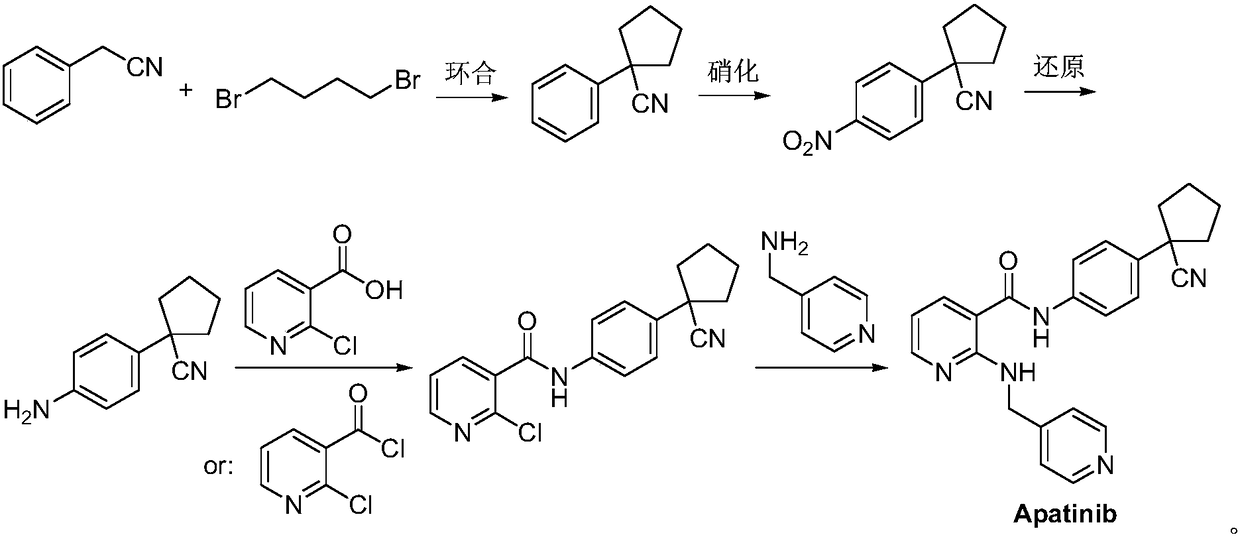
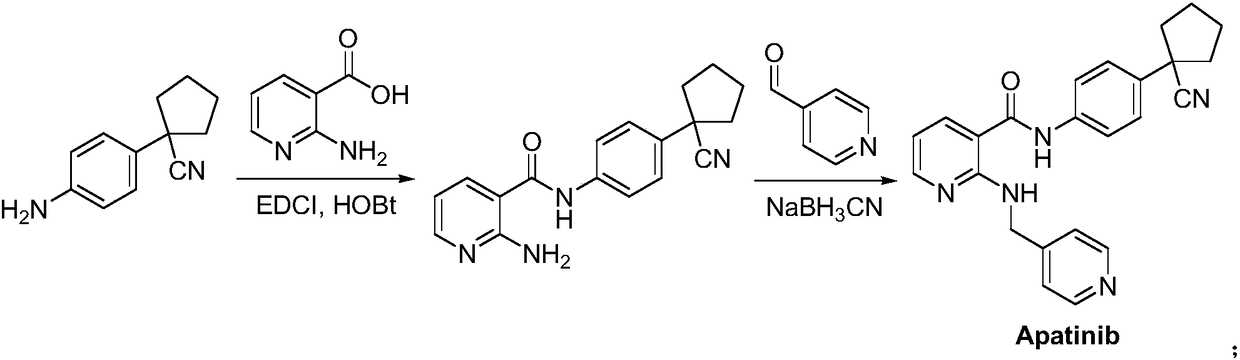
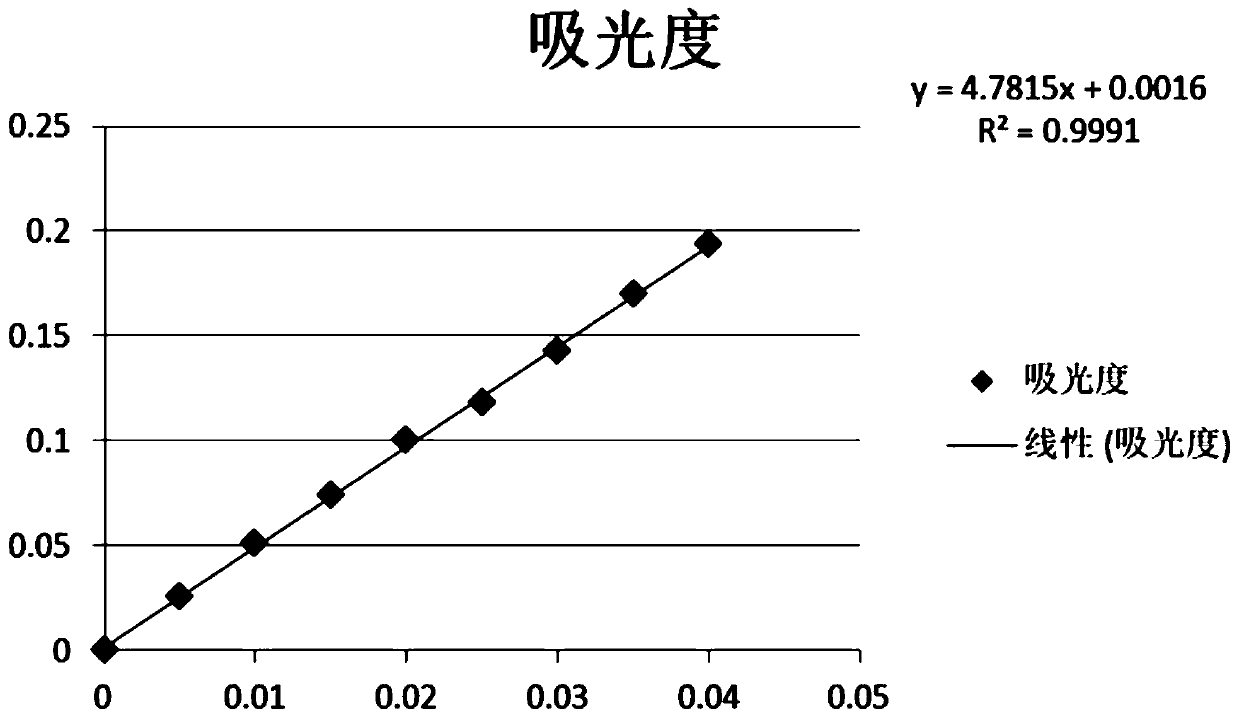
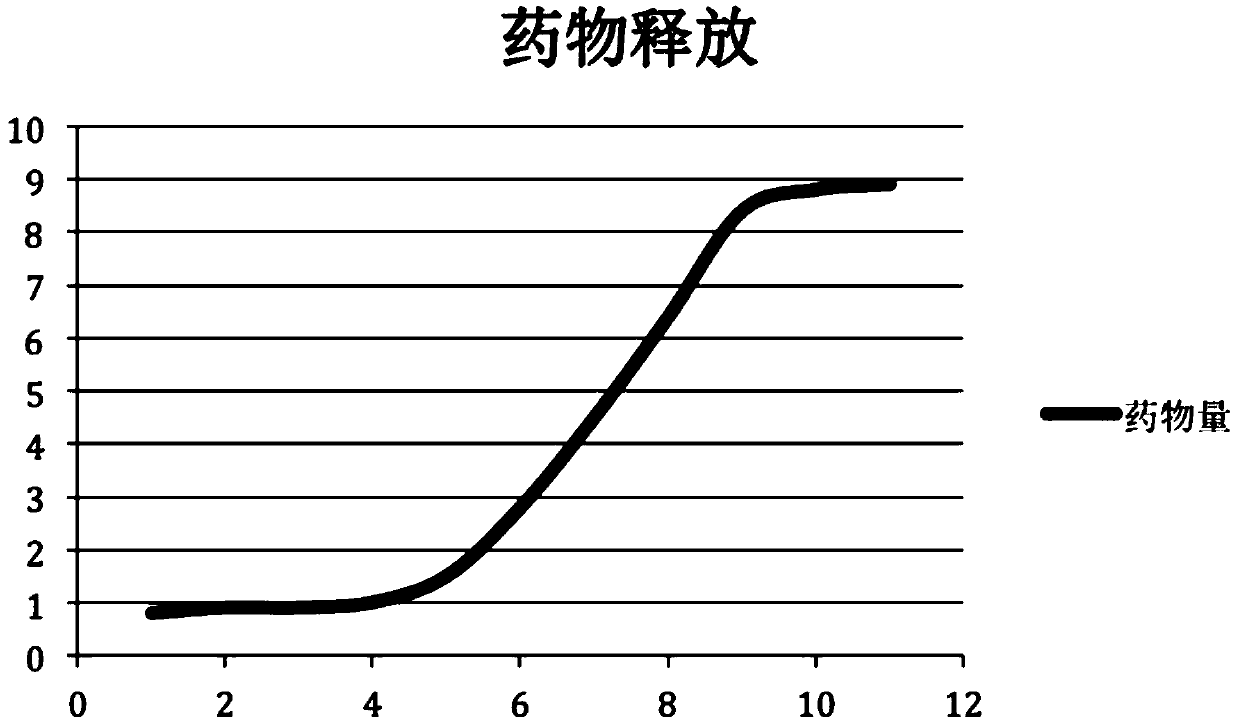
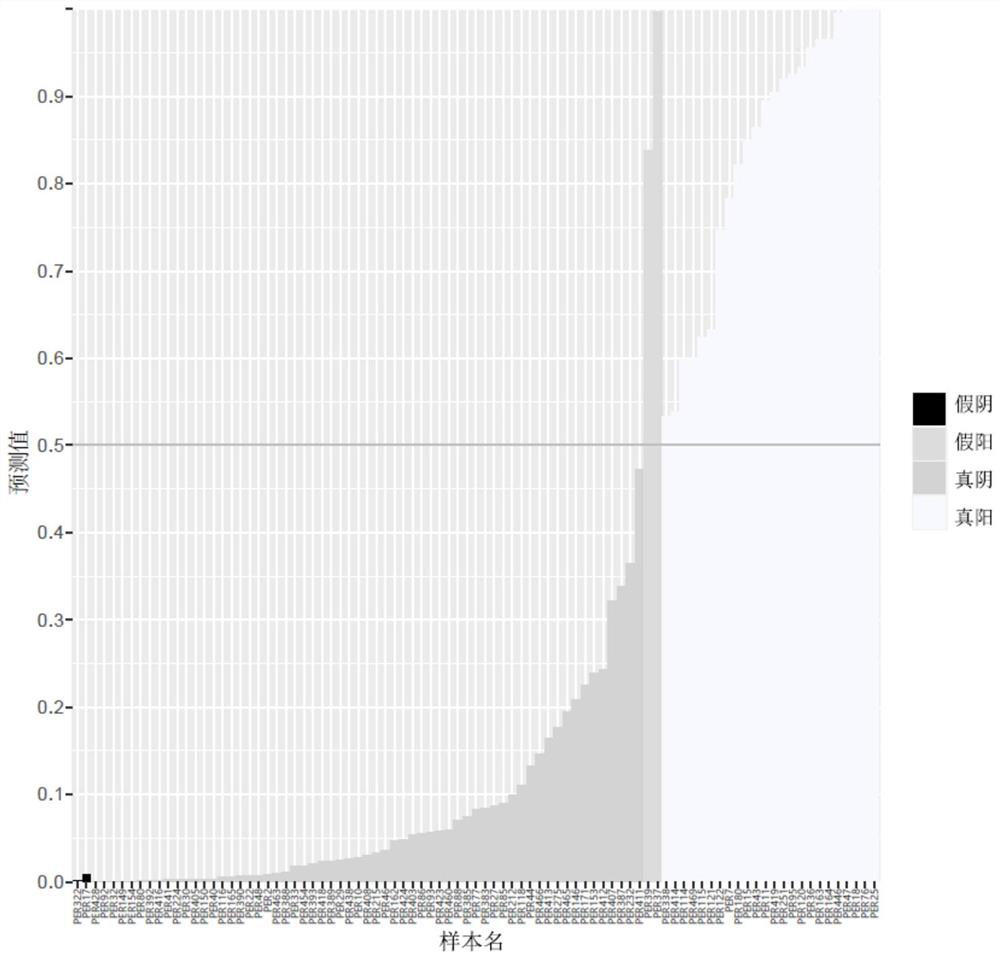
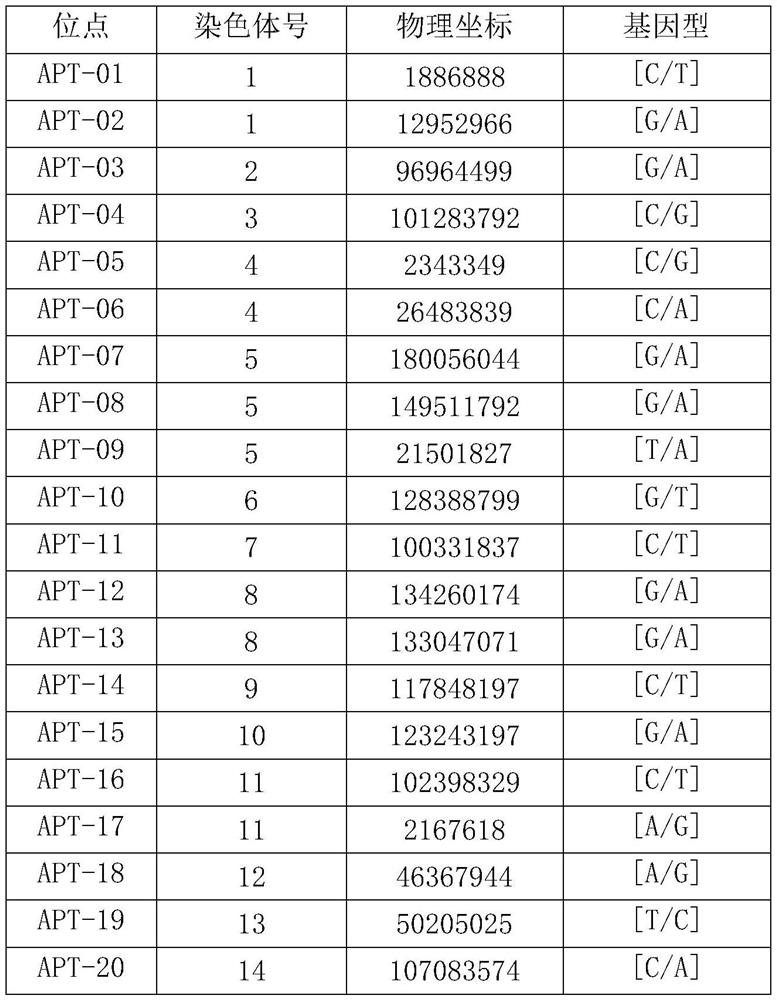
Patents
Literature
61 results about "Apatinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
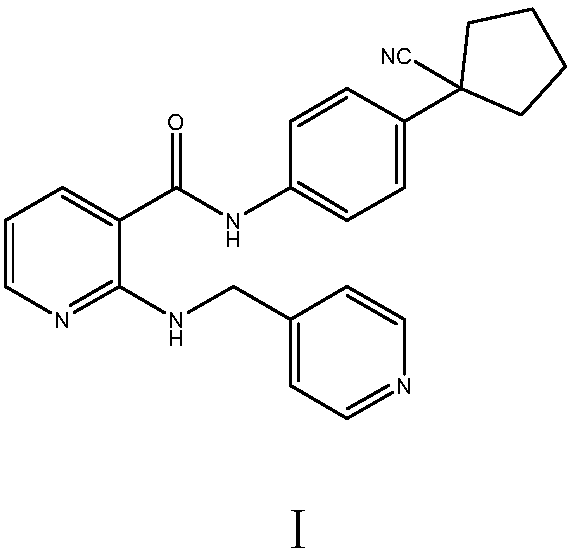
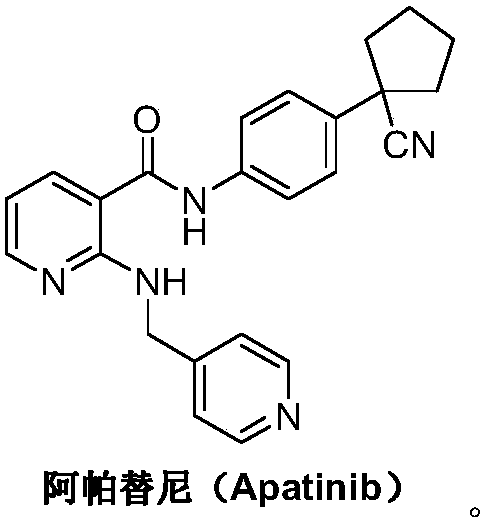
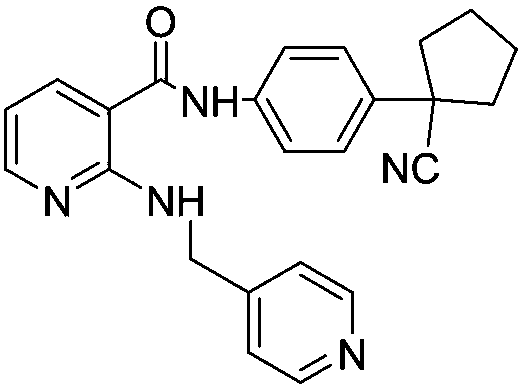
Apatinib, also known as YN968D1, is a tyrosine kinase inhibitor that selectively inhibits the vascular endothelial growth factor receptor-2 (VEGFR2, also known as KDR). It is an orally bioavailable, small molecule agent which is thought to inhibit angiogenesis in cancer cells; specifically apatinib inhibits VEGF-mediated endothelial cell migration and proliferation thus blocking new blood vessel formation in tumor tissue. This agent also mildly inhibits c-Kit and c-SRC tyrosine kinases.
