New crystal of apatinib sulfate
A technology of sulfuric acid and crystal form, which is applied in the field of new crystal forms of apatinib sulfate, can solve the problems such as no patent of crystal form of apatinib sulfate, and achieves excellent stability, good solubility, and is conducive to preservation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1. Preparation of new crystal form of apatinib sulfate
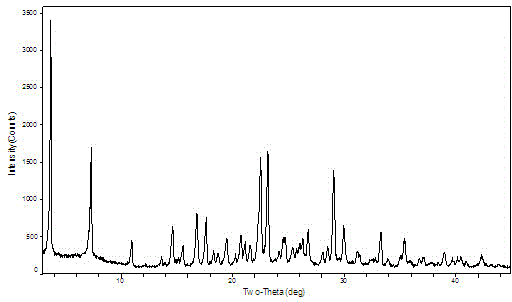
[0014] Weigh 500mg of apatinib sulfate into a container, add 100ml of acetone, suspend at 35°C for 48 hours, filter and vacuum dry to obtain a white powder, which is detected by XRPD, and the spectrum is as follows: figure 1 , the new crystal form is crystal form A.
Embodiment 2
[0015] Embodiment 2. Preparation of new crystal form of apatinib sulfate
[0016] Dissolve 500mg of apatinib sulfate in 100mL of acetonitrile, shake at 35°C for 48h, filter, dry, and vacuum-dry at room temperature to obtain a white powder, which is detected by XRPD, and the spectrum is as follows: figure 1 , the new crystal form is crystal form A.
[0017] It can be seen from the XRPD pattern that the diffraction peaks are about 3.681, 7.319, 10.96, 14.62, 15.54, 16.8, 17.62, 18.3, 18.699, 19.48, 20.74, 21.1, 21.58, 22.5, 23.159, 24.16, 24.7, 25.39, 26.77 28.079, 28.5, 29.06, 29.979, 31.181, 33.28, 35.42, where the error range of 2θ value is 0.2.
Embodiment 3
[0018] Example 3. Investigation of high-temperature stability of crystal form Apatinib sulfate
[0019] Place A crystal form sulfate Apatinib sample in 60 o C oven, after 5 days, the sample was taken out for XRPD test to investigate the stability of the crystal form of the sample to temperature. The results show that the crystal form A sample is stable under this condition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com