Anti-PD-1 (Programmed Death-1) antibody and application thereof
A PD-1 and antibody technology, applied in the direction of antibodies, applications, anti-inflammatory agents, etc., can solve the problem of low response rate and achieve the effects of high activity, low production cost, and high binding affinity
Active Publication Date: 2017-03-22
LUNAN PHARMA GROUP CORPORATION
View PDF13 Cites 41 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Although a variety of monoclonal antibodies against immune checkpoints such as PD-1, PD-L1 and CTLA4 have been used clinically, the response rate of these antibodies as a single drug is still low, with an average of only 15-20%
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0049] Abbreviations and Definitions
[0050] hPD-1 human PD-1 protein
[0051] CDR Complementarity-determining region in the variable region of an immunoglobulin defined by the Kabat numbering system
[0052] EC 50 Concentration that produces 50% efficacy or binding
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
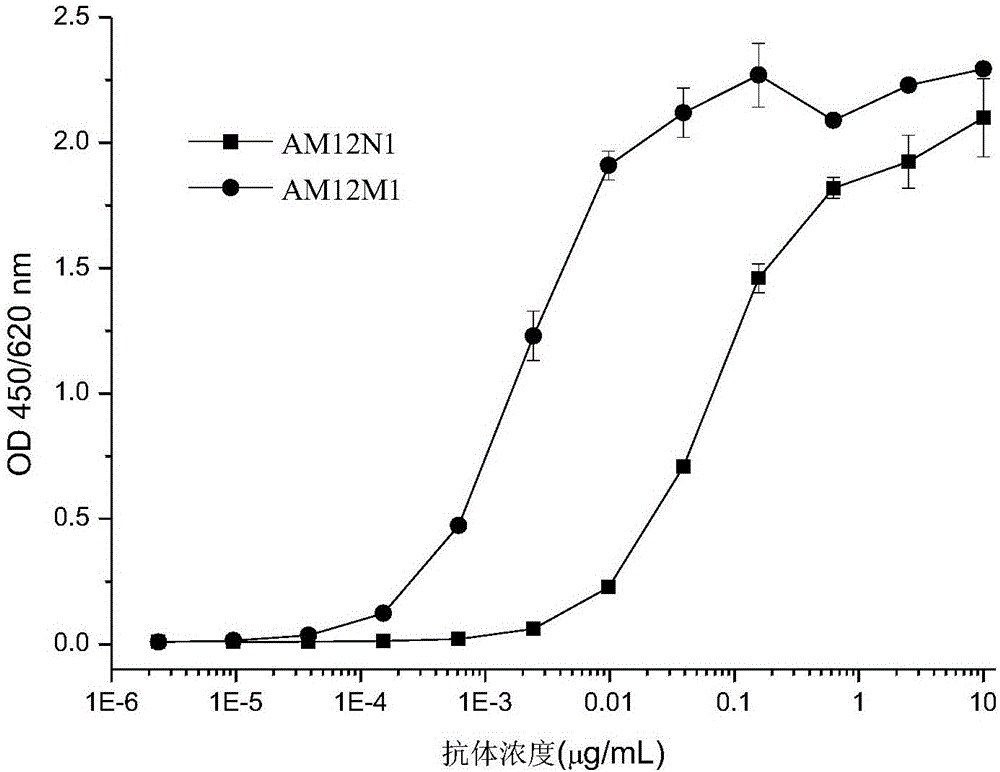
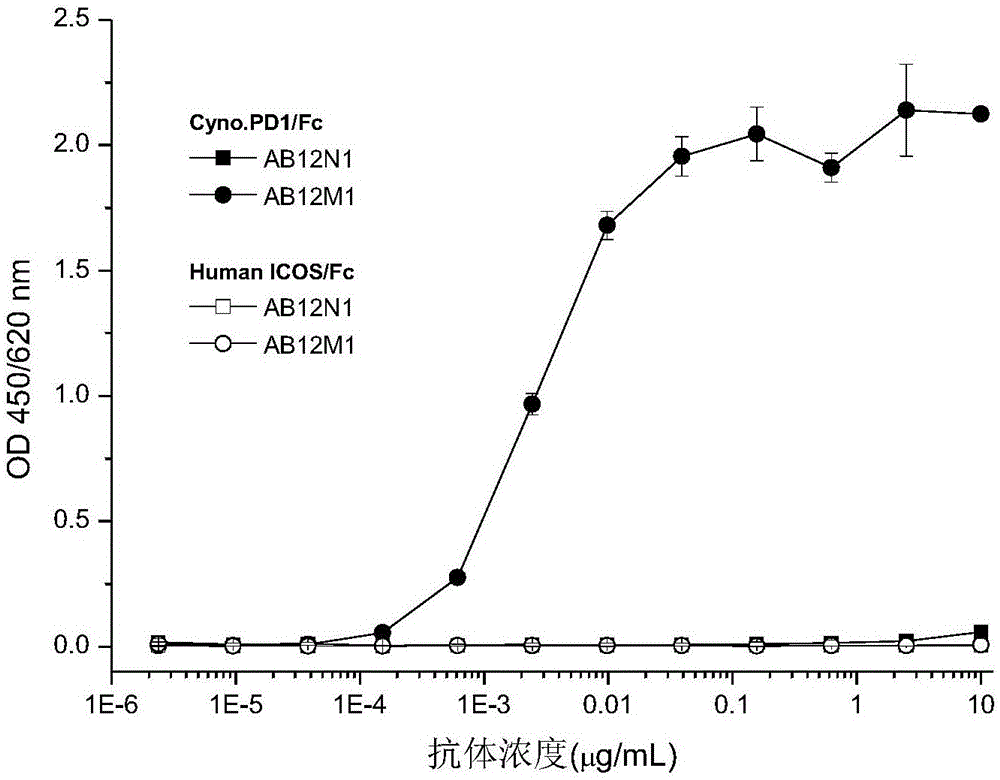
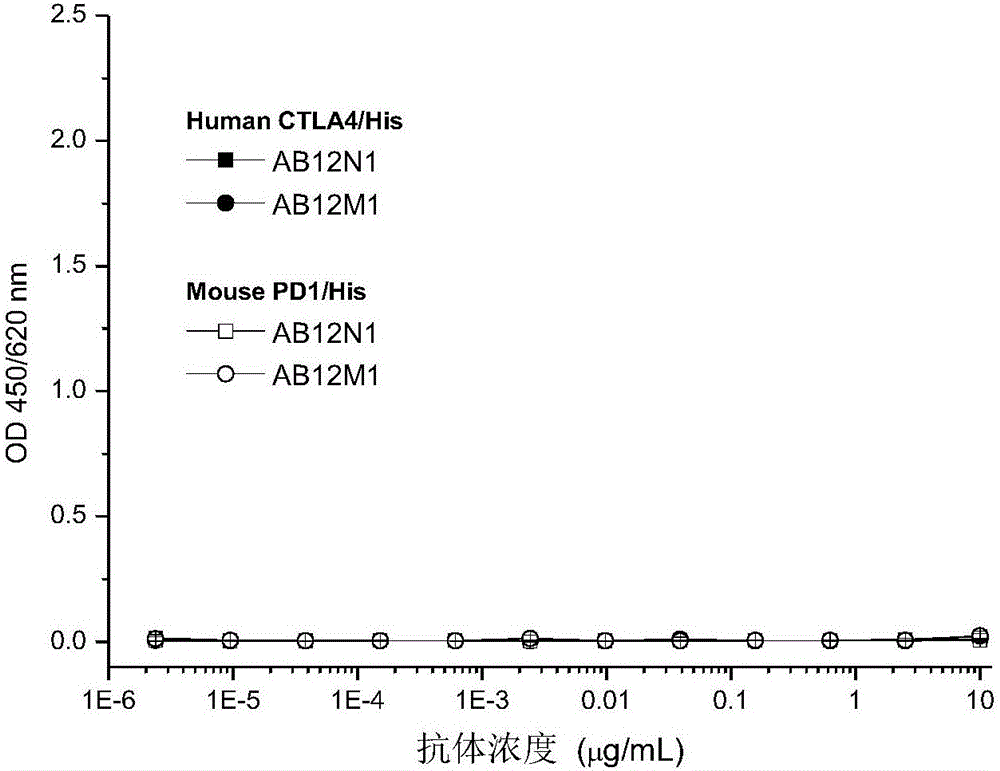
The invention provides an antibody specifically combined with PD-1 (Programmed Death-1) with high affinity, and also provides a nucleic acid molecule for coding the antibody, an expression vector and a host cell for expressing the antibody, and a production method of the antibody. In addition, the invention also provides an immune conjugate and a medicinal composition containing the antibody, and application of the antibody to the preparation of a medicine for treating multiple diseases (including a cancer, an infectious disease and an inflammatory disease).