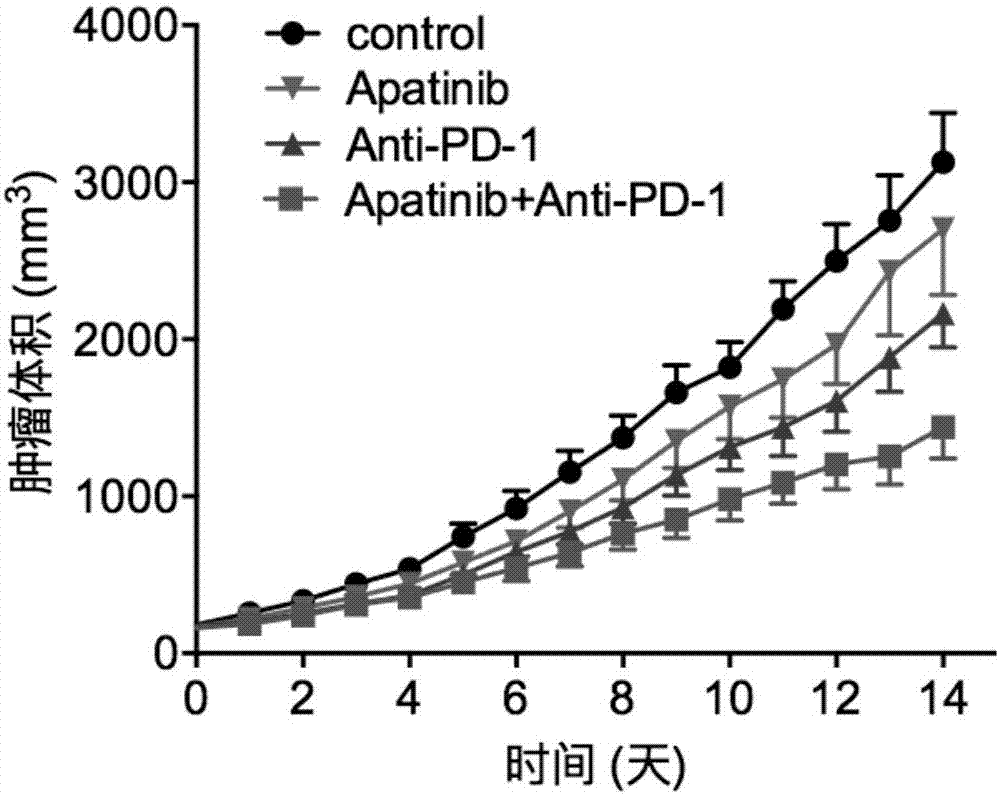
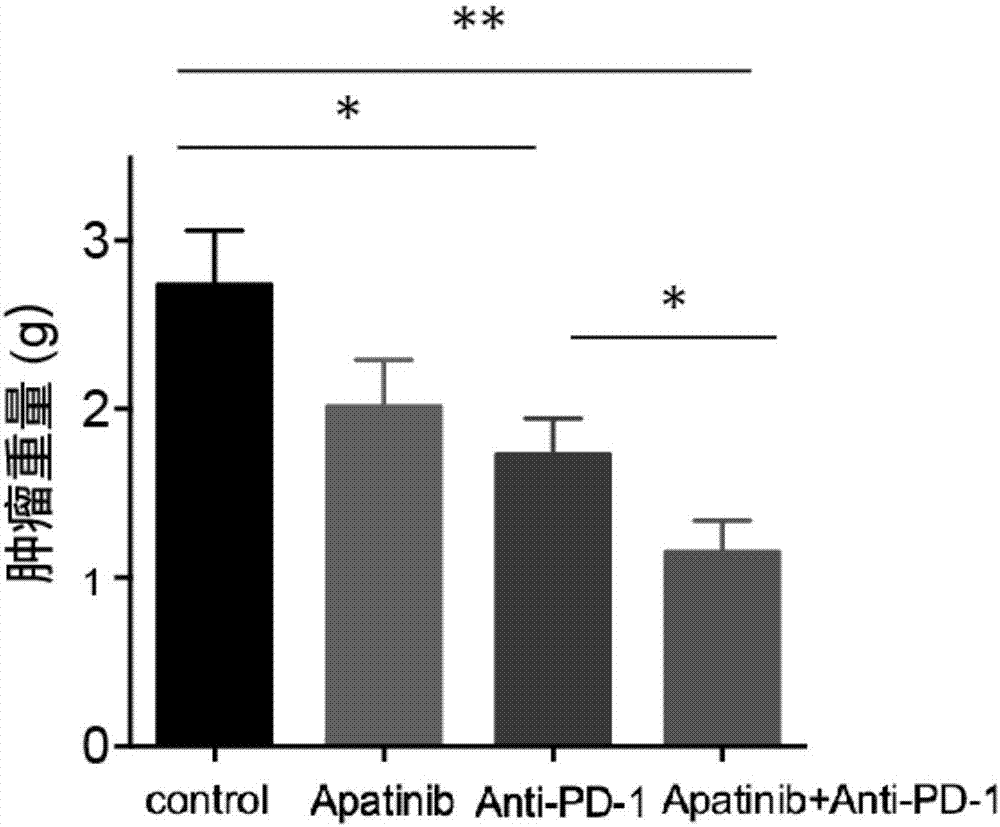
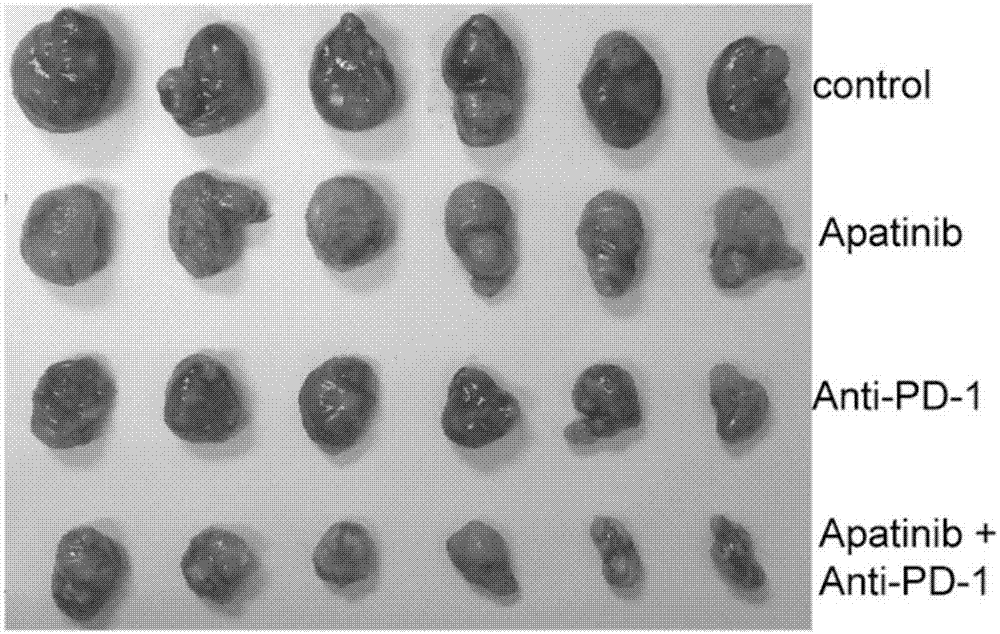
Application of apatinib and anti-PD-1 antibody combination to preparation of colon cancer medicines
InactiveCN106963948AHigh tumor inhibition rateIncreased area of necrosisOrganic active ingredientsAntibody ingredientsWilms' tumorCancer research
The invention discloses application of apatinib and anti-PD-1 antibody combination to preparation of colon cancer medicines. The colon cancer is treated through combined medication of apatinib and an anti-PD-1 antibody; through experiments, the tumor inhibition rate of apatinib and anti-PD-1 antibody combination is 53.97 percent, the tumor inhibition rate is increased by 40.4 percent as compared with that of a single apatinib group (53.97 percent vs 13.57 percent, P is less than 0.05, Mann-Whitneytest), and the tumor inhibition rate is increased by 23.17 percent as compared with that of a single anti-PD-1 antibody group (53.97 percent vs 13.57 percent, P is less than 0.05, Mann-Whitneytest) (the tumor inhibition rate is equal to 1-expreiment group tumor volume / control group tumor volume). After the apatinib and the anti-PD-1 antibody are combined, the tumor necrosis area can be obviously increased as compared with that of single groups.
Owner:顾艳宏
Method for simultaneously detecting multiple anti-tumor drugs in blood sample
InactiveCN110927297AEasy to handleImprove throughputComponent separationBusulfanTandem mass spectrometry
The invention discloses a method for simultaneously detecting multiple anti-tumor drugs in a blood sample. A pretreated sample to be detected is detected by adopting ultra-high performance liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). The pretreatment process comprises the following steps: adding serum into a mixed solution of methanol and acetonitrile, oscillating and centrifuging,taking out the centrifuged supernatant, drying, dissolving the dried powder into a methanol aqueous solution, and filtering to obtain a sample to be detected. The method can be used for simultaneously detecting 13 kinds of anti-tumor drugs such as methotrexate, 5-fluorouracil, apatinib, busulfan, carboplatin, cyclophosphamide, docetaxel, gemcitabine, imatinib, illinotecan, lenalidomide, oxaliplatin, paclitaxel and the like in blood.
Owner:JINAN YING SHENG BIOTECH
Synthesis method of apatinib for treating stomach cancer
InactiveCN107056695AHigh yieldHigh purityOrganic chemistryAntineoplastic agentsSodium bicarbonateSynthesis methods
