anti-PD-1 (programmed cell death 1) monoclonal antibody as well as pharmaceutical composition and application thereof
InactiveCN106632674ABinding blockRelief of immunosuppressionRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsMolecular ImmunologyHeavy chain
The invention belongs to the fields of oncotherapy and molecular immunology, and relates to an anti-PD-1 (programmed cell death 1) monoclonal antibody as well as pharmaceutical composition and an application thereof, in particular to a monoclonal antibody or an antigen binding fragment thereof. The heavy chain variable region of the monoclonal antibody contains CDR with amino acid sequences represented as SEQ ID NO: 13-15; and / or the light chain variable region of the monoclonal antibody contains CDR with amino acid sequences represented as SEQ ID NO: 16-18. The monoclonal antibody can be specifically bound with PD-1 very well, specifically remove immunosuppression of PD-1 on an organism and activate T lymphocytes.
Owner:ZEDA BIOPHARMACEUTICALS INC
Novel monoclonal antibody against PD-1
ActiveCN107840887AHigh binding affinityPromote proliferationGenetically modified cellsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainNucleic acid sequencing
The invention provides a monoclonal antibody against PD-1, especially a human monoclonal antibody against PD-1. The antibody specifically binds to PD-1 in virtue of high affinity and comprises a heavychain and a light chain. The invention also provides a nucleic acid sequence coding the antibody, a cloning or expression vector, a host cell, a method for expressing or isolating the antibody, and an immunoconjugate and therapeutic composition containing the antibody. The invention further provides application of the anti-PD-1 antibody to treatment of various cancers.
Owner:CSTONE PHARM (SUZHOU) CO LTD +2
Anticancer agent comprising Anti-pd-1 antibody or Anti-pd-l1 antibody
InactiveUS20100086550A1Recovery of iNKT cell responsivenessAntibody ingredientsImmunoglobulinsAnticarcinogenLymphatic Spread