Description
technical field [0001] The present invention belongs to the field of therapeutic monoclonal antibodies, more specifically, the present invention relates to an antibody against programmed death receptor (PD-1); , inflammatory diseases). Background technique [0002] Programmed Death-1 (PD-1), a member of the CD28 family, is an immunosuppressive receptor expressed on the surface of activated T cells and B cells (Yao Zhu et al., Nat Rev Drug Discov, 2013; 12(2):130-146), originally obtained by subtractive hybridization in apoptotic T-cell hybridomas. PD-1 is mainly expressed in CD 4+ T cells, CD 8+ The surface of T cells, NK-T cells, B cells and activated monocytes is mainly induced by T cell receptor (TCR) or B cell receptor (BCR) signaling, and TNFα can enhance the expression of PD-1 on the surface of these cells (Francisco LM et al., Immunol Rev, 2010; 236:219-242). The PD-1 molecule consists of an extracellular domain, a transmembrane domain and an intracellular domain...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07K16/28C07K16/46C12N15/13C12P21/02A61K39/395A61P35/00A61P31/00A61P31/14A61P31/04A61P31/20A61P31/18A61P33/00A61P29/00
CPCC07K16/2818C07K16/468A61K2039/505C07K2317/24C07K2317/565C07K2317/56C07K2317/92A61P35/00A61K39/395C07K16/2803C07K16/30A61K47/55C07K16/22C07K16/2863C07K16/2878C07K16/2887C07K16/32
Inventor 李强孙见宇郑云程杨璐马心鲁李媛丽
Owner LUNAN PHARMA GROUP CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com