The invention discloses a synthesis method of apatinib for treating stomach cancer, which comprises the following steps: (1) carrying out contact reaction between 2-chloro-3-methyl picolinate and 4-diazomethyl-pyridine in a mixed solution in the presence of an alkali to obtain a compound shown as formula I; (2) reacting the compound obtained in step (2) with the compound shown as formula II under stirring and under catalysis of tert-butyl alcohol, filtering reaction solution, concentrating filtrate, pouring into a sodium bisulfite solution, continuing stirring for reaction, regulating pH to 8-9 with saturated sodium bicarbonate after the reaction is complete to obtain apatinib. The method of the invention provides a new route for synthesis of apatinib, and has the following advantages: few reaction steps are involved, the yield of the target product is high, the raw materials are easily-accessible, the product purification is simple, and the method is suitable for large-scale industrial production.
Owner:QINGDAO CHENDA BIOLOGICAL SCI & TECH
Crystal form II of Apatinib mesylate as well as preparation method and application of crystal form II
InactiveCN105801476AImprove stabilityLow hygroscopicityOrganic active ingredientsOrganic chemistry methodsSolubilityX-ray
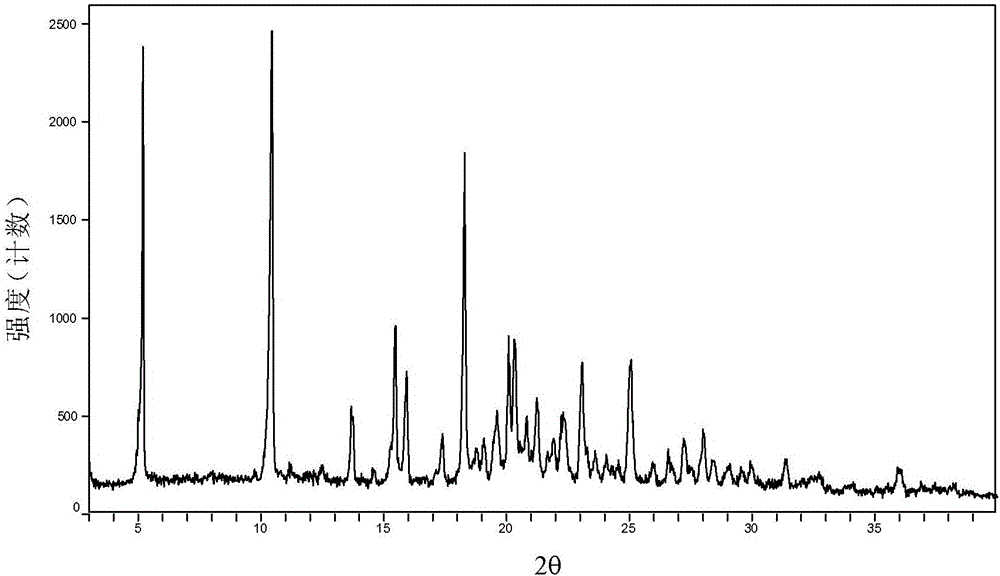
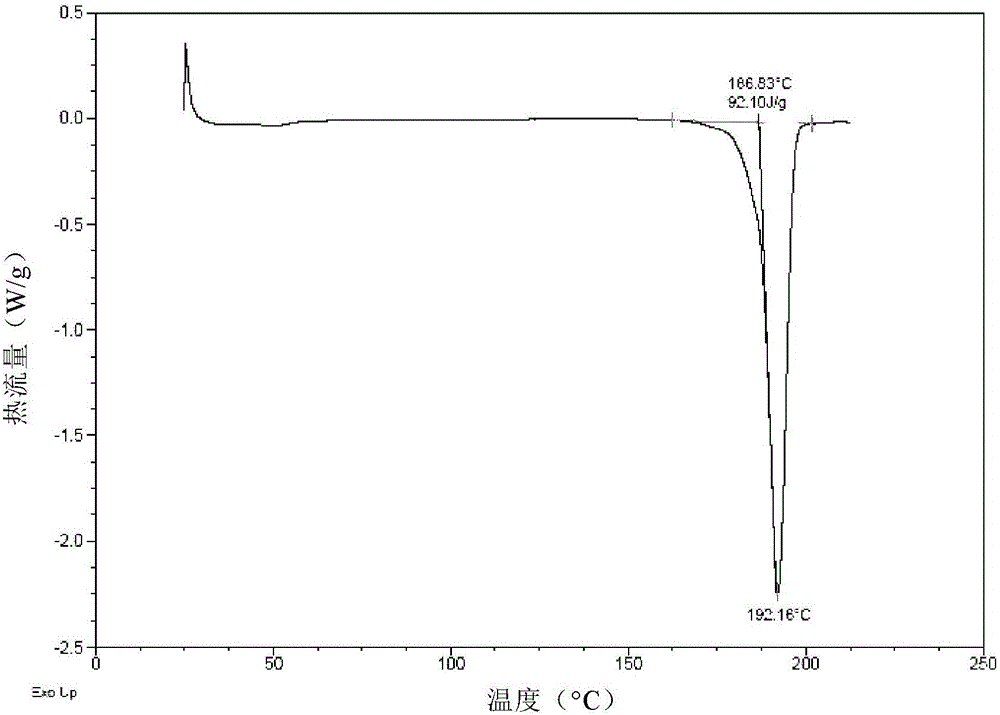
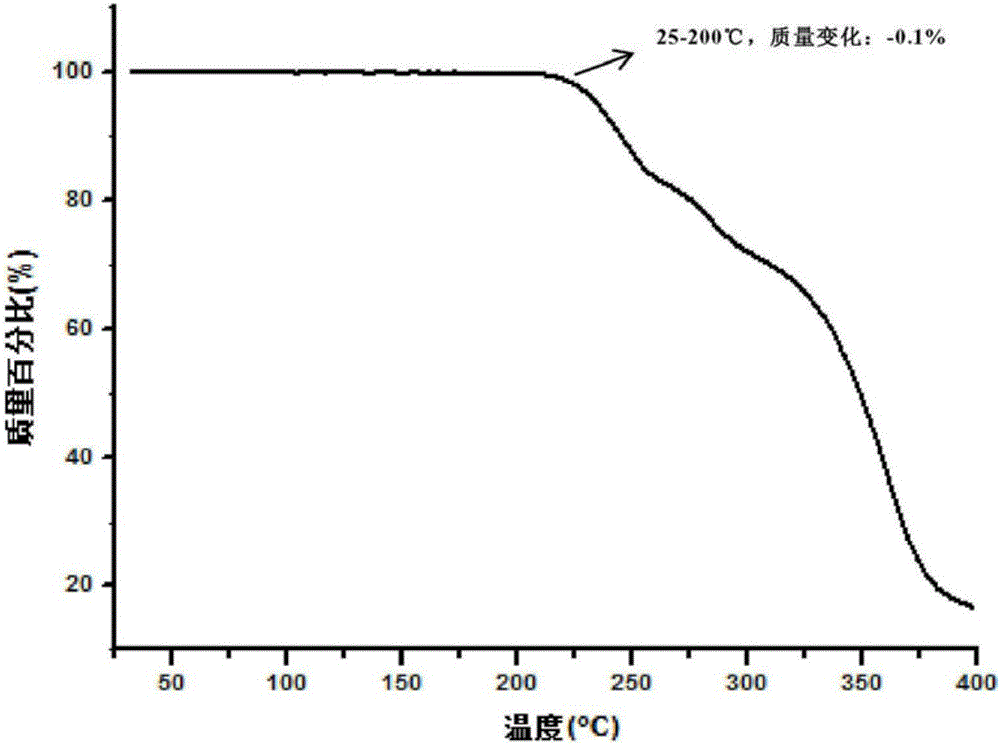
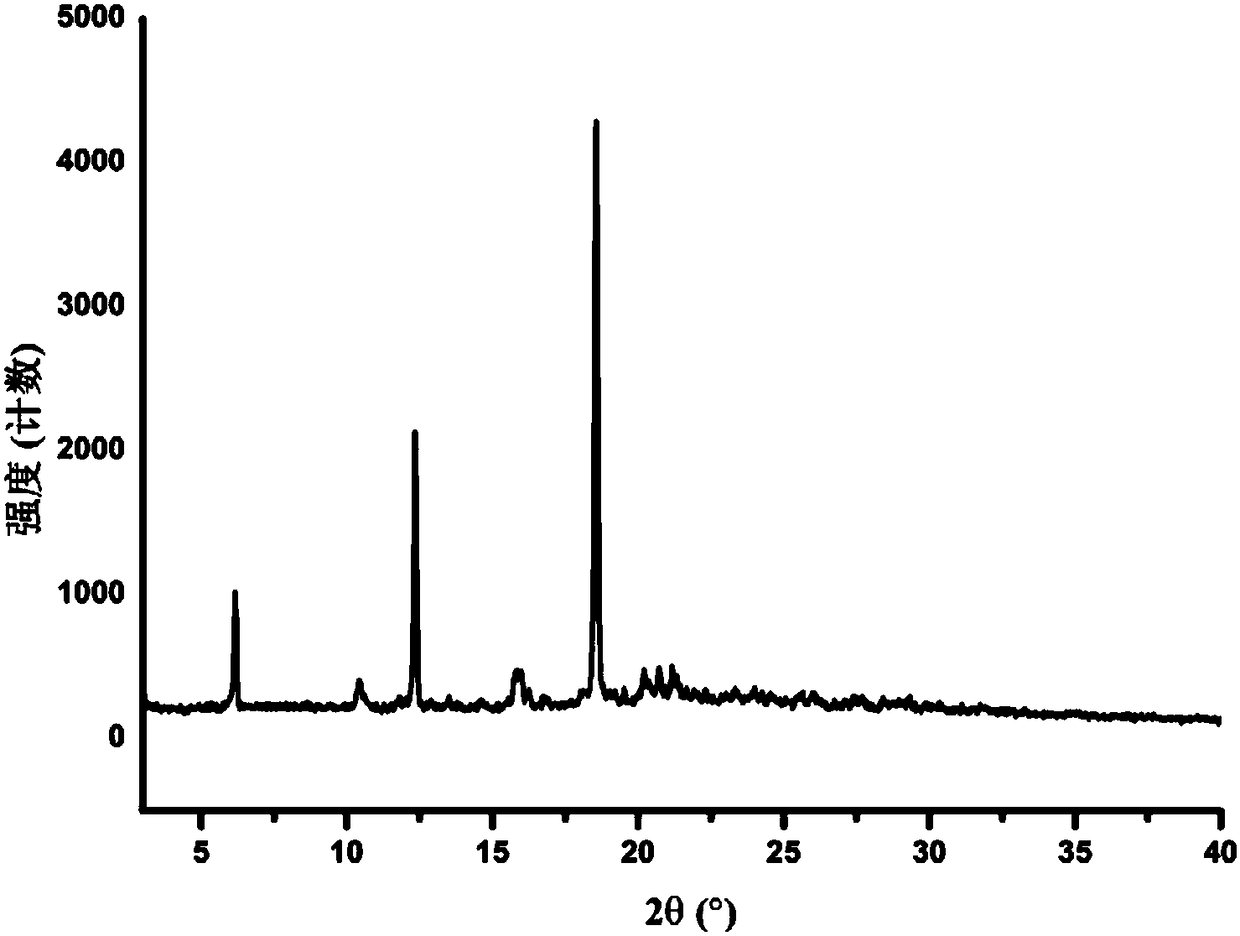
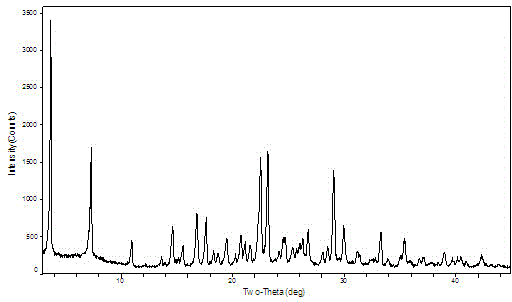
The invention provides a crystal form II of Apatinib mesylate. The crystal form II is characterized in that Cu-Kalpha 1 rays are adopted for radiation, X-ray powder diffraction represented by a 2theta angle has diffraction peaks when the 2theta angle is 5.02 degrees, 10.28 degrees, 11.13 degrees, 13.75 degrees, 15.65 degrees, 18.18 degrees, 20.35 degrees, 20.86 degrees, 21.58 degrees and 24.96 degrees, and an error range of a 2theta value is plus or minus 0.2. The crystal form II of Apatinib mesylate has the advantages of good stability, low hygroscopicity and high solubility.
Owner:SHANGHAI SUNTRONG BIOTECH
Preparation method of apatinib
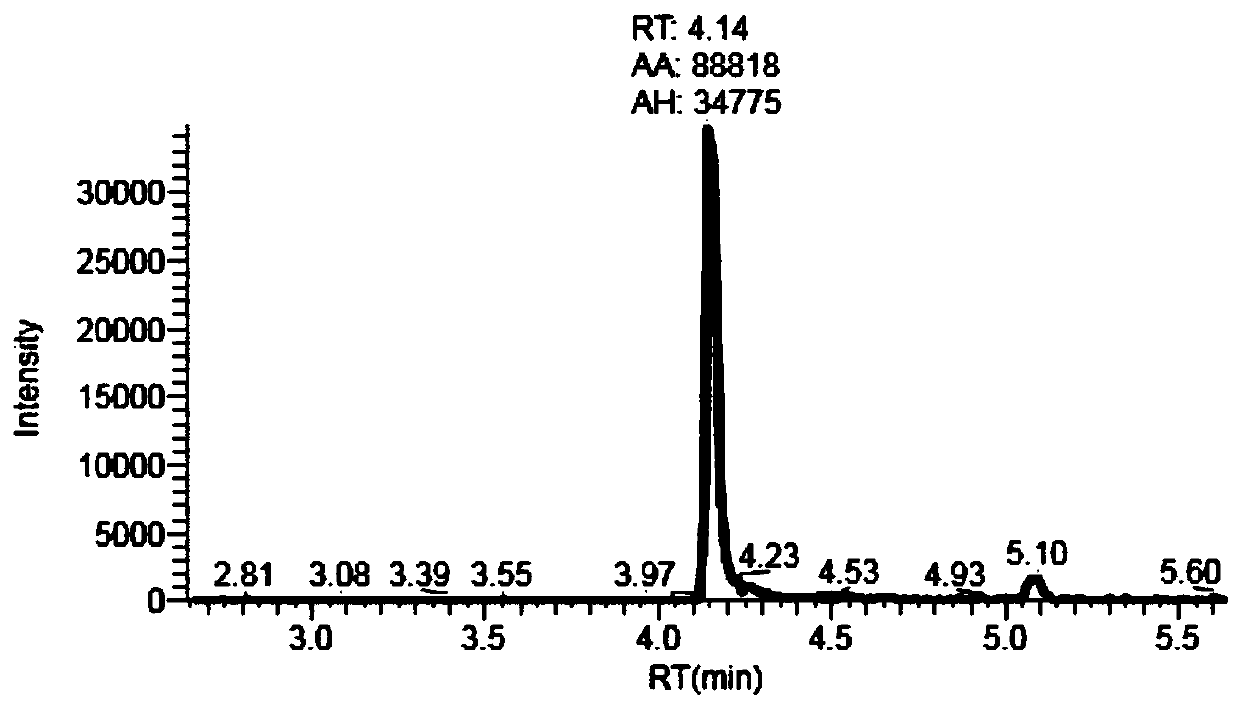
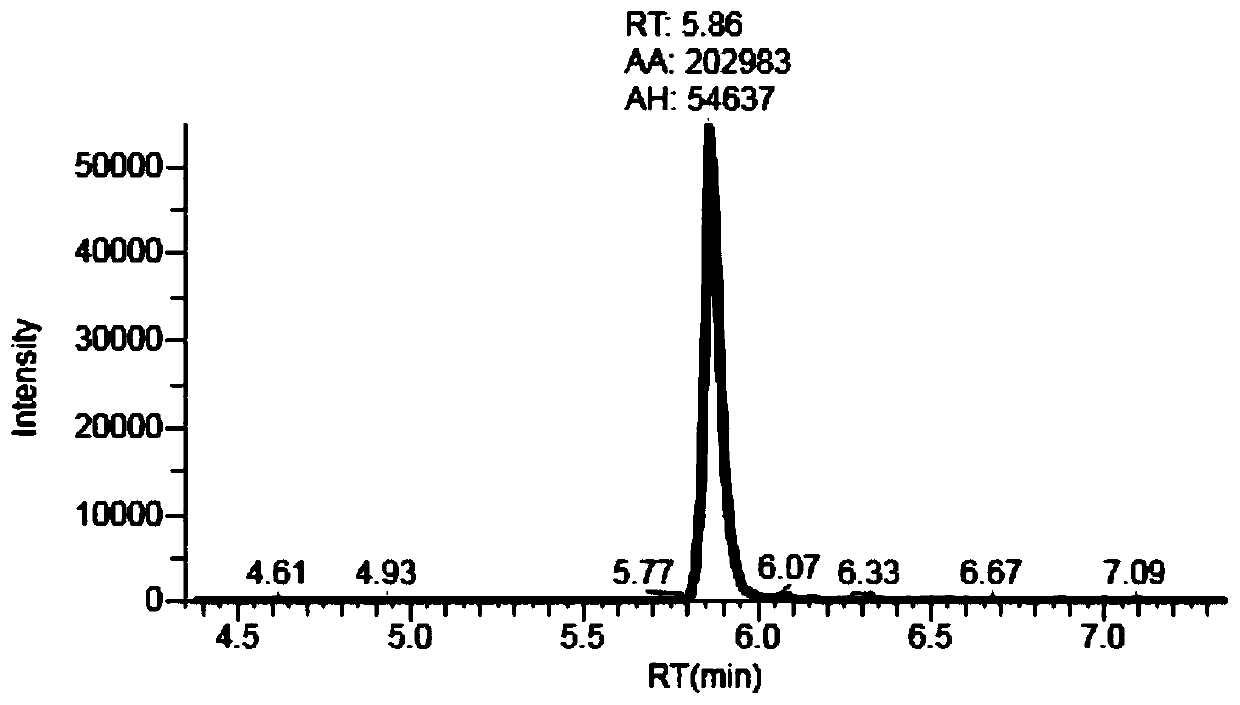
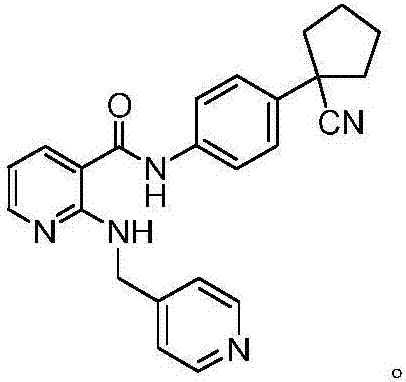
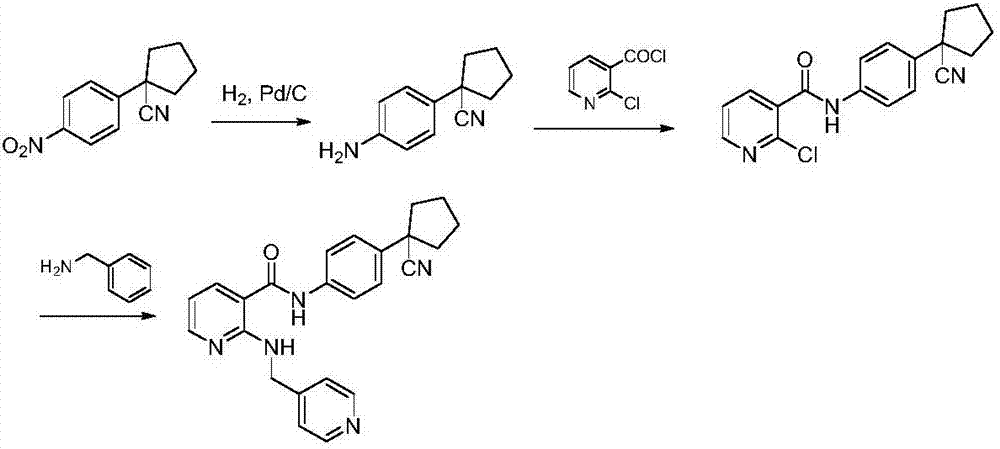
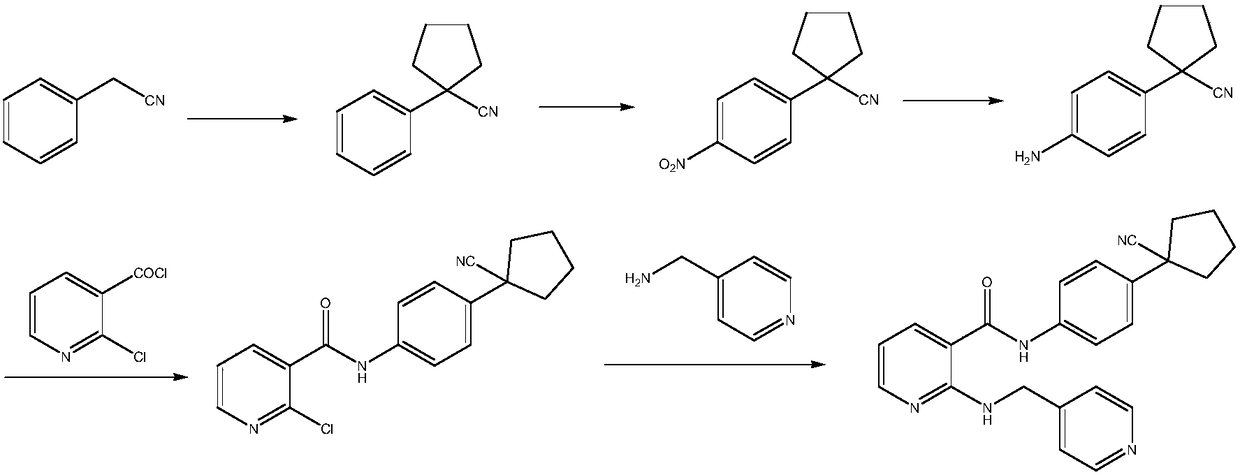
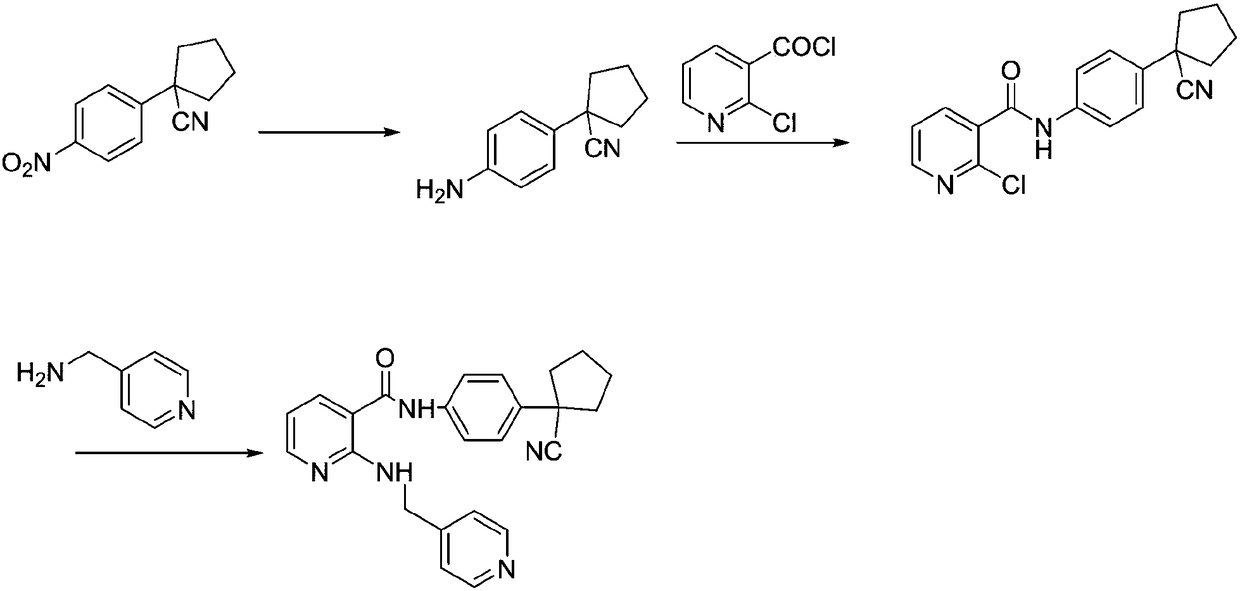
The invention provides a preparation method of apatinib. The preparation method comprises that 2-halogenated-3-cyanopyridine as a raw material and 4-aminomethylpyridine undergo a substitution reactionto produce N-(pyridin-4-yl-methyl)-2-amino-3-cyanopyridine, the N-(pyridin-4-yl-methyl)-2-amino-3-cyanopyridine undergoes an esterification reaction to produce N-(pyridin-4-yl-methyl)-2-amino-3-picolinate, and the N-(pyridin-4-yl-methyl)-2-amino-3-picolinate and 1-(4-aminophenyl)cyclopentylformonitrile undergo an amidation reaction to produce apatinib (I). The preparation method has the advantages of low cost and easy availability of raw materials, simple processes, low cost, less waste water generation, safety and environmental protection, easy realization of reaction conditions, high reactivity and selectivity, few side reactions, few apatinib impurities, high purity and high yield.