Provided is an anticancer agent which comprises an anti-PD-1 antibody or an anti-PD-L1 antibody as an active ingredient, functioning to reverse the unresponsiveness of iNKT cells in which anergy has been induced by administration with an iNKT cell ligand. The anti-PD-1 or anti-PD-L1 antibody blocks the PD-1 / PD-L1-mediated signaling pathway not only to prevent the iNKT cell ligand-induced iNKT cell anergy, but also to reverse the unresponsiveness of already anergic iNKT cells to produce cytokines. In addition, the anti-PD1 or anti-PD-L1 antibody ensures the potent anti-tumor activity of iNKT cells as demonstrated by a significant reduction in the number of metastatic nodules in B16F10 melanoma metastasis models in vivo. Collectively, the anticancer agent can be very useful in the treatment of cancer, particularly metastatic cancer.
Owner:ANTICANCER AGENT COMPRISING ANTI PD 1 ANTIBODY OR ANTI PD L1 ANTIBODY
Humanized anti-PD-1 and c-MET bispecific antibody, and preparation method and application thereof
InactiveCN105085680ALow immunogenicityEasy to manufactureImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSequence signalSequence analysis
The invention belongs to the biotechnical field, and concretely relates to a recombinant humanized anti-PD-1 and c-MET bispecific antibody, and a preparation method and an application thereof. Humanized antibody database comparison, computer molecular structure simulation, molecular docking simulation and sequence analysis are carried out, and recombinant humanized anti-PD-1 and c-MET bispecific antibody molecule designing comprising affinity maturation, structure optimization, humanization and codon optimization is carried out, and an expression plasmid of the recombinant humanized anti-PD-1 and c-MET bispecific antibody is constructed on the basis of an optimized signal peptide sequence and a plasmid expression vector replicon. The bispecific antibody has the characteristics of low immunogenicity, simple and safe preparation process, high yield, high purity and high activity, and can be used to further prepare new specific drugs for treating non-small cell lung cancer.
