Apatinib polymeric micelle and preparation method thereof
A technology of apatinib and polymer glue, which is applied in the field of apatinib polymer micelles and its preparation, can solve the problems of poor solubility, large side effects, and low bioavailability of apatinib, and improve the curative effect , increased solubility, and high biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of a kind of PEG-PLA entrapped apatinib micelles
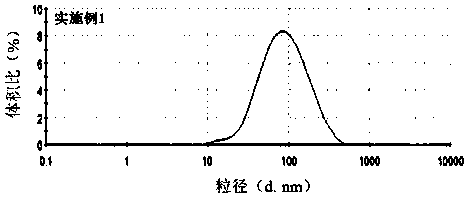
[0030] 40mgPEG 5K -PLA 10K Dissolve together with 4mg of apatinib in 5ml of acetone solvent as the organic phase; mix 5ml of water and 5ml of ethanol to form the water phase; drop the organic phase into the water phase at a speed of 1ml / min, stir at a low speed of 300r / min to form a shallow The blue nanoemulsion was reacted for 2 minutes, then transferred to a rotary evaporator, and treated for 5 minutes under vacuum rotary evaporation at 37° C., and the acetone solution was removed to obtain apatinib micelles. The average particle size measured by the laser dynamic scattering instrument is 76.53±0.58nm, and the particle size distribution results are as follows: figure 1 shown. The encapsulation efficiency of nanoparticles is 89.11±1.04%, and the dispersion coefficient is 0.361.
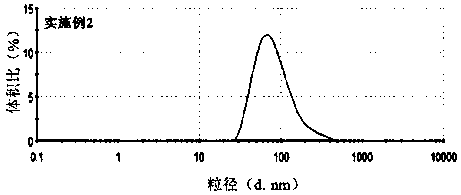
[0031] Example 1: Preparation of apatinib micelles loaded with PEG-PLA and TPGS
[0032] 20mgPEG 5K -PLA ...
Embodiment 3
[0033] Embodiment 3: In vitro drug release experiment of micelles containing Apatinib
[0034] Dissolve the prepared Apatinib micelles (Example 1 and Example 2) in an appropriate amount of PBS buffer (pH7.4, containing 0.2% Tween 80), and dilute to Apatinib 0.1 mg / mL , mix well. Take 9mL and put it in the dialysis bag, and tighten the dialysis bag. Put the dialysis bag into 50mL PBS buffer solution (pH7.4, containing Tween 800.2%), 37°C, 100r / min, and take 1.0mL of PBS solution outside the dialysis bag at different time points. Determine the content of Apatinib respectively (chromatographic column: ODS2 (Lichrospher-C18, 250 × 4.6mm, 5 μ m); mobile phase: methanol-acetonitrile-water (30:40:32); flow rate: 1.0mL / min, detection wavelength : 226nm; column temperature: 30°C), see the results figure 2 .
Embodiment 4
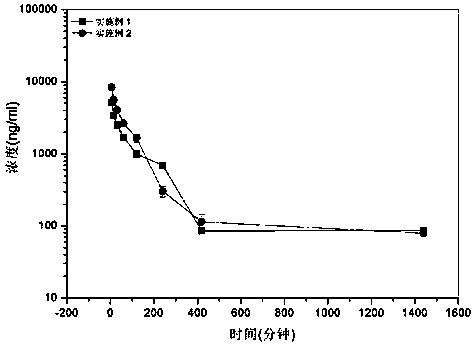
[0035] Embodiment 4: In vivo pharmacokinetic experiment of apatinib micelles
[0036] In vivo pharmacokinetic experiments were carried out on the apatinib micelles prepared in Example 1 and Example 2. A total of 8 healthy female SD rats (200±10) grams were taken and randomly divided into 2 groups with 4 rats in each group. The dose of 10 mg / kg was administered to the tail vein of each group of rats, and 200 μL of blood was collected after 5 minutes, 15 minutes, 30 minutes, 45 minutes, 1, 2, 4, 7, 12, and 24 hours, and centrifuged at 5000 rpm. separate. After acetonitrile treatment, the content of apatinib in plasma was detected by high performance liquid chromatography. Figure 5 is the obtained drug-time curve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com