Owner:XINFA PHARMA
Preparation method and intermediate of apatinib
The invention discloses a preparation method and an intermediate of apatinib. The preparation method comprises the steps as follows: the intermediate 2-[(pyridine-4-methyl)amino]alkane nicotinate and1-(4-aminophenyl)-1-cyanocyclopentane are subjected to an amidation reaction in the presence of an alkaline substance in a reaction solvent to produce apatinib. By the means of the method, the reaction process can be milder, the operation is simple, no pollutants are produced, and more ideal yield and purity can be realized.
Owner:江苏美迪克化学品有限公司
Composition for promoting hair cell regeneration and hearing recovery and application thereof
ActiveCN111643671APromote regenerationPromote maturitySenses disorderNervous system cellsHair cell differentiationRegorafenib
The invention belongs to the field of biological medicines, and discloses a composition for promoting hair cell regeneration and hearing recovery and an application thereof. The composition comprisesa Wnt agonist and one or more of the following reagents: (a) a VEGFR inhibitor; (b) a Tgfbr inhibitor; and (c) an ERG inhibitor. The VEGFR inhibitor comprises regorafenib, apatinib mesylate, cabozantinib, pazopanib hydrochloride and medicinal salts or derivatives of the regorafenib, the apatinib mesylate, the cabozantinib and the pazopanib hydrochloride. An organoid platform verifies that after any inhibitor in a signal axis of EGFR-TGFB1-ERG is combined with the Wnt agonist to prepare the composition, efficient hair cell differentiation, maturation and survival can be realized, and the composition has important value for hearing recovery.
Owner:NANJING UNIV
Novel application of Apatinib for preparing medicine for treating acute myelogenous leukemia
InactiveCN110292578AGood biocompatibilityImprove targetingOrganic active ingredientsAntineoplastic agentsSide effectPhosphorylation
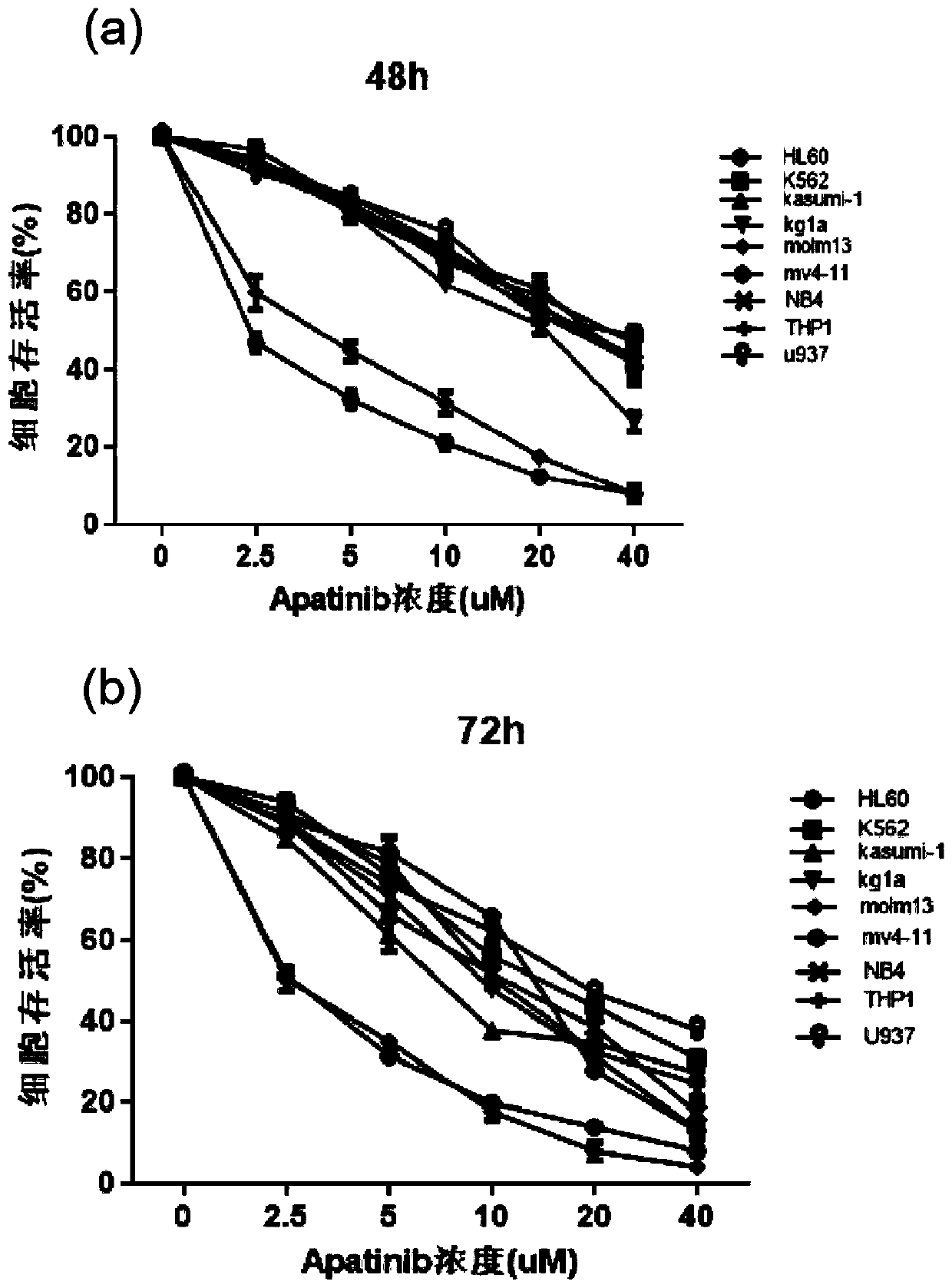
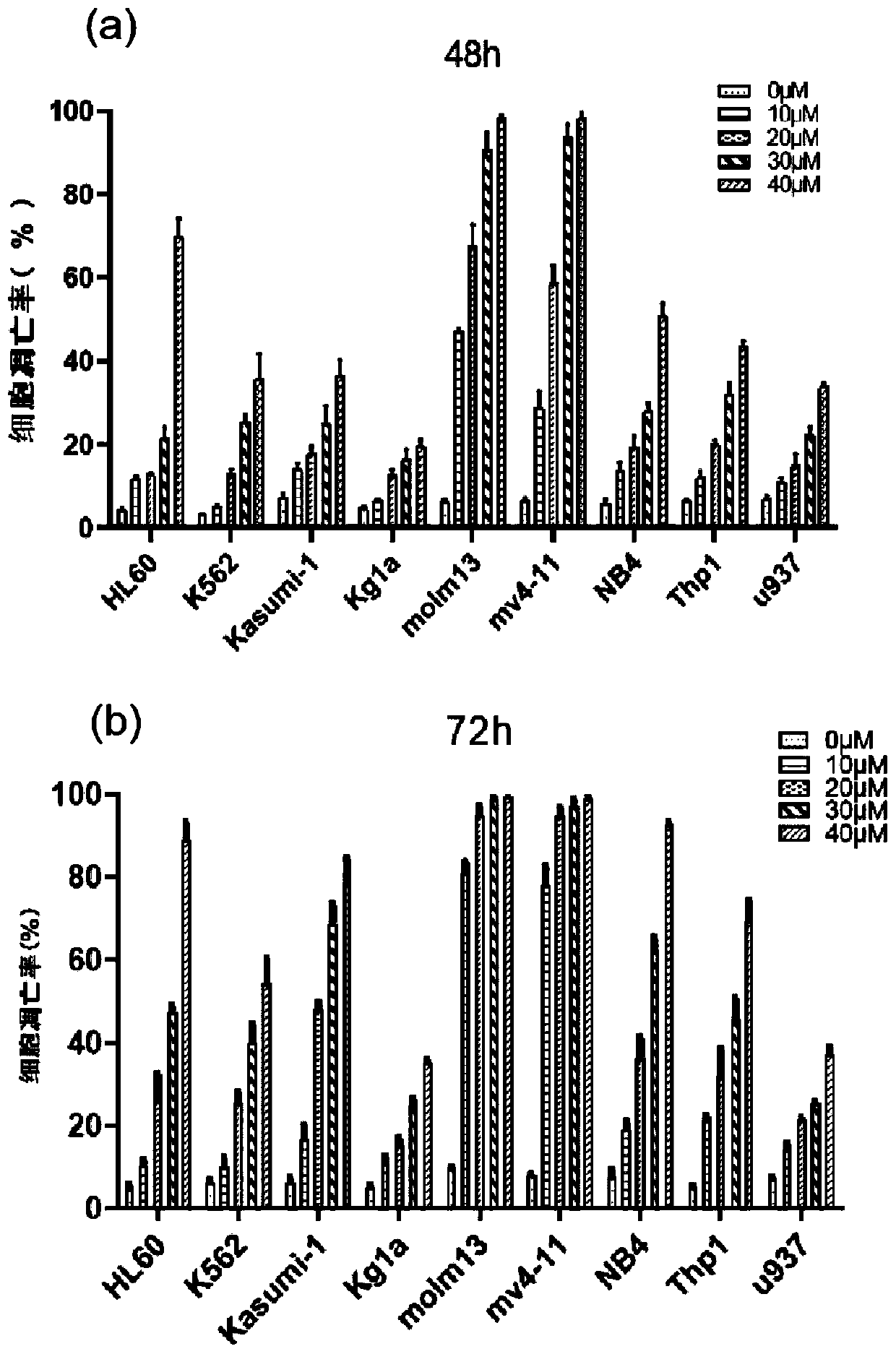
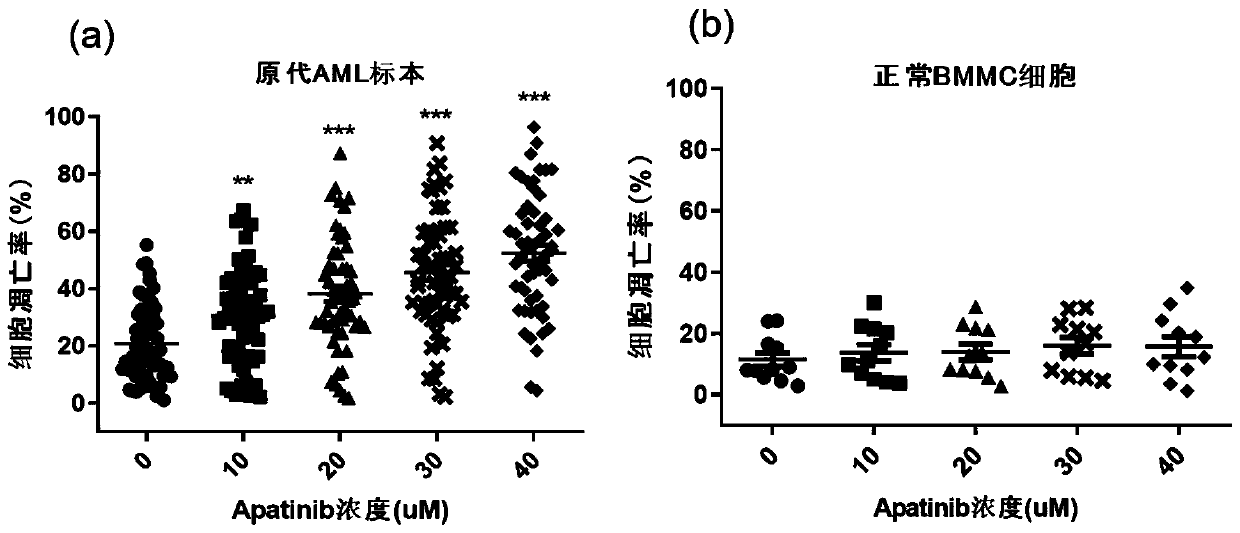
The invention relates to novel application of Apatinib or pharmaceutically acceptable salts, ester and solvent compounds thereof for preparing a medicine for treating acute myelogenous leukemia. The Apatinib restrains acute myelogenous leukemia cell amplification and induces apoptosis of cells by activating the mitochondrion-mediated endogenesis apoptosis way and lowering the VEGFR2 phosphorylation level and the PLCgama / PKC / S6 signal channel of the downstream thereof, and finally the effect of treating acute myelogenous leukemia is obtained; Apatinib or pharmaceutically acceptable salts, esterand solvent compounds thereof have no toxic or side effect on normal marrow mononuclear cells, the safety is good, the theoretical basis is provided for researching the treatment strategy for acute myelogenous leukemia, and an embedded point is supplied to preparation of the medicine for treating the acute myelogenous leukemia.