Owner:FUDAN UNIV
Stable antibody formulation
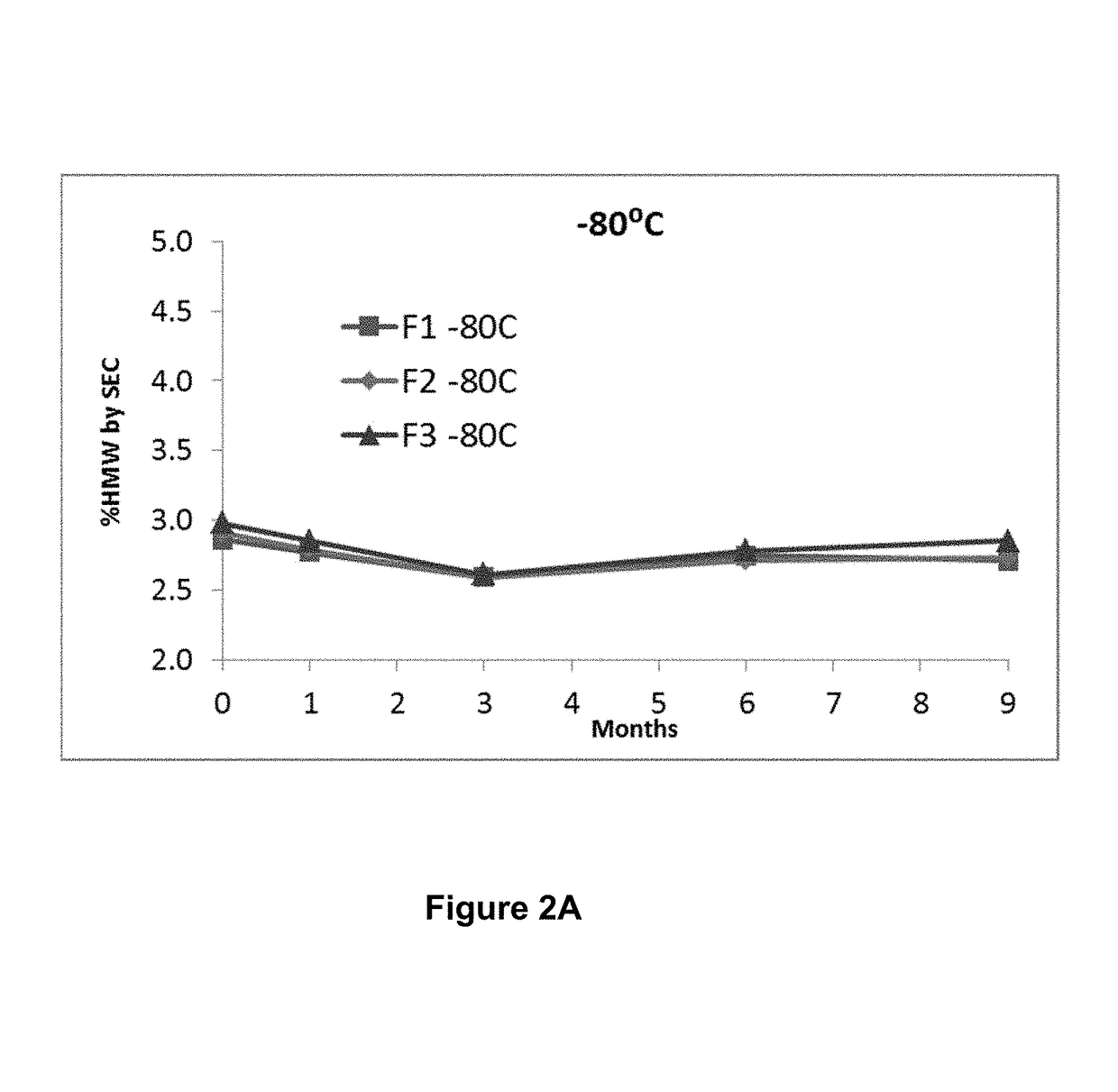
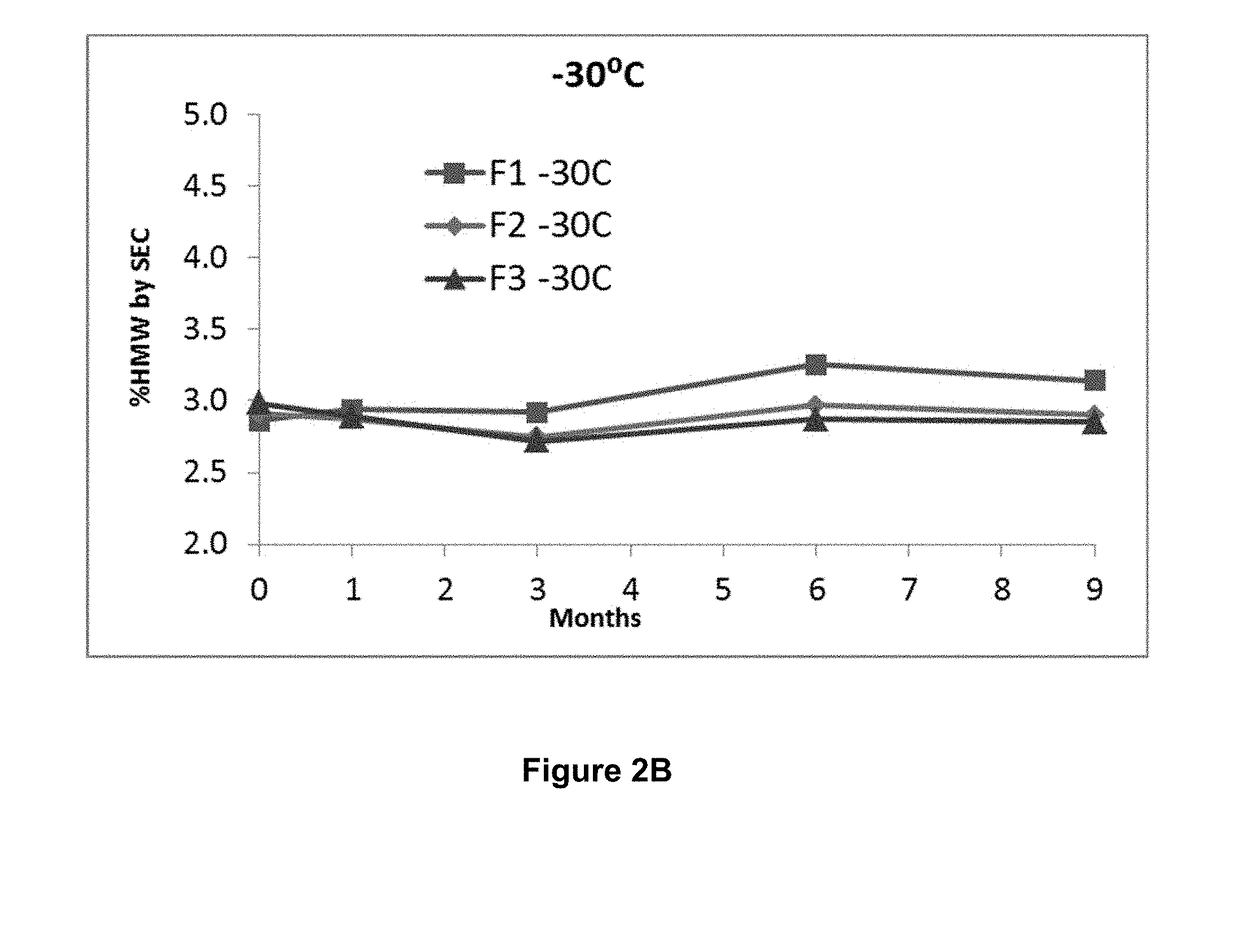
ActiveUS20190040137A1Pharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsProgrammed deathPharmaceutical formulation
The present invention provides stable pharmaceutical formulations comprising a human antibody that specifically binds to human programmed death-1 protein (PD-1). In certain embodiments, the formulations contain, in addition to an anti-PD-1 antibody, a buffer, an amino acid, a non-ionic surfactant, and a sugar. The pharmaceutical formulations of the present invention exhibit a substantial degree of antibody stability upon stress and storage.
Owner:REGENERON PHARM INC
Cancer immunotherapy
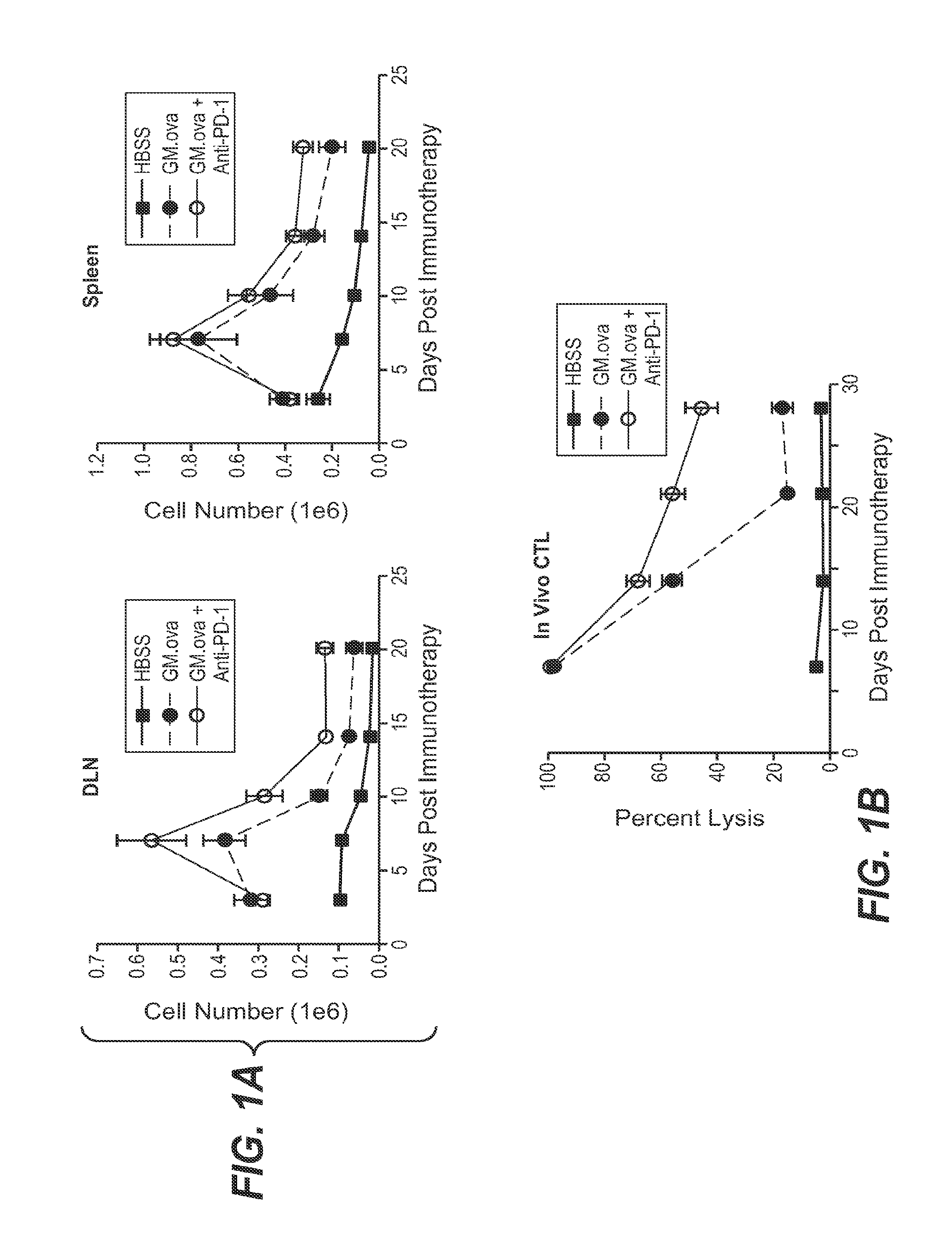
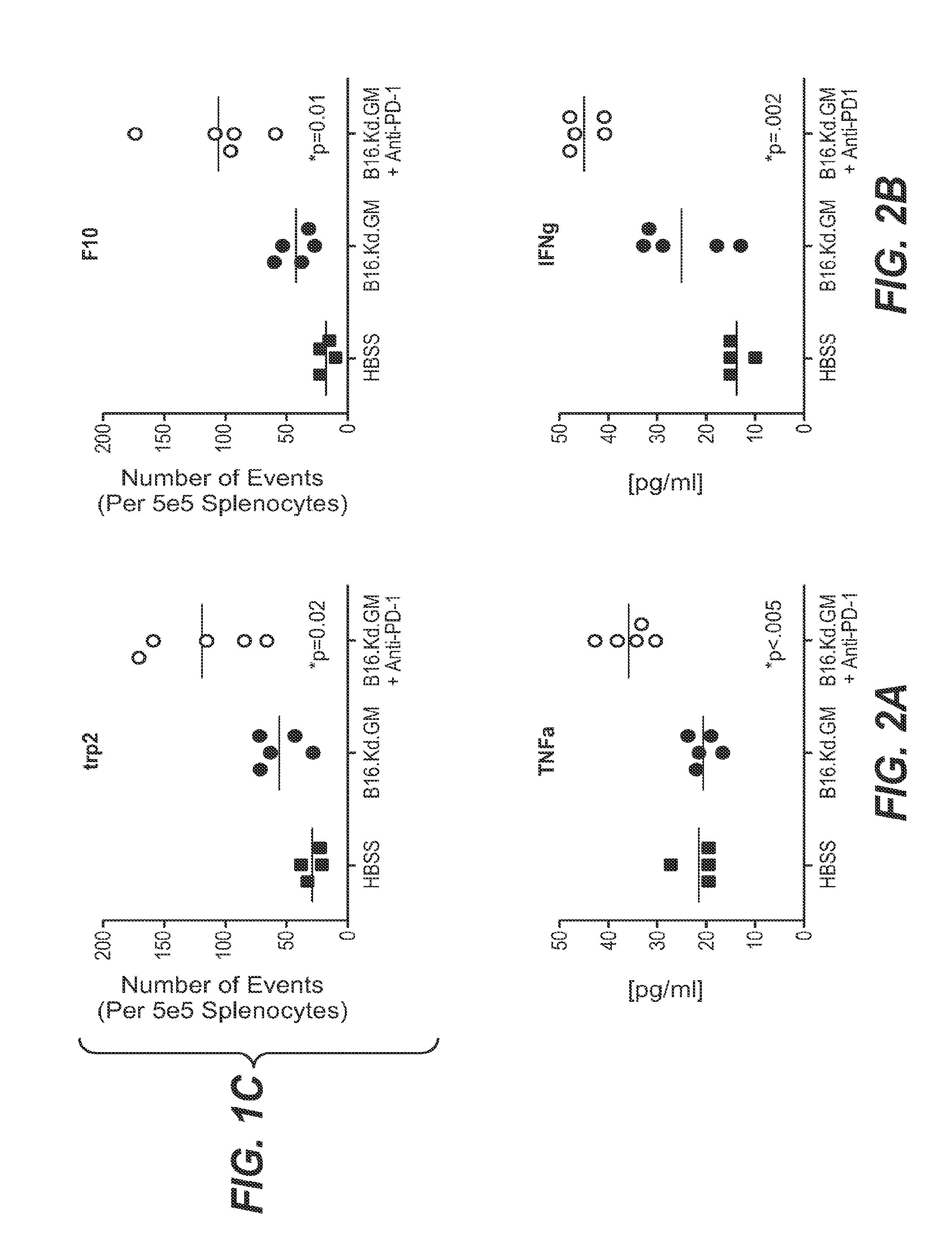
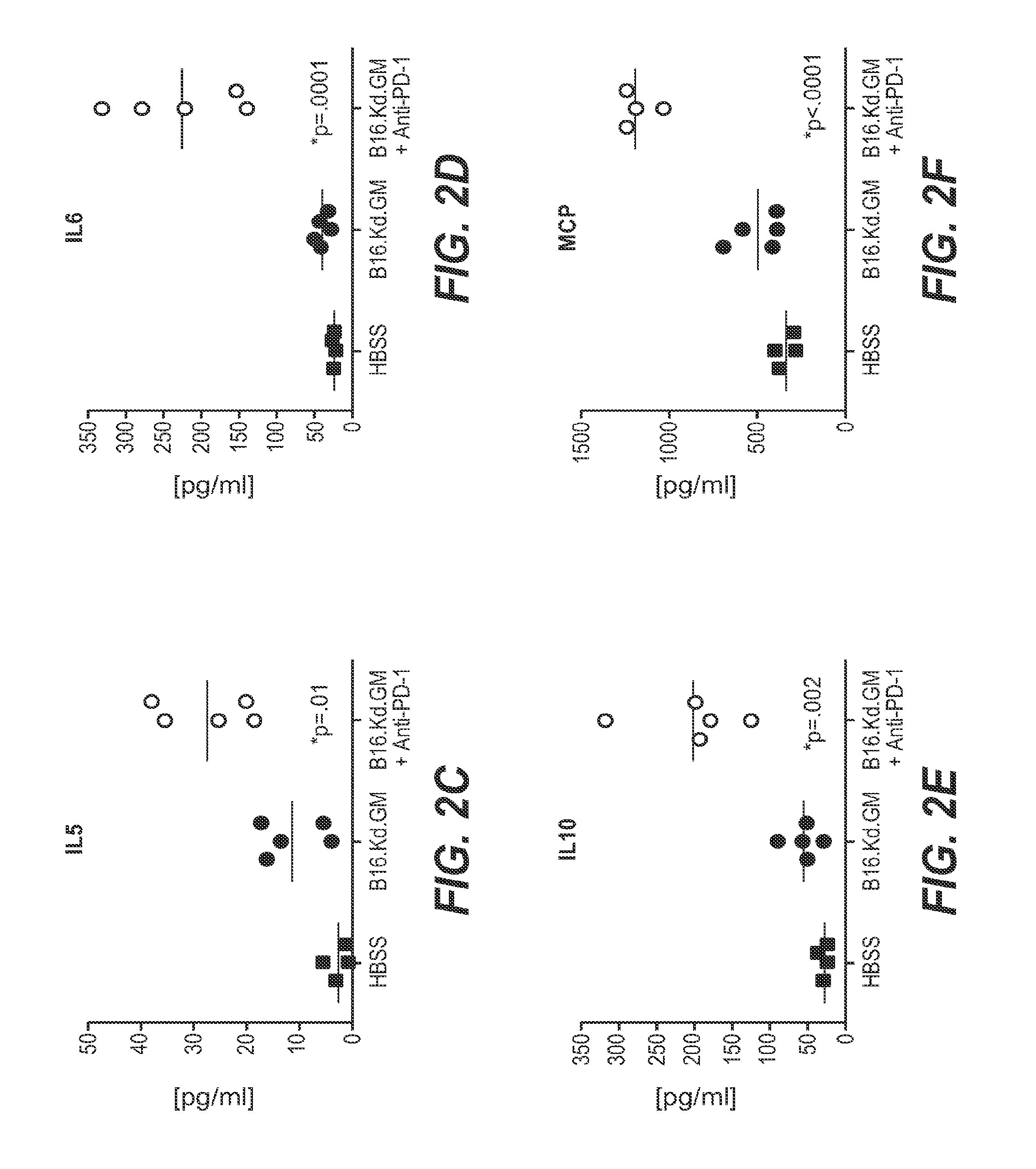
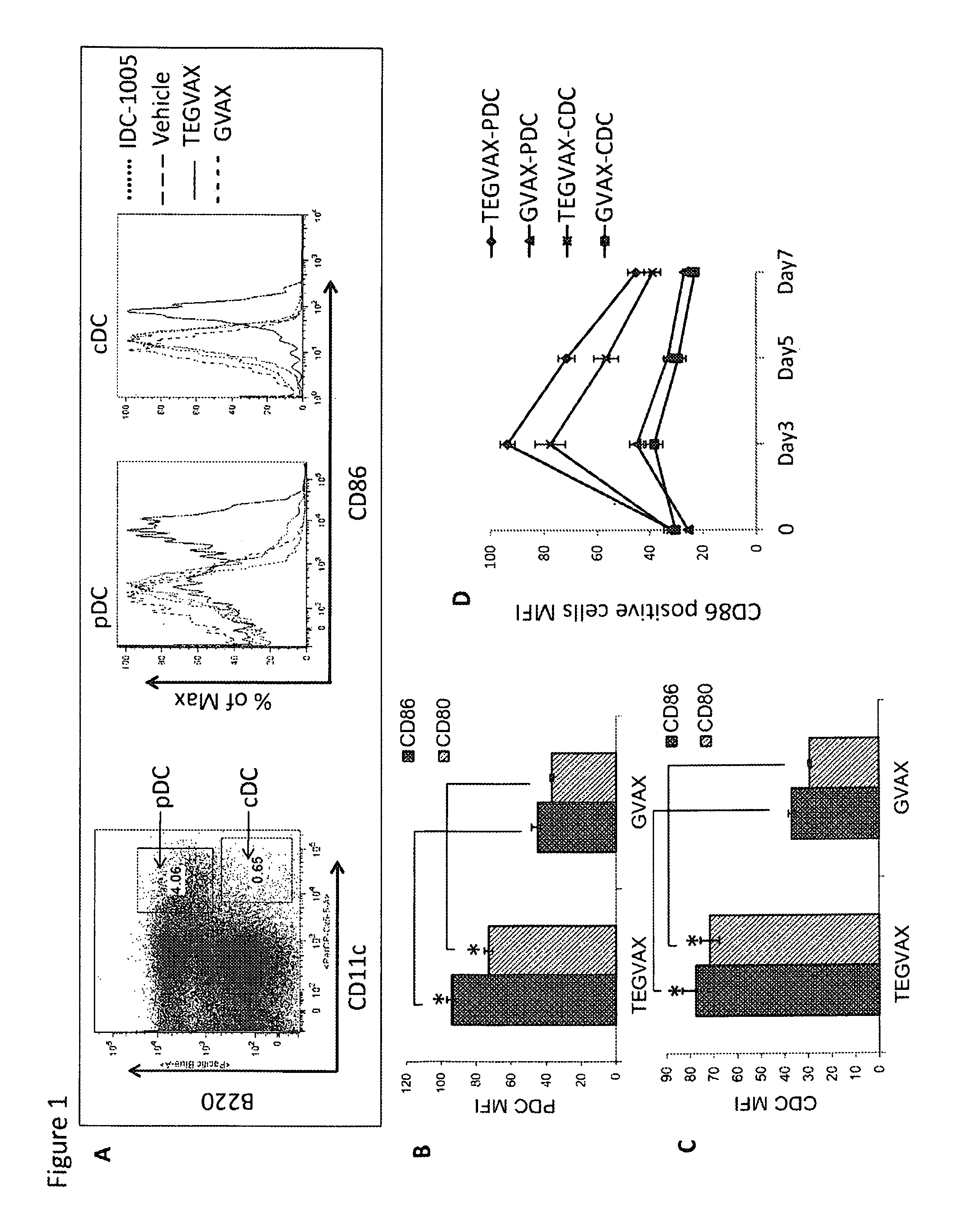