Owner:THE FIRST AFFILIATED HOSPITAL OF XIAMEN UNIV +1
Pharmaceutical composition with synergistic anti-osteosarcoma effect and application thereof
ActiveCN108186643AGrowth inhibitionGood anti-tumor effectOrganic active ingredientsAntineoplastic agentsOsteosarcoma TumorHistone demethylation
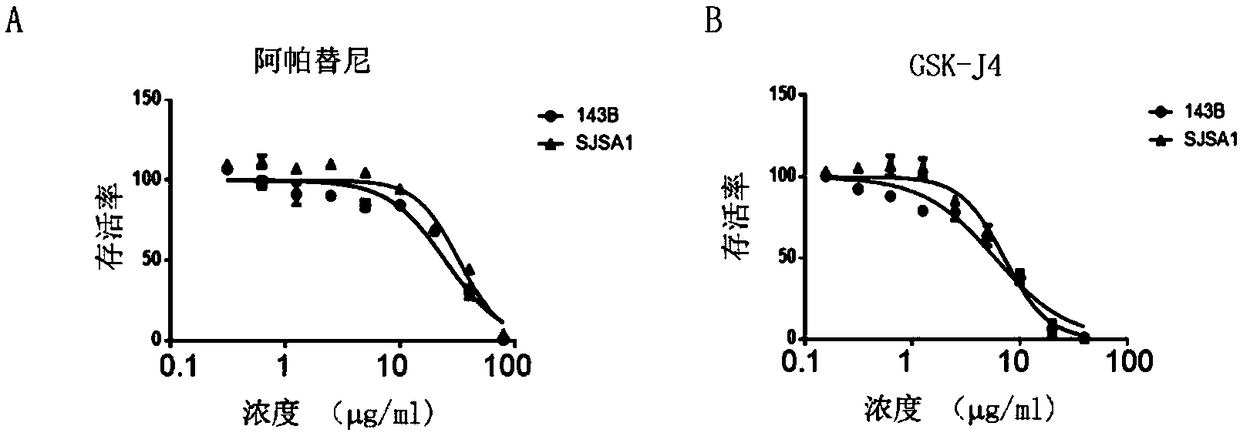
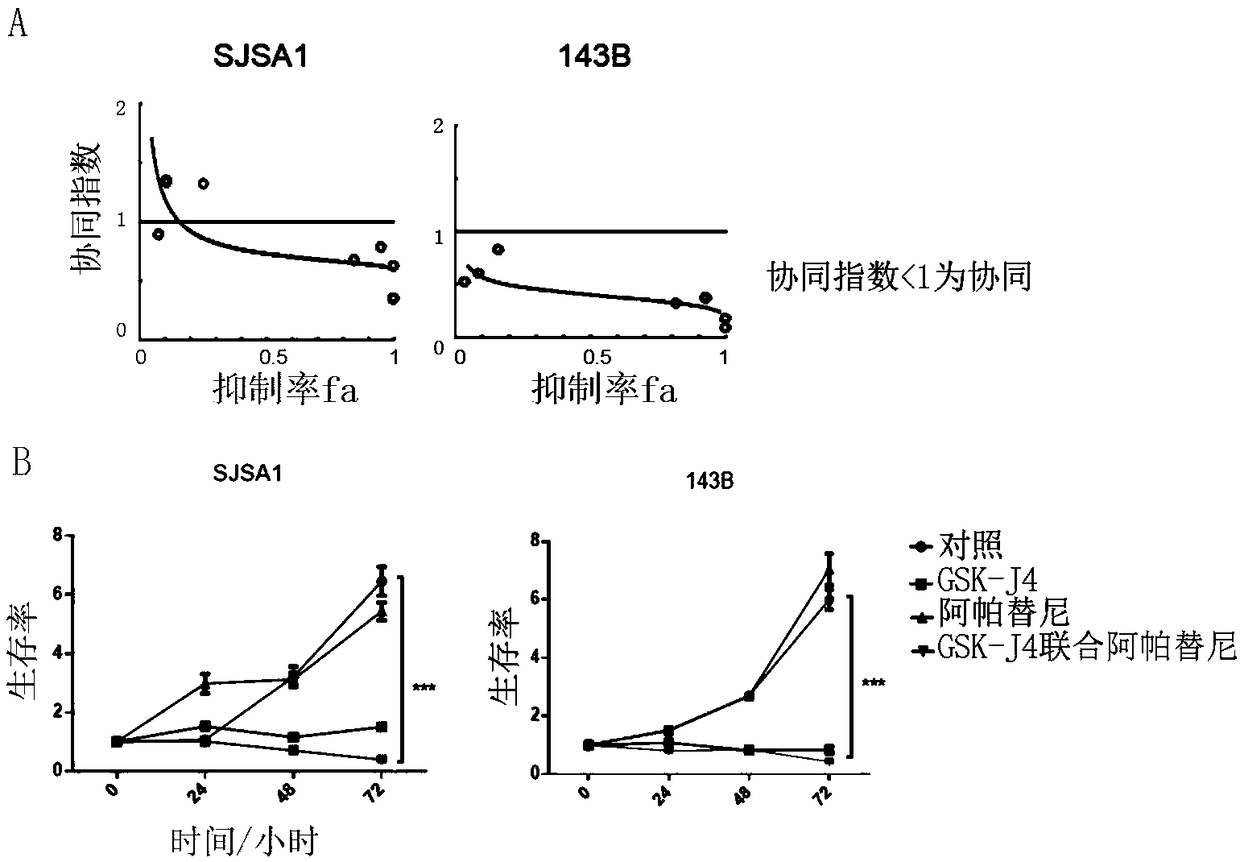
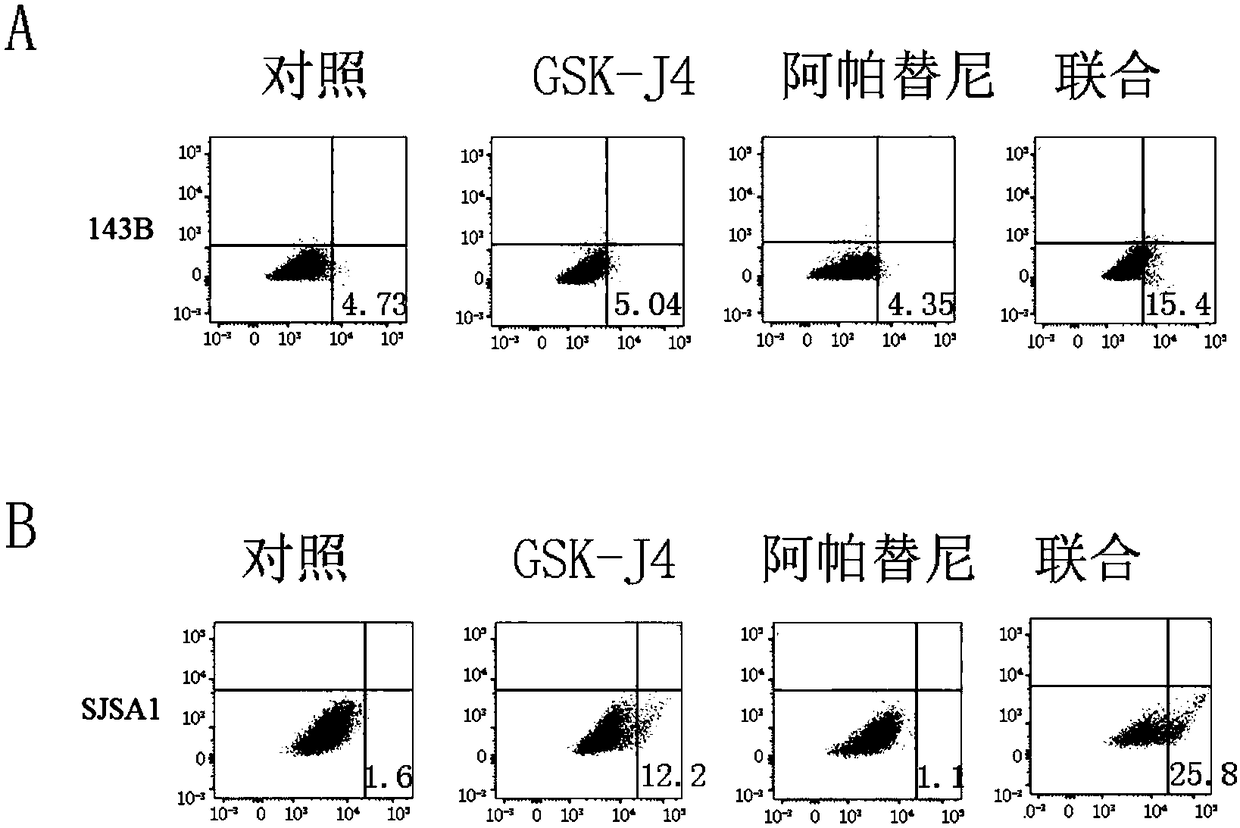
Provided are a pharmaceutical composition with a synergistic anti-osteosarcoma effect and application thereof. The invention discloses the pharmaceutical composition containing a VEGFR2 selective inhibitor Apatinib and a histone demethylase inhibitor GSK-J4 and the application thereof. Apatinib and GSK-J4 are used in combination to achieve the synergistic anti-osteosarcoma effect, the osteosarcomacell growth can be obviously inhibited, and the pharmaceutical composition is used for treatment of tumors like osteosarcoma.
Owner:徐州维康生物科技有限公司
Preparation method of apatinib
ActiveCN108409647AReduce usageLow priceOrganic active ingredientsCarboxylic acid nitrile preparationMedicinal chemistryApatinib
The invention discloses a preparation method of apatinib and belongs to the field of medicine and fine chemical industry. The preparation method is a novel preparation scheme; a target compound that is satisfactory can be acquired with any intermediate. The preparation method has the advantages the advantages of short procedure, simple reacting steps, good safety and reliability, high yield, low cost, high purity, low pollution, good operational simplicity and the like.
Owner:YANCHENG TEACHERS UNIV +1
Preparation method for apatinib
ActiveCN109879805AAvoid expensiveMild reaction conditionsOrganic chemistryFormateTert-Butyloxycarbonyl protecting group
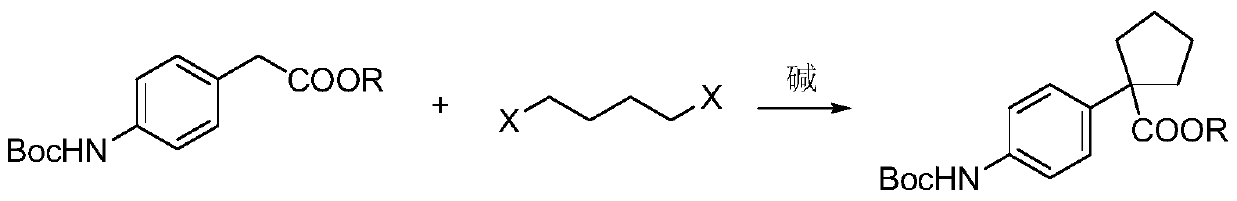
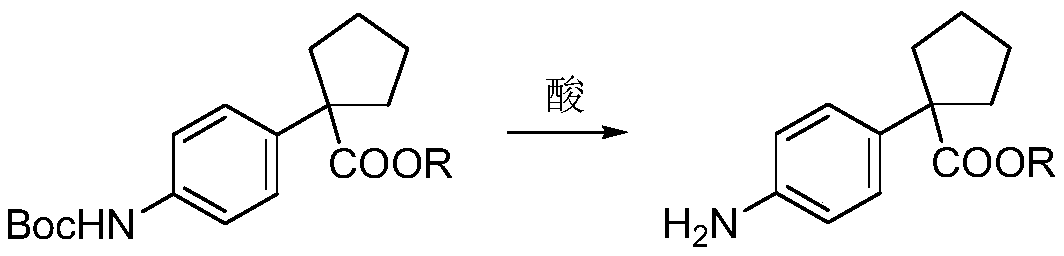
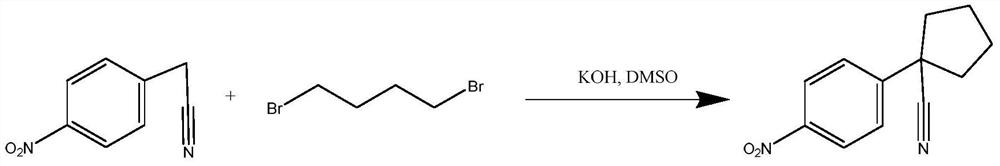
The invention relates to a preparation method for apatinib. The preparation method comprises the following steps: performing condensation reaction on 4-(tert-butoxycarbonyl group) alkyl phenylacetateand 1,4-butane dihalide, thereby acquiring 1-[4-(tert-butoxycarbonyl group) phenyl] alkyl cyclopentane formate; performing deprotection reaction on the acquired product, thereby acquiring 1-(4-aminophenyl) alkyl cyclopentane formate; performing amidation reaction on the acquired product and 2-chloronicotinoyl chloride, thereby acquiring 1-{4-[(2-chlorine pyridine-3-group) carbonyl amino] phenyl} alkyl cyclopentane formate; performing substitution reaction on the acquired product and 4-aminomethyl pyridine, thereby acquiring 1-{4-[(2-((4-pyridyl methyl) amino) pyridine-3-group) carbonyl amino]phenyl} alkyl cyclopentane formate; performing amidation reaction on the acquired product, thereby acquiring 1-{4-[(2-((4-pyridyl methyl) amino) pyridine-3-group) carbonyl amino] phenyl} cyclopentaneformamide; lastly, dehydrating, thereby acquiring an end product.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Method for determining apatinib drug concentration in human plasma
The invention relates to the technical field of clinical plasma concentration detection for anti-tumor drugs and especially relates to a method for determining apatinib drug concentration in human plasma. HPLC-MS / MS method is adopted for determining apatinib drug concentration in human plasma and comprises the steps of pretreatment of a blood sample and HPLC-MS / MS (High Performance Liquid Chromatography-Mass Spectrum / Mass Spectrum) analysis. An internal standard method is adopted for determining the apatinib drug concentration in the to-be-determined blood sample. In the invention, the internal standard method is combined with the HPLC-MS / MS method, so that the accuracy of the determining result is improved, the system error is eliminated, the analysis time is shortened, the detection process is simple, convenient and quick, the detection for the apatinib drug concentration in patient body in the clinic treatment is facilitated, and an experimental basis is supplied for the individualadministration of the apatinib and the reduction of toxic and side effects.
Owner:沈阳和合医学检验所有限公司
Pharmaceutical composition containing sunitinib as well as preparation and application of pharmaceutical composition
ActiveCN111558044ADelay drug resistancePrevent proliferationOrganic active ingredientsAntineoplastic agentsCancer cellSide effect
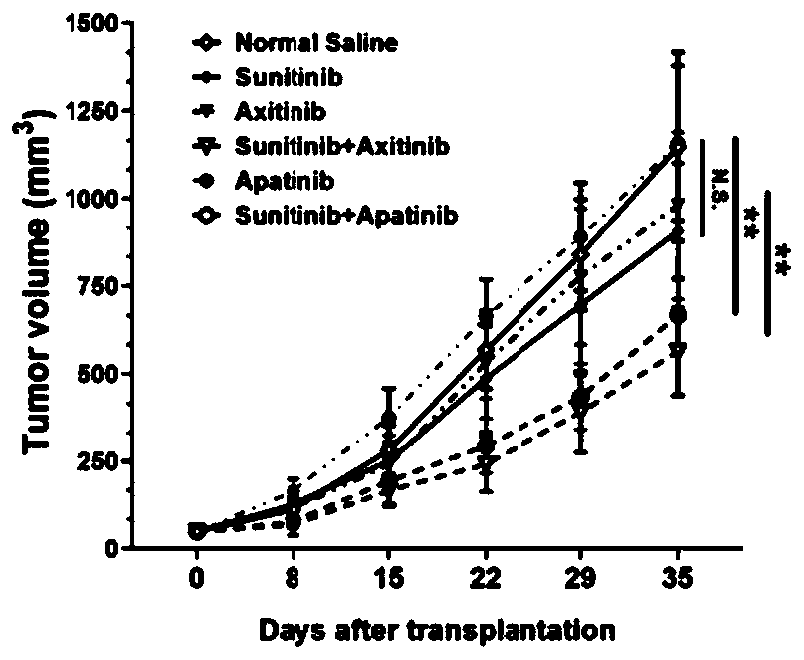
The invention provides a pharmaceutical composition containing sunitinib as well as a preparation and an application of the pharmaceutical composition. On the basis of the original sunitinib, at leastone tyrosine kinase receptor inhibitor, especially apatinib and / or axitinib, is further added to generate a very significant synergistic effect with the sunitinib, so that the proliferation of cancercells is obviously inhibited, and the sunitinib has very significant killing efficiency on the cancer cells. The pharmaceutical composition provided by the invention can obviously improve the problemof serious drug resistance caused by singly adopting sunitinib in the prior art and the problem of obviously reduced treatment effect when the conventional use amount of sunitinib is reduced; clinical treatment efficiency is greatly improved, treatment toxic and side effects of a patient are reduced, a new scheme is provided for clinical treatment, and the traditional Chinese medicine compositionhas very wide market prospects and extremely important social significance.
Owner:SUN YAT SEN UNIV CANCER CENT
Apatinib polymeric micelle and preparation method thereof
InactiveCN108354902AGood water solubilityGood biocompatibilityOrganic active ingredientsPharmaceutical non-active ingredientsOrganic solventSide effect
The invention discloses apatinib polymeric micelle which is prepared from apatinib as a main medicine and an amphiphilic polymer material as a carrier. A preparation method comprises the following steps: 1) dissolving apatinib and a polymer into an organic solvent so as to obtain a co-solution; 2) dropping the co-solution into stirred water, and stirring at the same time; 3) evaporating off the organic solvent; 4) filtering off medicines which are not packaged and can be separated out by using a filtering membrane, thereby obtaining the apatinib polymeric micelle. The apatinib polymeric micelle disclosed by the invention is low in side effect, high in biocompatibility and simple, convenient and feasible in preparation process.
Owner:杭州市肿瘤医院
Crystal form A of apatinib, and preparation method and application thereof
InactiveCN108250138AExcellent high humidity stabilityOrganic active ingredientsOrganic chemistry methodsHigh humidityApatinib
The invention provides a crystal form A of apatinib, and a preparation method and application thereof. The crystal form A is as shown in a formula (I) which is described in the specification. In an XRPD pattern of the crystal form A of apatinib, a diffraction peak occurs when the angle 2theta is equal to 11.69, 16.13, 17.13, 17.82, 20.74, 21.81, 23.51, 23.87 or 27.01 degrees, and the error range of the value of the 2theta is + / -0.2 degree. The crystal form A of apatinib provided by the invention has good high-temperature stability and high-humidity stability.
Owner:SHANGHAI SUNTRONG BIOTECH
Preparation method of nano-drug and application of nano-drug in treatment of osteosarcoma
ActiveCN110922587AEasy to synthesizeQuick Restore ResponsivenessOrganic active ingredientsAntineoplastic agentsApoptosisPharmaceutical drug
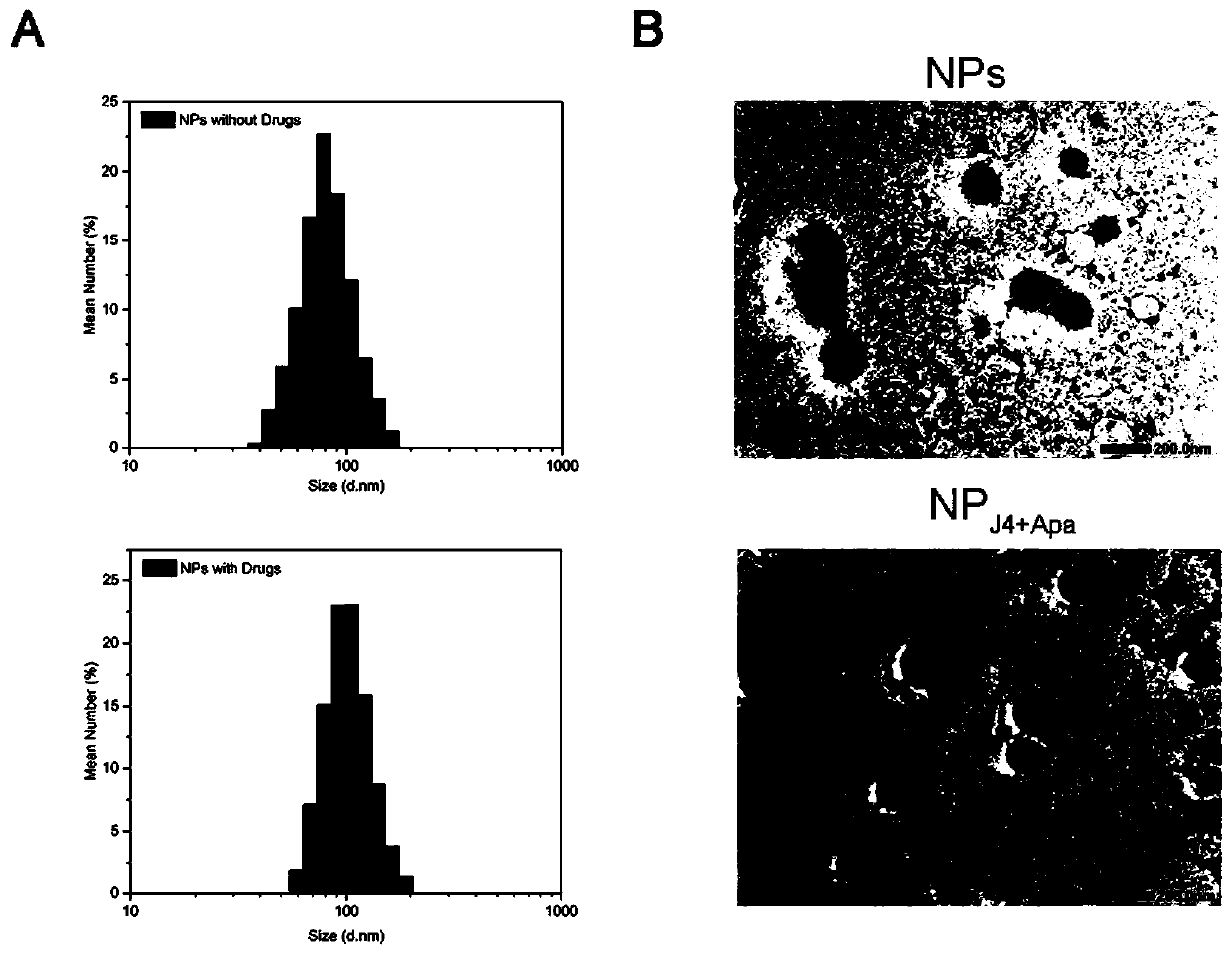
The invention discloses a preparation method of a nano-drug and application of the nano-drug in treatment of osteosarcoma. A Cys-8E material synthesized in the invention is good in biocompatibility and strong in drug entrapment capacity. After Apatinib and GSK-J4 are entrapped by a nano-carrier to prepare a nano-drug (NPJ4+Apa), then the nano-drug (NPJ4+Apa) can target a tumor site of osteosarcoma, and has the better drug delivery capability for osteosarcoma stem cells which cannot be acted by a traditional drug. The nano-drug NPJ4+Apa can induce apoptosis of the osteosarcoma stem cells, and the treatment effect of Apatinib and GSK-J4 is remarkably improved. Meanwhile, the nano-drug NPJ4+Apa can prevent Apatinib and GSK-J4 from acting on normal cells, so that the toxic and side effects ofApatinib and GSK-J4 are reduced. The nano-drug NPJ4+Apa has the advantages of targeting the osteosarcoma stem cells, being small in side effect and the like, and has a good application prospect and awide development space in the clinical field of osteosarcoma.
Owner:SUN YAT SEN UNIV
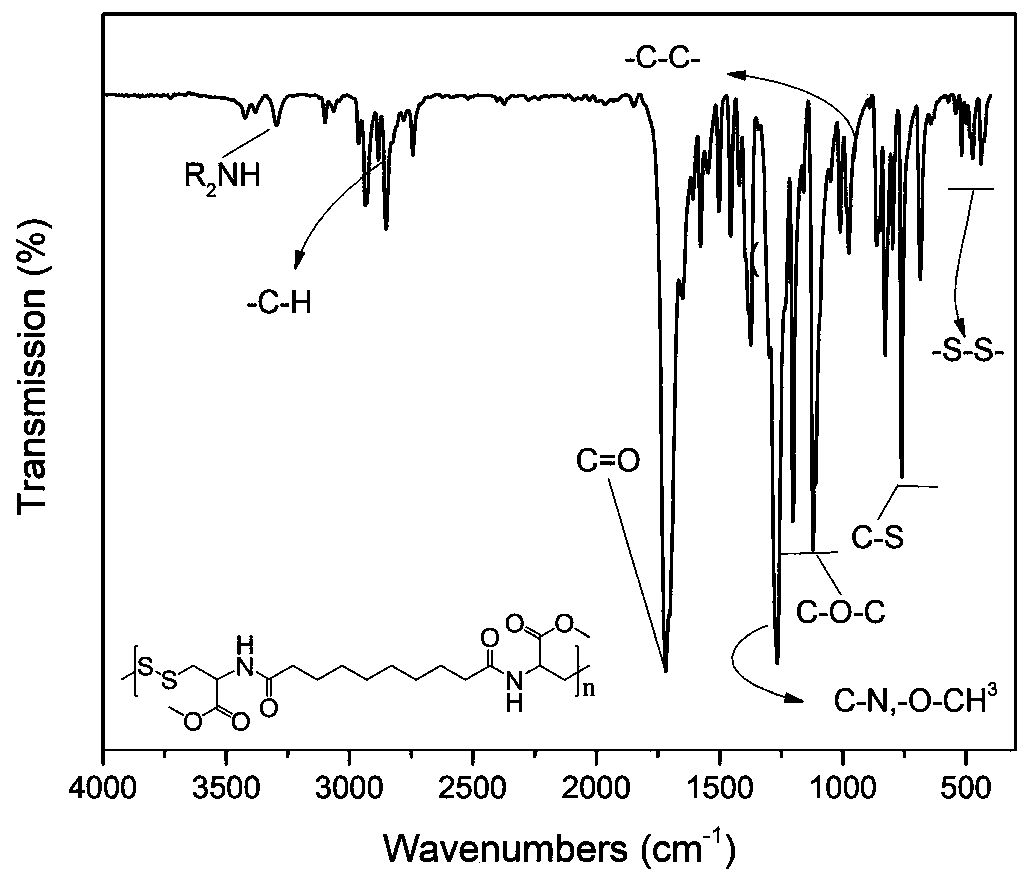
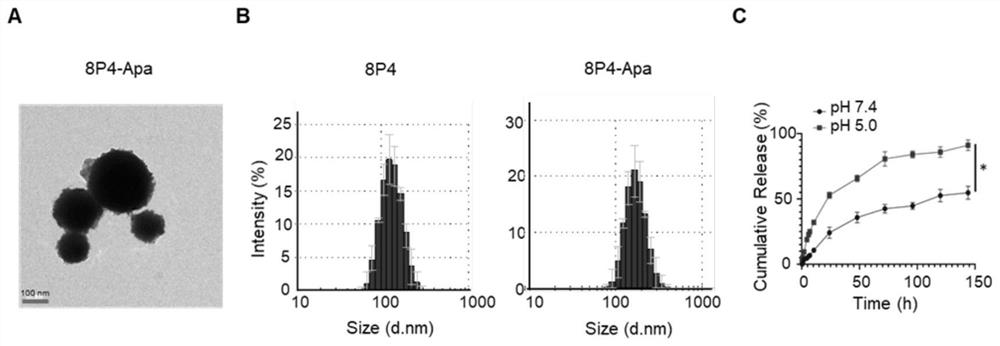
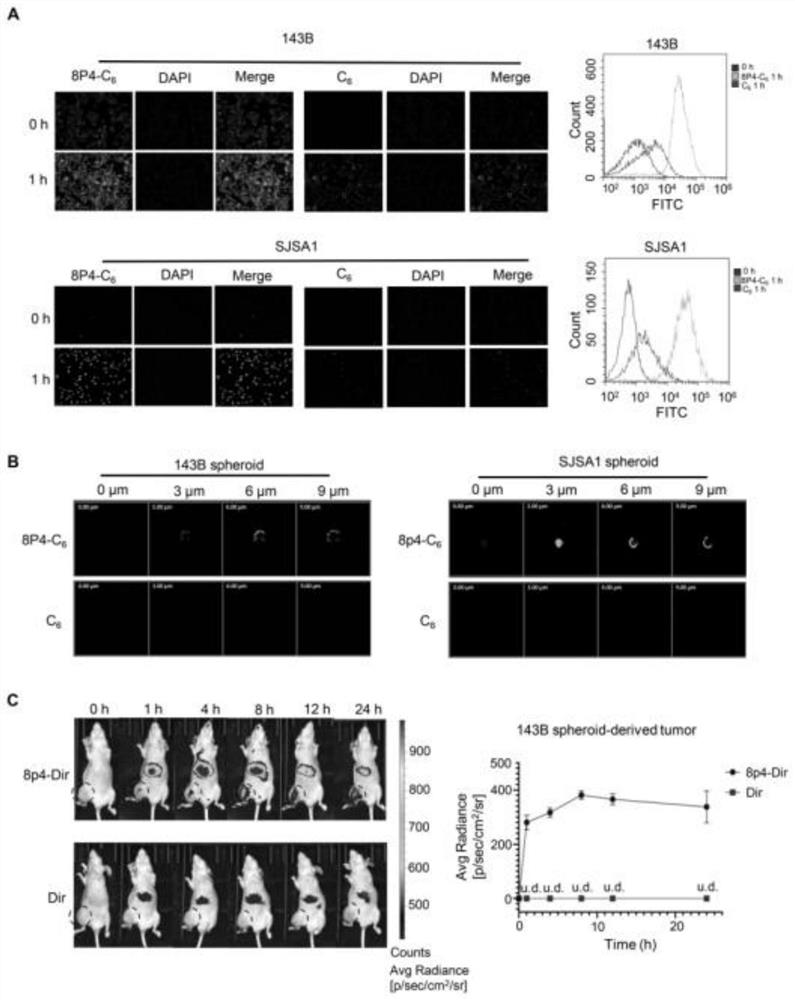
Novel polyester compound, nano-drug taking novel polyester compound as carrier and application of nano-drug
ActiveCN111763315AFacilitated releaseEasily brokenPowder deliveryOrganic active ingredientsPolyesterPharmacology
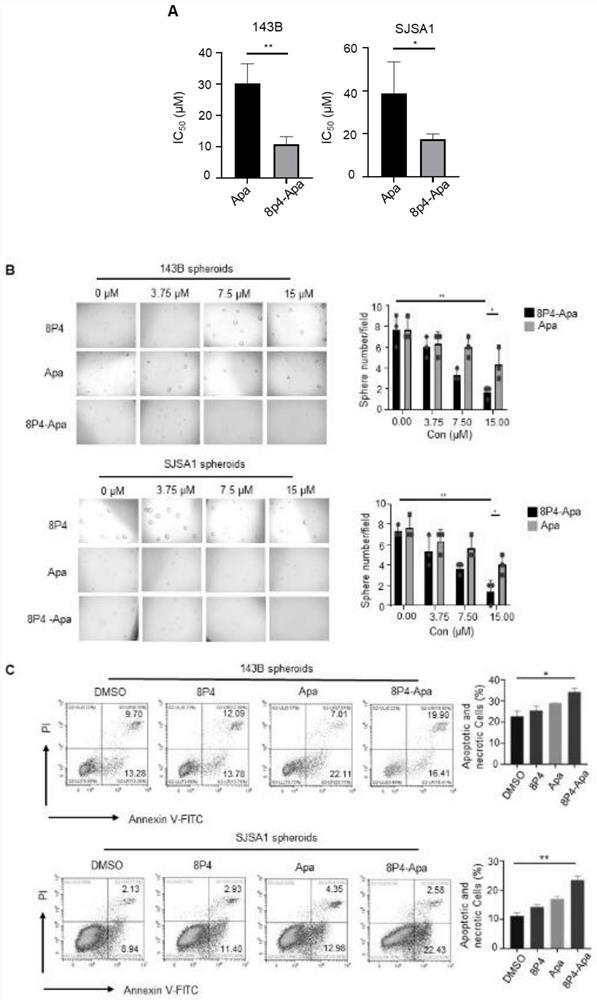
The invention discloses a novel polyester compound, a nano-drug taking the novel polyester compound as a carrier, and application of the nano-drug. According to the novel polyester compound, the nano-drug taking novel polyester compound as the carrier and the application of the nano-drug, a nano material 8P4 encapsulates a compound Apatinib, so that the nano-drug (8P4-Apa) can be prepared, and then the nano-drug (8P4-Apa) can target a tumor site of osteosarcoma; in addition, the nano-drug has good drug delivery capability on the stem cells of the osteosarcoma on which traditional drugs just exert poor effects; the treatment effect of the compound Apatinib is remarkably improved; the apoptosis of the stem cells of the osteosarcoma is induced; the nano material does not have obvious cytotoxicity under the application concentration; and meanwhile the toxic and side effects of the Apatinib on normal cells can be reduced through a nano drug delivery system. The nano-drug 8P4-Apa has the advantages of capability of targeting the osteosarcoma, small side effect and the like, and has a good application prospect and a wide development space in the field of clinical treatment of the osteosarcoma.
Owner:CHANGZHI MEDICAL COLLEGE
Kit for evaluating treatment sensitivity and/or drug resistance of apatinib
ActiveCN114395627ASimple and fast operationShort detection timeMicrobiological testing/measurementAgainst vector-borne diseasesOncologyPersonalized therapy
The invention discloses a kit for evaluating treatment sensitivity and / or drug resistance of apatinib, and provides a kit which can help a clinician to determine whether a colorectal cancer patient is suitable for fluorouracil drug treatment or not according to the detection condition of PEX3 gene mRNA in tumor tissue of the colorectal cancer patient. The kit provides a basis for formulating a personalized treatment scheme, is simple and convenient to operate, short in detection time, capable of realizing high-throughput detection and low in price, is mainly applied to detection of the PEX3 gene, and can be used for guiding clinicians to take apatinib for colorectal cancer patients.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Medicine for treating advanced refractory solid tumors with TP53 mutation and application
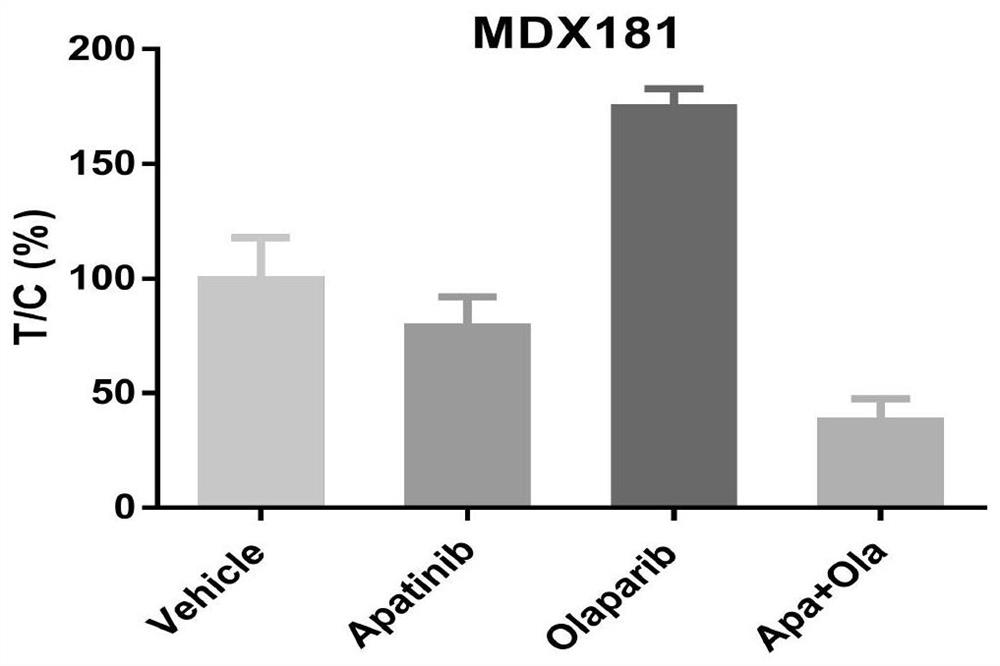
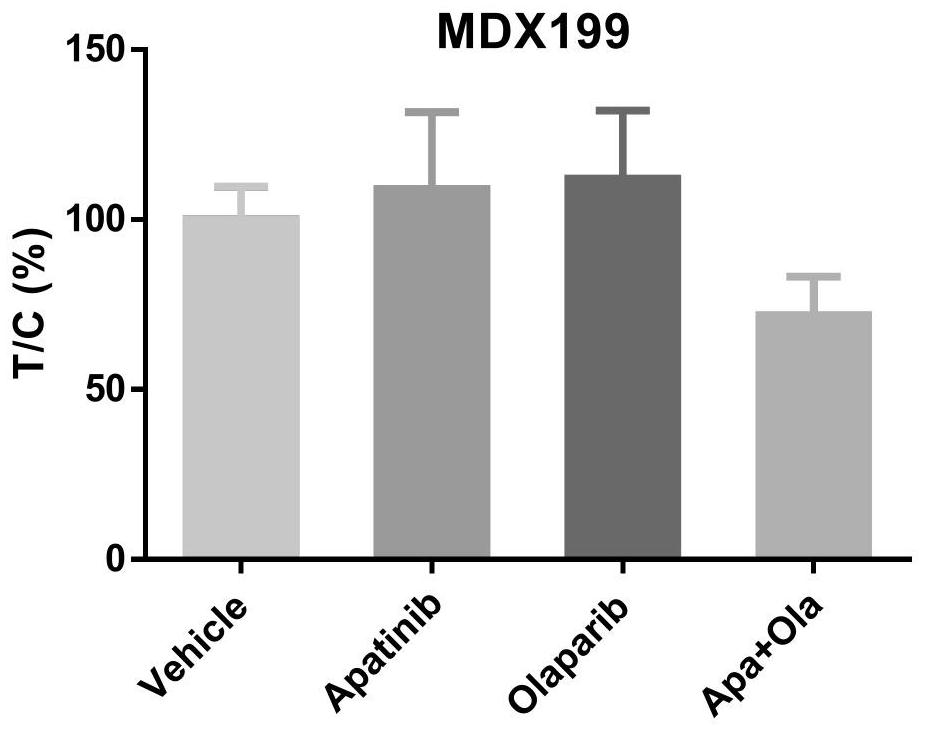
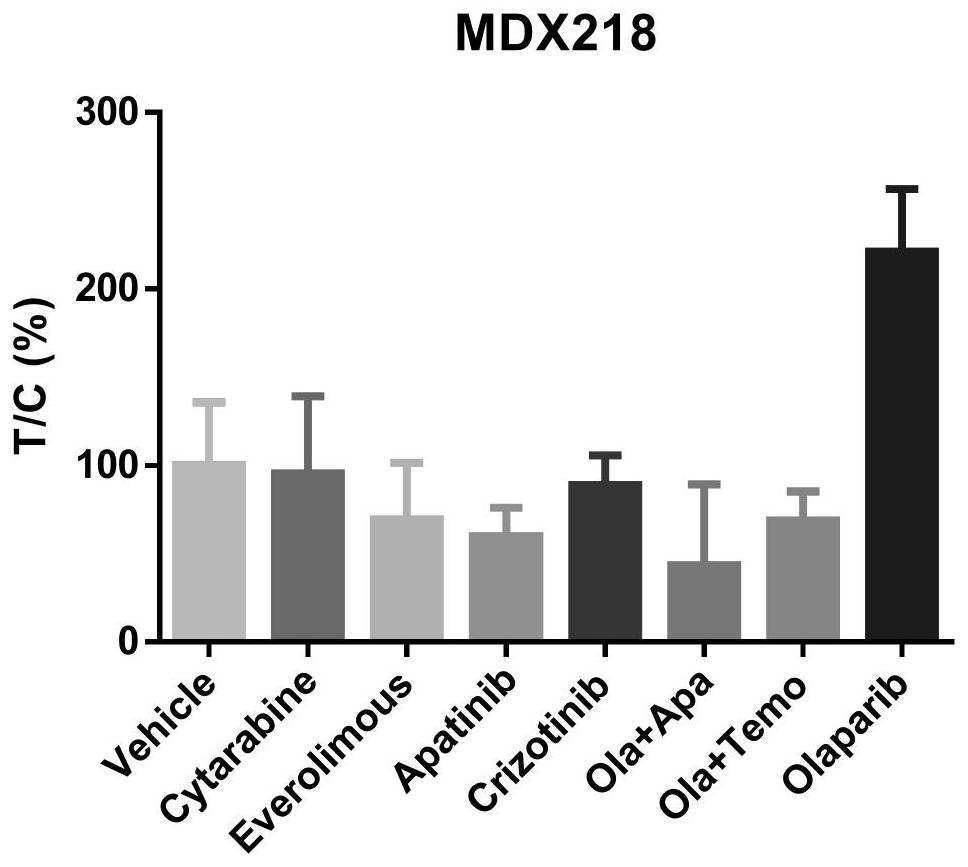
PendingCN112316149AGood treatment effectThe curative effect is miraculousOrganic active ingredientsAntineoplastic agentsRAD51Therapeutic effect
The invention belongs to the technical field of precision medicine, and discloses a medicine for treating advanced refractory solid tumors with TP53 mutation and application. The medicine for treatingthe advanced refractory solid tumors with TP53 mutation comprises apatinib and olaparib. AN anti-angiogenesis medicine is combined with a PARP inhibitor to treat the advanced solid tumors with TP53 mutation, and magical curative effects are achieved. The anti-angiogenesis medicine combined with the PARP inhibitor shows a certain anti-cancer curative effect in preclinical research. The anti-angiogenesis medicine can down-regulate homologous recombination repair related genes such as BRCA and RAD51, can make cells in a hypoxic state, and can enhance the treatment effect of the PARP inhibitor.