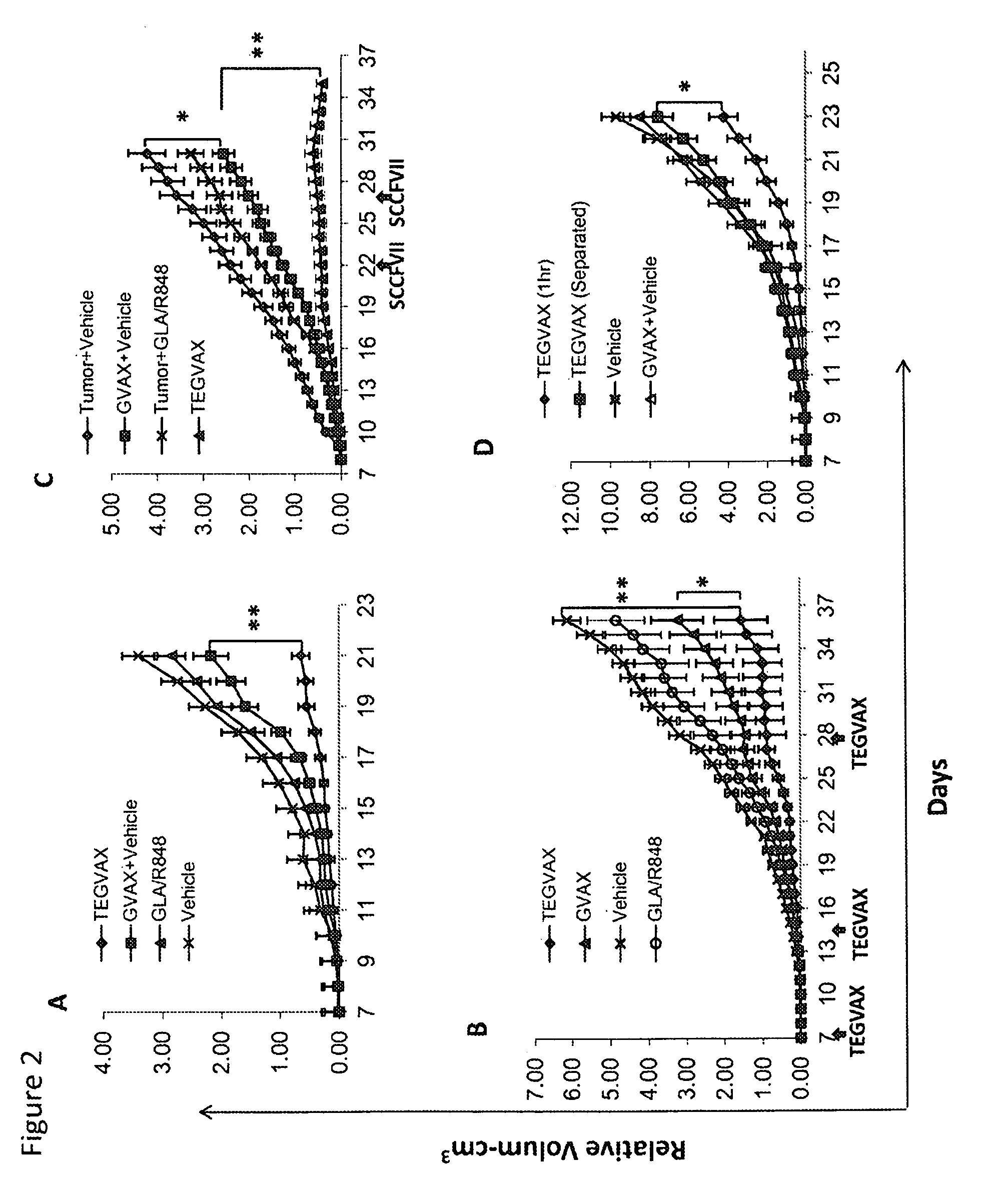
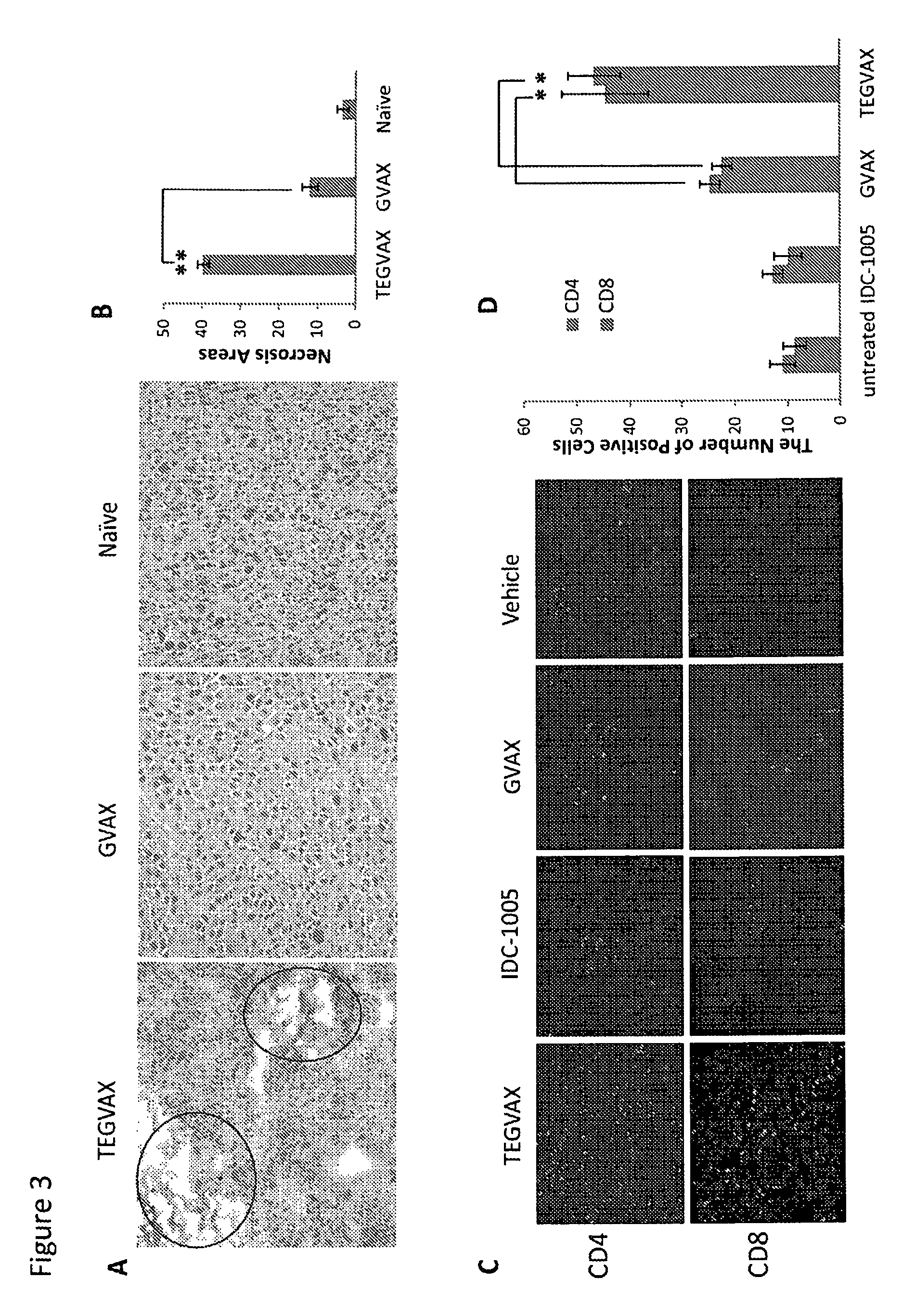
We formulated multiple TLR agonists into GVAX (lethally irradiated tumor cell vaccines engineered to secrete GM-CSF). Specifically, GLA and R848, TLR4 and TLR7 / 8 agonists found to be safe in patients, were formulated with GVAX (TEGVAX—for TLR agonists enhanced GVAX), and this formulation was effective in producing anti-tumor responses in 3 different preclinical models, including palpable B16. These anti-tumor responses were correlated with increased CD4 and CD8 T-cells that can secrete IFNγ circulating in the tumor microenvironment as well as significantly higher level of p15E specific CTL mediated cell killing in mice treated with TEGVAX in comparison to controls. When combined with anti-PD-1 antibody, TEGVAX was able to induce regression of established B16 tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
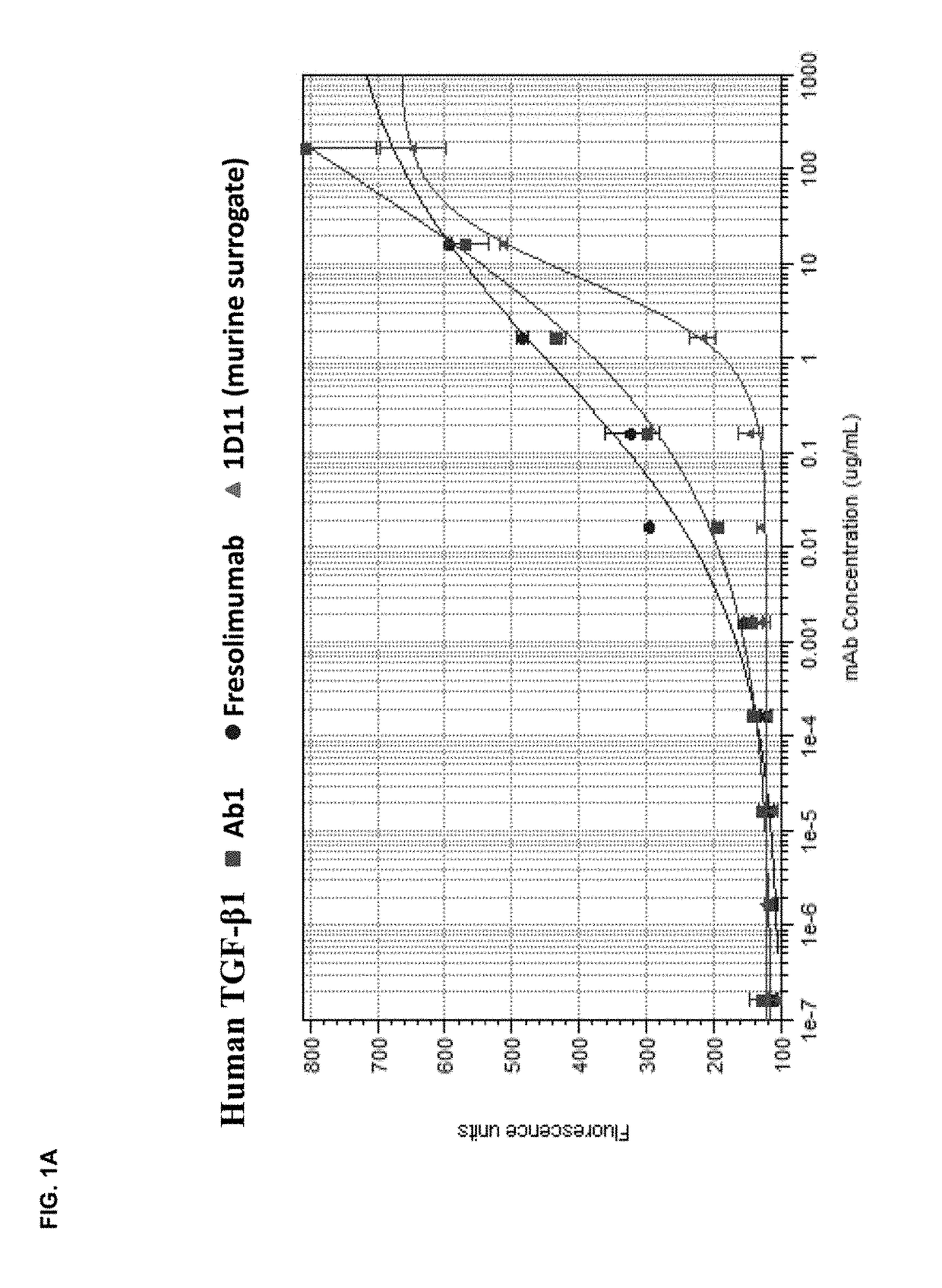
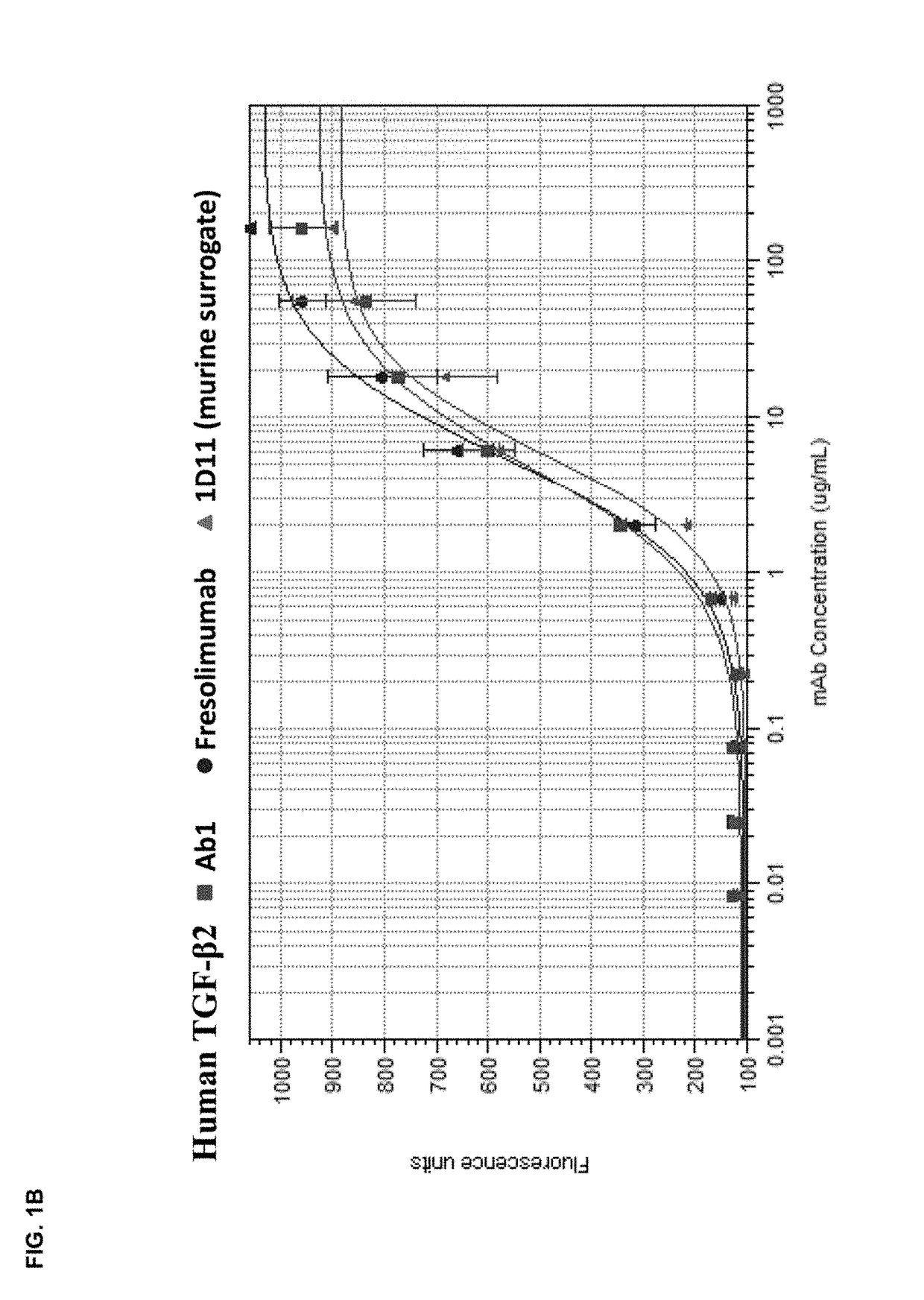
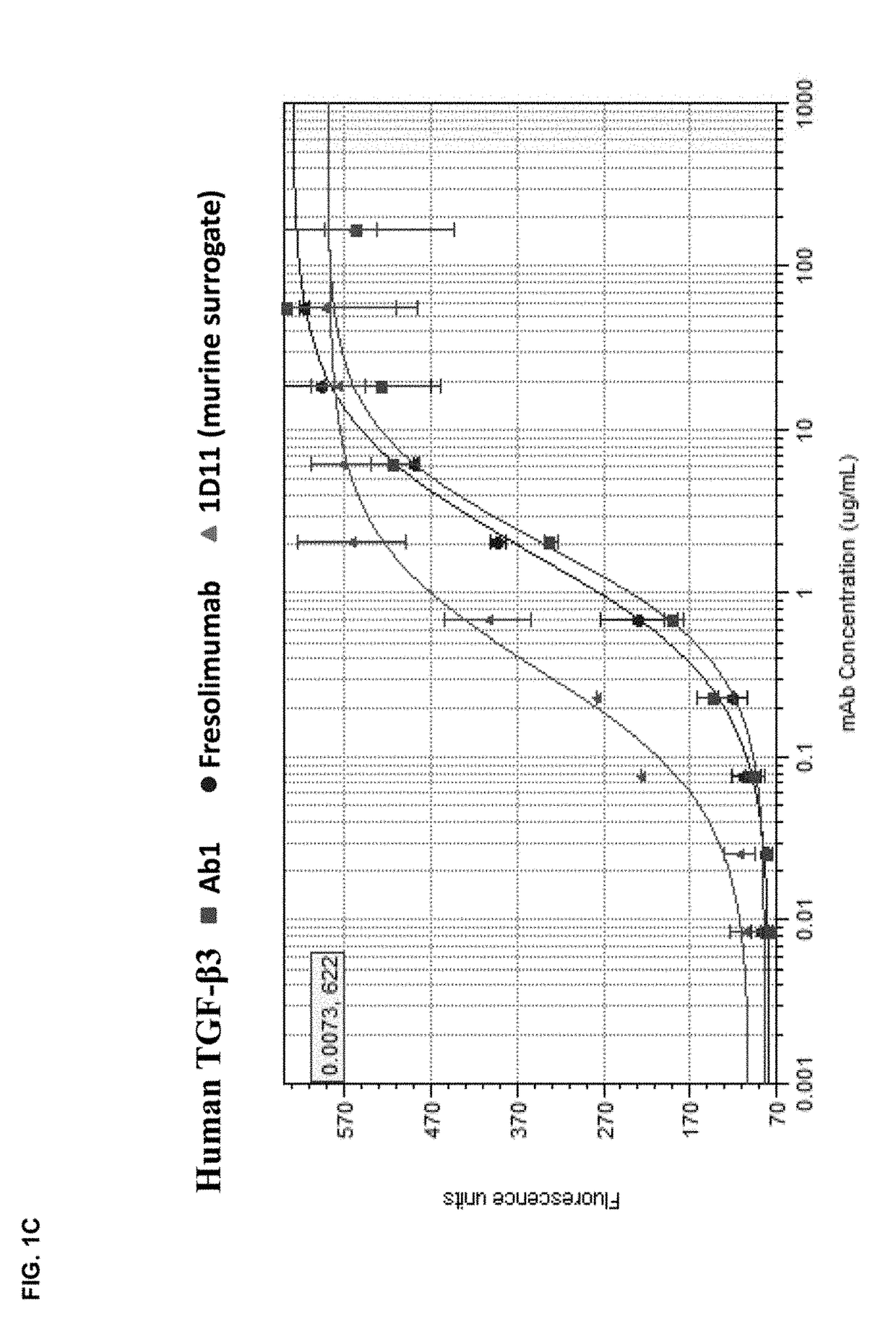
Anti-tgf-beta antibodies and their use
ActiveUS20180244763A1Alleviates immunosuppressive microenvironmentImprove efficacyImmunoglobulins against growth factorsImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune conditionAutoimmune disease
The invention provides an improved pan-TGF-β antibody for treatment of conditions that are mediated by TGF-β, including autoimmune diseases, fibrotic conditions, and cancers. Also provided are methods and uses of the antibody in conjunction with other immunomodulatory agents such as an anti-PD-1 antibody.
Owner:SANOFI SA
Antagonists of pdl-1 and pd-1 for the treatment of hpv-negative cancers
InactiveUS20160031990A1Enhance immune responseAvoid interactionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenCancer research
Provided herein are methods of treating HPV-negative tumors comprising administering an effective amount of an antagonist of the PDL-1 / PD-1 interaction (e.g., an anti-PDL-1 or anti-PD-1 antibody antigen binding fragment thereof).