Owner:王海涛
Preparation method of apatinib intermediate
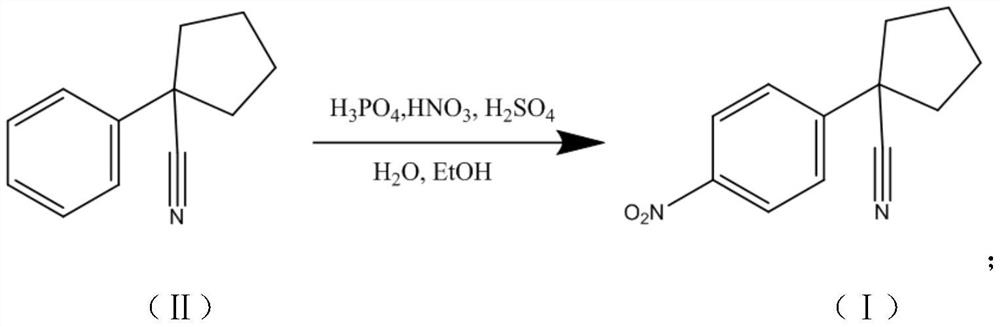
InactiveCN111763156AReduce usageRelieve pressureOrganic compound preparationCarboxylic acid nitrile purification/separationChemical synthesisO-Phosphoric Acid
A preparation method of an apatinib intermediate belongs to the technical field of chemical synthesis of medicines. The preparation method comprises the following steps: nitration reaction: dropwise adding 1-phenyl-1-cyclohexonitrile as a compound II into a mixed acid of phosphoric acid, nitric acid and sulfuric acid, and stirring for reaction to obtain an apatinib intermediate primary product asa compound I; and refining: refining the obtained apatinib intermediate primary product by using a refining solvent to obtain an apatinib intermediate finished product serving as a compound I. The usage amount of nitric acid and concentrated sulfuric acid is remarkably reduced, and the pressure on the environment influence is reduced; the purity is high; the preparation cost is reduced; the stepsare simple and reasonable, the operation is convenient, and tedious post-treatment is avoided; and the preparation method is beneficial for obtaining raw material medicines with higher quality.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Pharmaceutical combination
PendingCN113491769AReduce anemiaReduced plateletsOrganic active ingredientsAntibody ingredientsDiseaseAntiendomysial antibodies
The disclosure relates to pharmaceutical combinations. Specifically, the invention provides application of a PD-1 antibody combined with paclitaxel and a VEGFR inhibitor such as apatinib in preparation of drugs for treating triple negative breast cancer, the combined treatment scheme shows better disease control rate and objective response rate, embodies a synergistic effect of combined medication, and significantly improves the curative effect of immunotherapy.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Crystal form B of apatinib, and preparation method and application thereof
The invention provides a crystal form B of apatinib, and a preparation method and application thereof. The crystal form B is as shown in a formula (I) which is described in the specification. In an XRPD pattern of the crystal form B of apatinib, a diffraction peak occurs when the angle 2theta is equal to 6.17, 10.66, 12.35, 14.62, 18.56, 20.21, 20.84 or 21.21 degrees, and the error range of the value of the 2theta is + / -0.2 degree. The crystal form B of apatinib provided by the invention has good high-temperature stability and high-humidity stability.
Owner:SHANGHAI SUNTRONG BIOTECH
Application of apatinib to preparing BRAF V600E protein kinase inhibitors
The invention relates to the technical field of medicines, in particular to application of apatinib to preparing BRAF V600E protein kinase inhibitors and application of the apatinib to preparing medicines for treating BRAF V600E mutation tumor. Melanoma, thyroid papillary carcinoma, borderline ovarian tumor, colorectal cancer, non-small cell lung cancer and hairy cell leukemia can be treated by the medicines. The application has the advantages that novel targets and indication can be provided for the apatinib; novel thinking can be provided for treating tumor such as BRAF V600E mutation melanoma, colorectal cancer and non-small cell lung cancer.
Owner:SECOND AFFILIATED HOSPITAL SECOND MILITARY MEDICAL UNIV
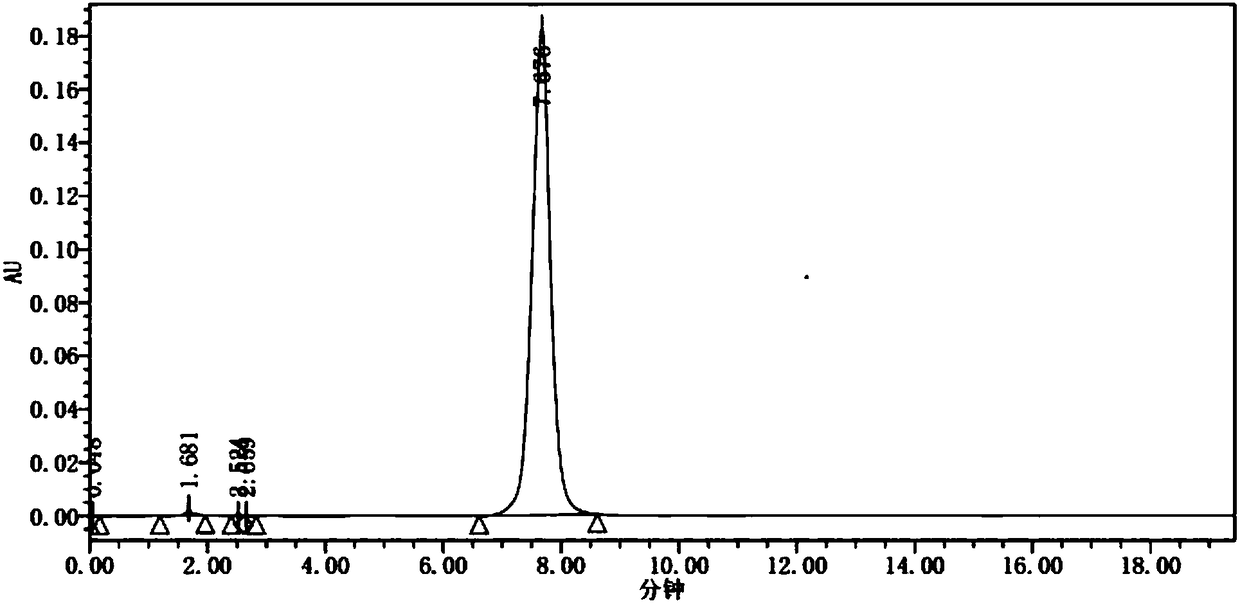
New crystal of apatinib sulfate
The invention discloses a new crystal of apatinib sulfate; the new crystal has an XRPD pattern having diffraction peaks at 2 theta values approximately: 3.681, 7.319, 10.96, 14.62, 15.54, 16.8, 17.62, 18.3, 18.699, 19.48, 20.74, 21.1, 21.58, 22.5, 23.159, 24.16, 24.7, 25.38, 26.779, 28.079, 28.5, 29.06, 29.979, 31.181, 33.28, and 35.42, wherein a 2 theta value error range is 0.2. The new crystal has the advantages that the obtained crystal A of apatinib sulfate has the stability superior to that of an amorphous form, has good solubility, and is conducive to preservation.
Owner:华东理工常熟研究院有限公司
Combined pharmaceutical composition for treating acute myelogenous leukemia and application thereof
ActiveCN113577070AInduce apoptosisEnhanced inhibitory effectOrganic active ingredientsAntineoplastic agentsPharmaceutical drugInducer Cells
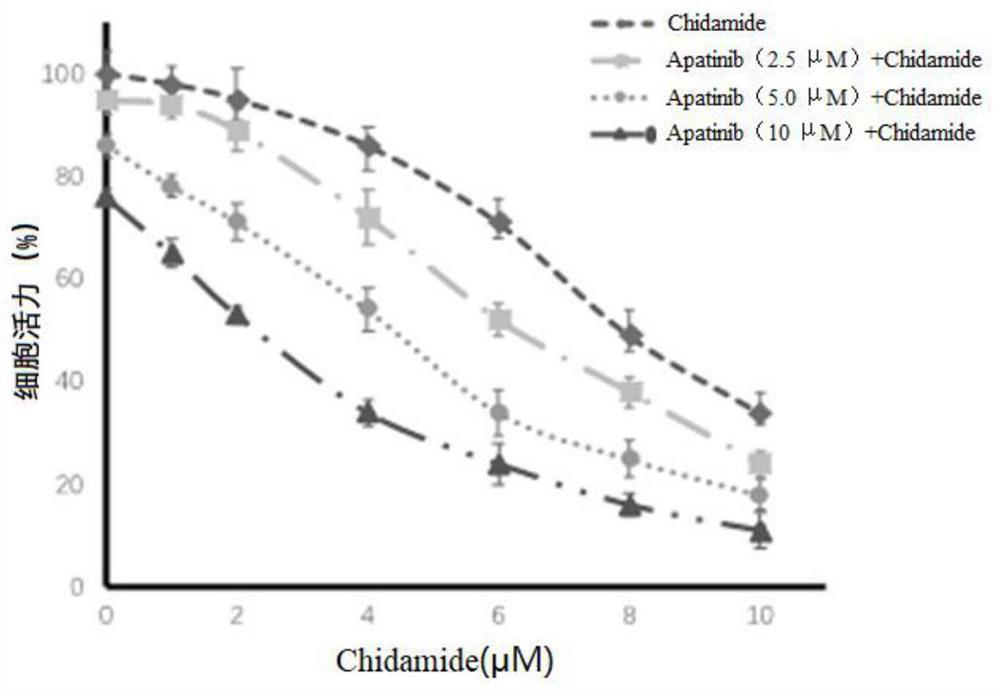
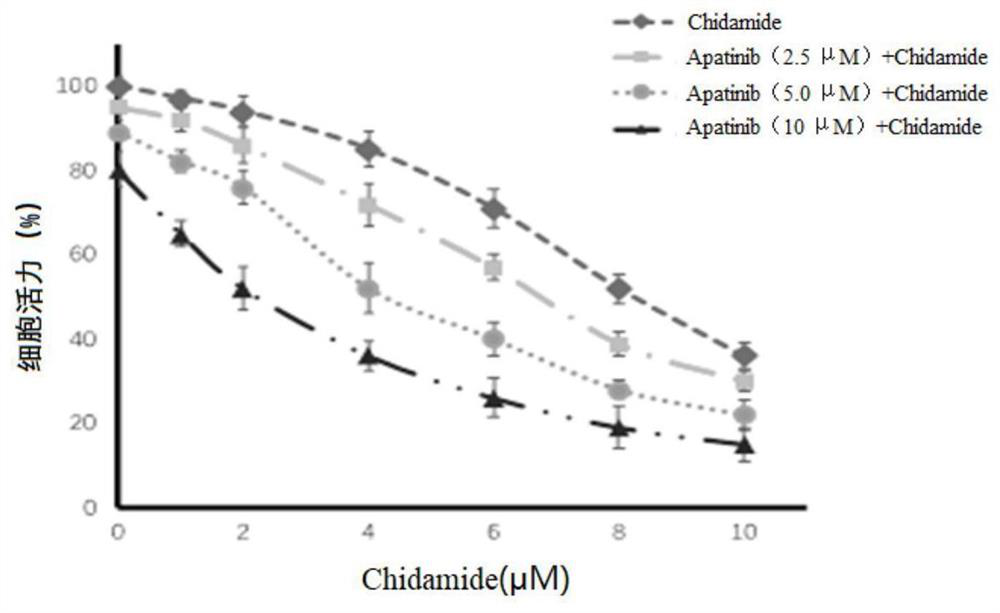
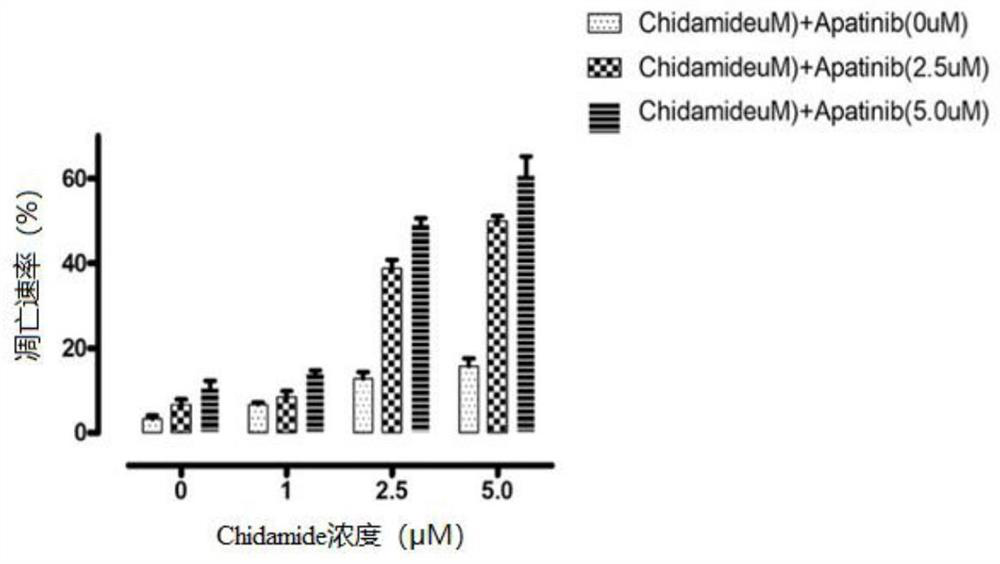
The invention provides a combined pharmaceutical composition for treating acute myelogenous leukemia and application of the combined pharmaceutical composition. The combined pharmaceutical composition for treating acute myelogenous leukemia comprises chidamide and apatinib. According to the combined medicine composition, the medicine chidamide and the medicine apatinib are creatively combined to serve as a medicine for treating acute myelogenous leukemia, and the research shows that the combined chidamide and apatinib have the effect of more remarkably inhibiting AML cell proliferation compared with single chidamide or apatinib; has the effect of more remarkably inducing AML cell apoptos compared with single chidamide or apatinib, and has the effect of more remarkably prolonging the survival time of an AML mouse model compared with single chidamide or apatinib. The combined pharmaceutical composition provides a new strategy and thought for the treatment of acute myelogenous leukemia, and has very significant significance.
Owner:THE FIRST AFFILIATED HOSPITAL OF XIAMEN UNIV +1
Preparation method and application of apatinib-coated chitosan sodium alginate microsphere
InactiveCN110063946APromote absorptionTargeted therapy for colorectal cancerOrganic active ingredientsPharmaceutical non-active ingredientsSide effectFreeze-drying
The invention discloses a preparation method and application of apatinib-coated chitosan sodium alginate microsphere. The method comprises the following steps of: 1, uniformly mixing 10mg / mL of apatinib solution and 0.0315% in mass concentration of alginate solution according to a volume ratio of 1: 23.5 to obtain a mixed solution, 2, dripping a calcium chloride solution with a mass concentrationof 0.2% into the mixed solution A prepared in step 1 at a speed of 0.1ml / min, and stirring to obtain a mixed solution B; wherein the volume ratio of the mixed solution A to the calcium chloride solution is 24.5: 1.5; 3, dripping a chitosan solution with a mass concentration of 0.07% into the mixed solution B at a speed of 0.1ml / min, fully reacting, centrifuging, washing, and performing vacuum freeze drying to obtain the apatinib-coated chitosan sodium alginate microsphere; the volume ratio of the mixed solution B to the chitosan solution is 13: 1; the microsphere can promote the absorption ofmedicines in intestinal tracts, can improve the absorption and utilization rate of the medicines, can reduce the oral intake of the medicines and reduce the side effect caused by the medicines.
Owner:SOUTHWEST JIAOTONG UNIV
Method and kit for determining effectiveness of targeted drug apatinib
PendingCN112280863AValidity judgmentPredict or assist in predicting the risk of drug efficacyMicrobiological testing/measurementProteomicsFOLFOX RegimenEfficacy
The invention discloses a method and a kit for determining effectiveness of a targeted drug apatinib. The method comprises the following steps: step 1, preparing a drug effect sample of the targeted drug apatinib; 2, carrying out DNA molecular marking on the drug effect sample obtained in the step 1; 3, establishing a multiple linear regression mathematical model; 4, calculating the correlation coefficient of the effective variable of the drug effect of the targeted drug apatinib; and step 5, determining the effectiveness of the targeted drug apatinib according to the correlation coefficient of the effective variable of the drug effect. According to the method and the kit for determining the effectiveness of the targeted drug apatinib, provided by the invention, the efficacy effectivenessof the targeted drug apatinib is researched from a gene level in a DNA molecular marking manner, so that the efficacy degree of a drug scheme is associated with the expression of a gene, and the efficacy effectiveness of the targeted drug apatinib can be better determined; and predicting or assisting in predicting of the risk of the efficacy of the chemotherapy FOLFOX regimen is carried out.
Owner:南京普恩瑞生物科技有限公司
Application of combination of clostridium ghonii and tumor angiogenesis inhibitor
PendingCN113855710AReduce TGFβ amountImprove the immune microenvironmentBacterial antigen ingredientsMicroorganismsTumor reductionOncology
The invention relates to an application of a combination of clostridium ghonii and a tumor angiogenesis inhibitor in cancer treatment. It is found for the first time that the combination of clostridium ghonii and the low-dose tumor angiogenesis inhibitor reduces infiltration of M2-like macrophages, MDSC and the like in tumors, reduces the number of TGF beta in the tumors and reduces the inhibition effect of anti-tumor immune response in TME. Meanwhile, by combining with the low-dose tumor angiogenesis inhibitor, immune cells such as CD8 < + >, CD3 < + > T and F4 / 80 < + > can be promoted to infiltrate into tumors, the immune microenvironment in the tumors is improved, and the anti-tumor curative effect is enhanced. The application can be applied to advanced malignant solid tumor types, the TME is converted into an immune activation state from an immunosuppression state by combining the clostridium ghonii with low-dose apatinib to reduce the anti-tumor immune response inhibition effect in the TME, and efficient anti-tumor is achieved.
Owner:SHANDONG XINCHUANG BIOLOGICAL TECH CO LTD
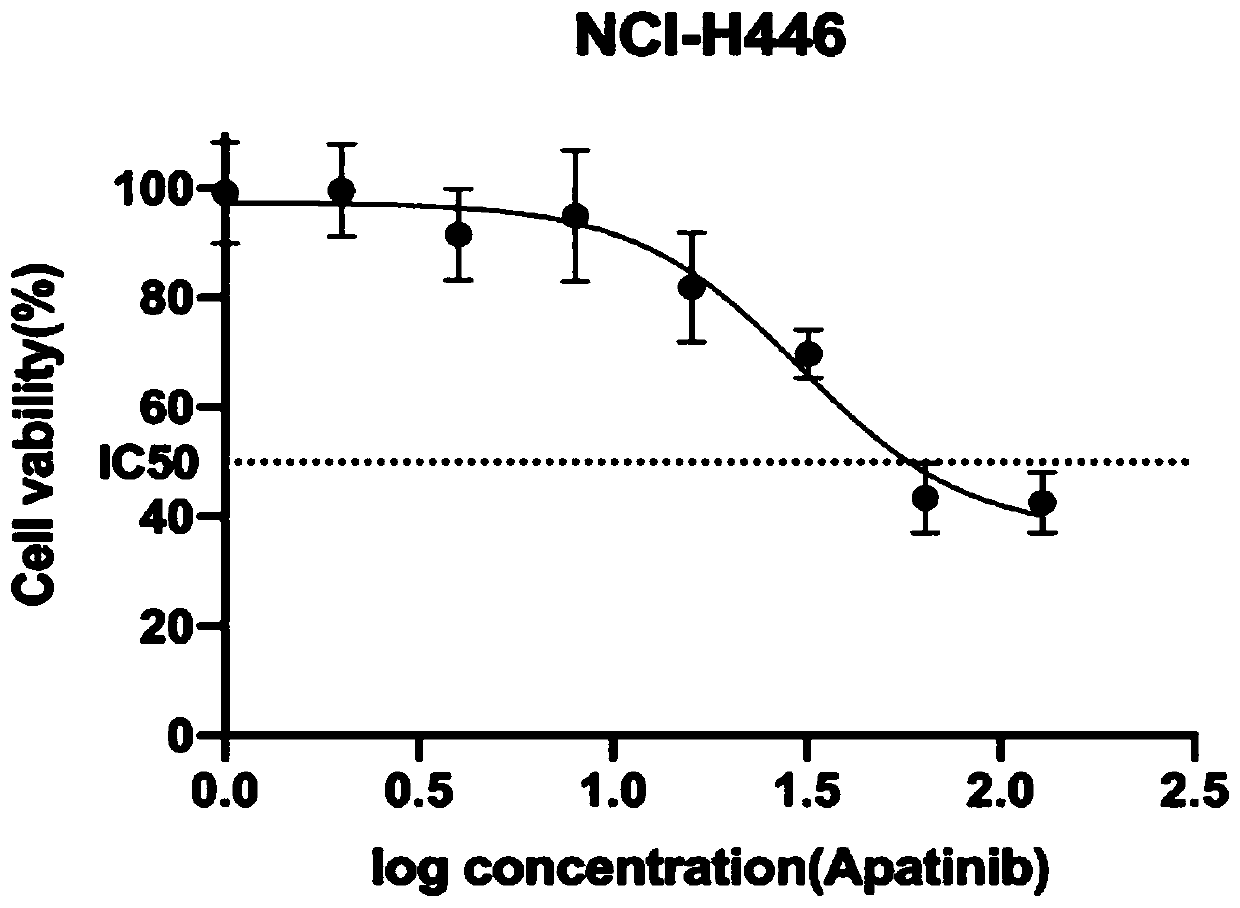
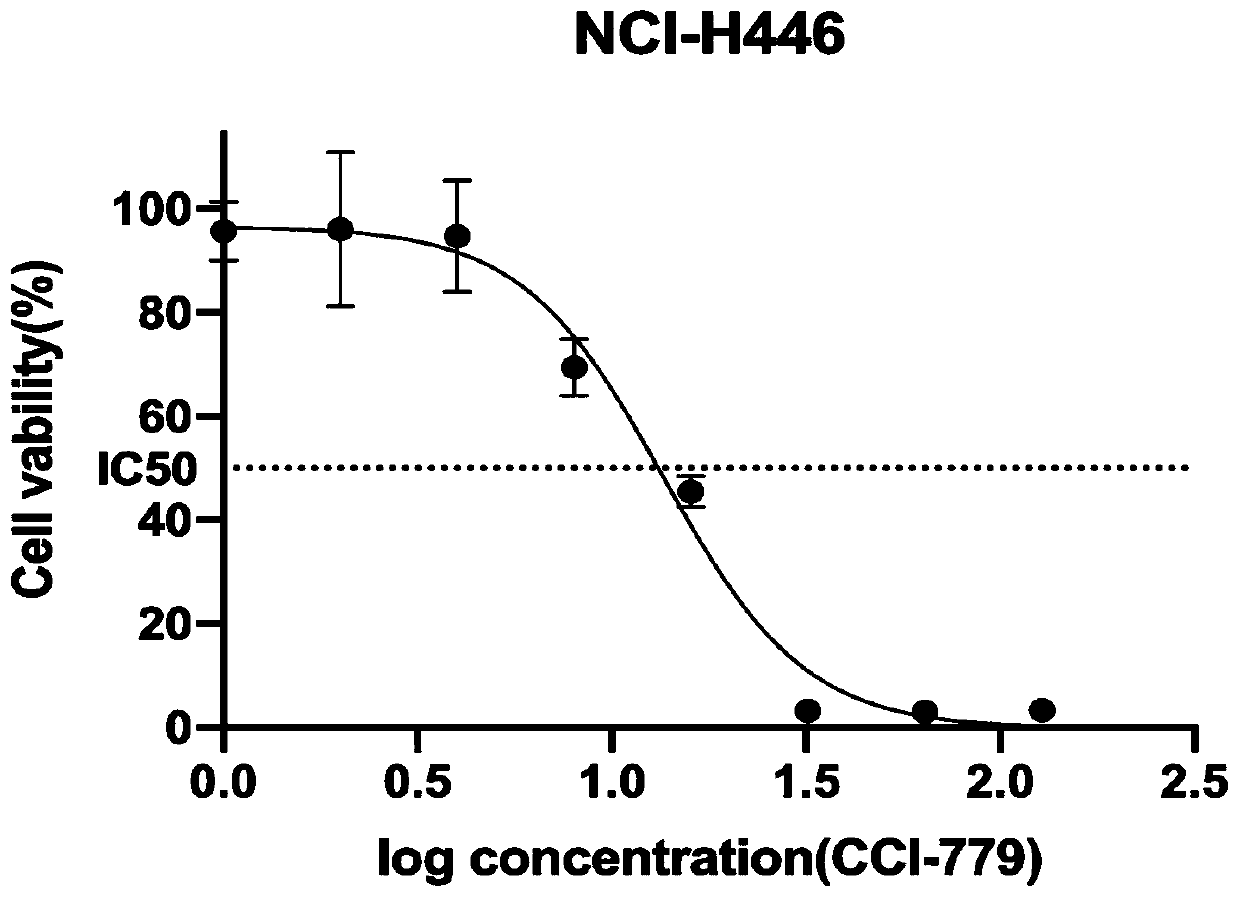
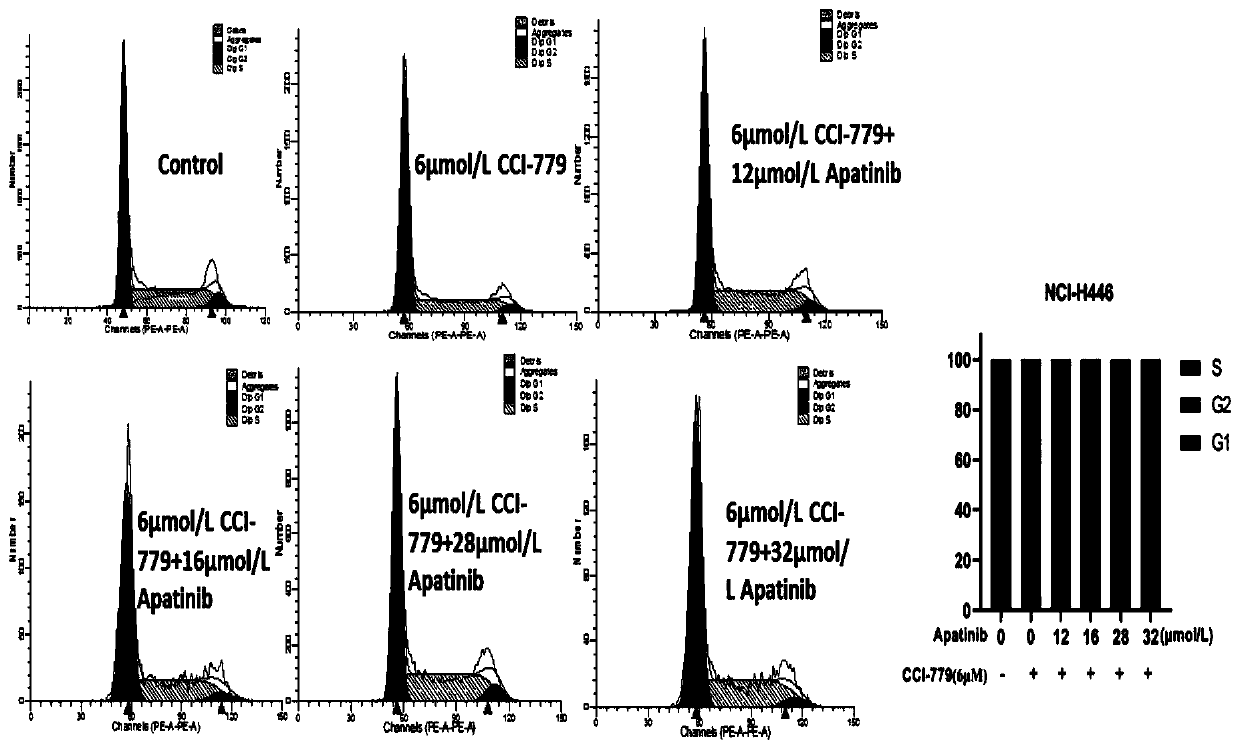
Application of apatinib and combination of apatinib and CCI-779 in preparation of drugs for treating lung cancer
PendingCN111110676AIncreased sensitivityPrevent proliferationOrganic active ingredientsAntineoplastic agentsInducer CellsOncology
The invention discloses application of apatinib and a combination of the apatinib and CCI-779 in preparation of drugs for preventing and treating small cell lung cancer, and relates to the technical field of pharmacy. In-vitro tests prove that the apatinib has a concentration-dependent characteristic on the action of a small cell lung cancer cell strain (NCI-H446), and high-concentration apatinibcan inhibit cell proliferation and migration of the small cell lung cancer cell strain and induce cell apoptosis, and can improve the sensitivity of small cell lung cancer cells to the apatinib when combined with an mTOR inhibitor CCI-779, and can inhibit proliferation and migration of the small cell lung cancer cells at a relatively low concentration to cause cell cycle arrest and induce cell apoptosis.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
Novel pharmaceutical application of apatinib or pharmaceutically acceptable salt thereof
InactiveCN111773220ASignificant effectImprove securityOrganic active ingredientsAntineoplastic agentsDownregulated geneSpleen
The invention relates to a novel pharmaceutical application of apatinib or a pharmaceutically acceptable salt thereof, in particular to an application of apatinib or a pharmaceutically acceptable saltthereof in preparation of a medicine for treating malignant mesothelioma. The in-vitro test result shows that apatinib mesylate inhibits MPM cell proliferation and activity, affects the G2 / M phase ofthe MPM cell cycle process and remarkably inhibits MPM cell movement and migration; an in-vivo test result shows that the apatinib mesylate obviously reduces the ePCI score and has no influence on the body weight of nude mice. The apatinib has no histological toxicity to lung, spleen, kidney and gastrointestinal tract, only has focal lymphocyte infiltration in liver and myocardial tissues, and has an obvious effect of down-regulating NPM2 gene expression. The new application of apatinib or the pharmaceutically acceptable salt thereof provided by the invention provides an important theoreticalbasis for application of apatinib or the pharmaceutically acceptable salt thereof in treatment of malignant mesothelioma, widens the treatment range of the drug, and provides a new drug solution fortreatment of malignant mesothelioma.
Owner:BEIJING SHIJITAN HOSPITAL CAPITAL MEDICAL UNIVERSTY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com