Owner:MEDIMMUNE LLC
Application of apatinib and anti-PD-1 antibody combination to preparation of colon cancer medicines
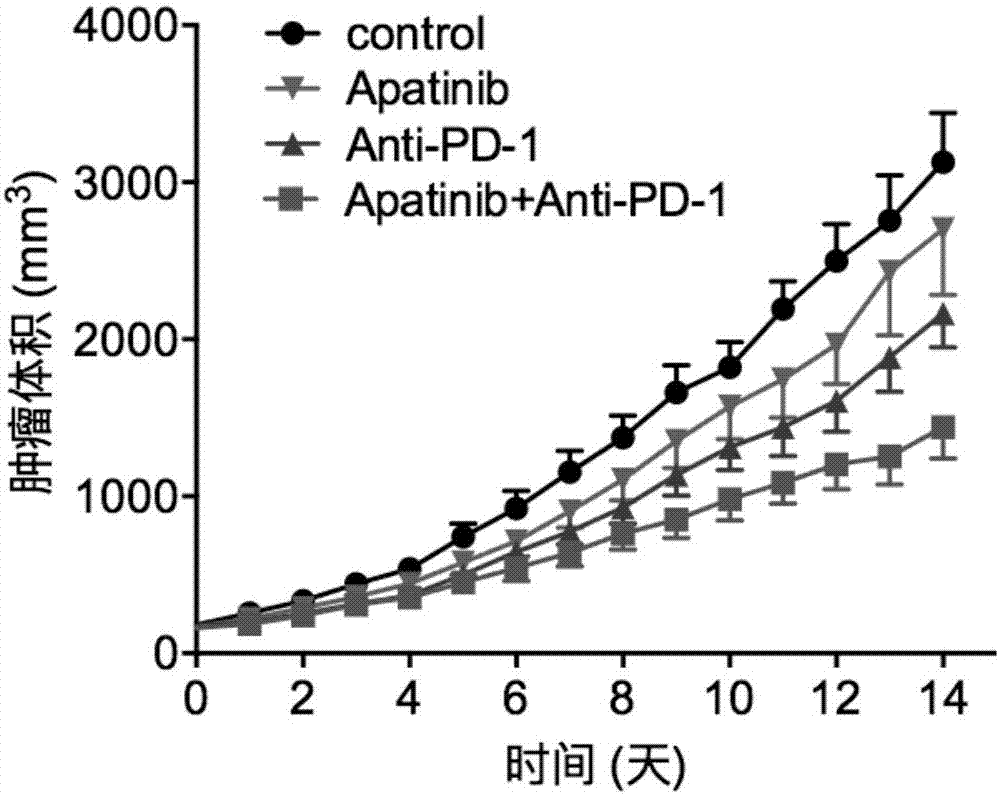
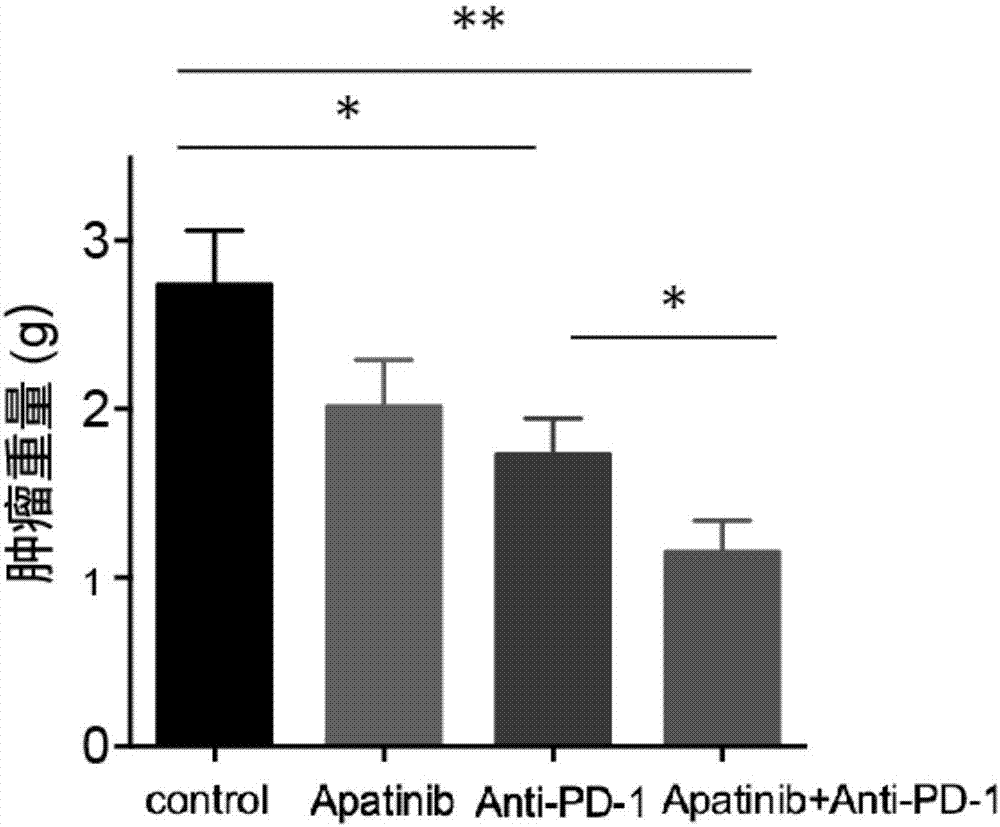
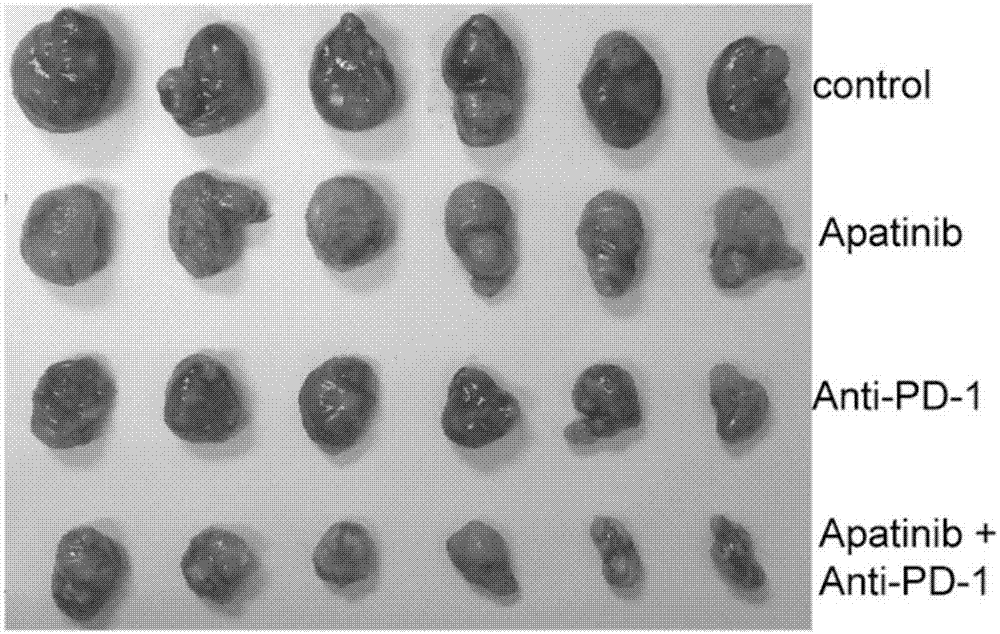
InactiveCN106963948AHigh tumor inhibition rateIncreased area of necrosisOrganic active ingredientsAntibody ingredientsWilms' tumorCancer research
The invention discloses application of apatinib and anti-PD-1 antibody combination to preparation of colon cancer medicines. The colon cancer is treated through combined medication of apatinib and an anti-PD-1 antibody; through experiments, the tumor inhibition rate of apatinib and anti-PD-1 antibody combination is 53.97 percent, the tumor inhibition rate is increased by 40.4 percent as compared with that of a single apatinib group (53.97 percent vs 13.57 percent, P is less than 0.05, Mann-Whitneytest), and the tumor inhibition rate is increased by 23.17 percent as compared with that of a single anti-PD-1 antibody group (53.97 percent vs 13.57 percent, P is less than 0.05, Mann-Whitneytest) (the tumor inhibition rate is equal to 1-expreiment group tumor volume / control group tumor volume). After the apatinib and the anti-PD-1 antibody are combined, the tumor necrosis area can be obviously increased as compared with that of single groups.
Owner:顾艳宏
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com