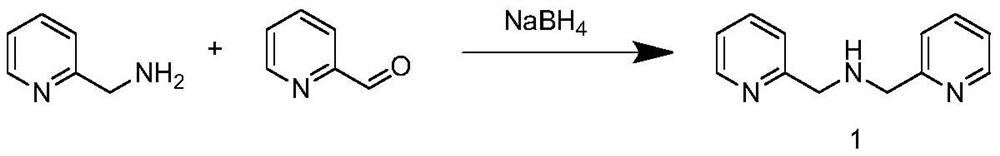Patents
Literature
56results about How to "No physiological toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Prepn process of microcapsule with included anticancer medicine
InactiveCN1771917AGood biocompatibilityGood tissue compatibilityOrganic active ingredientsUnknown materialsNanometer sizeWater soluble
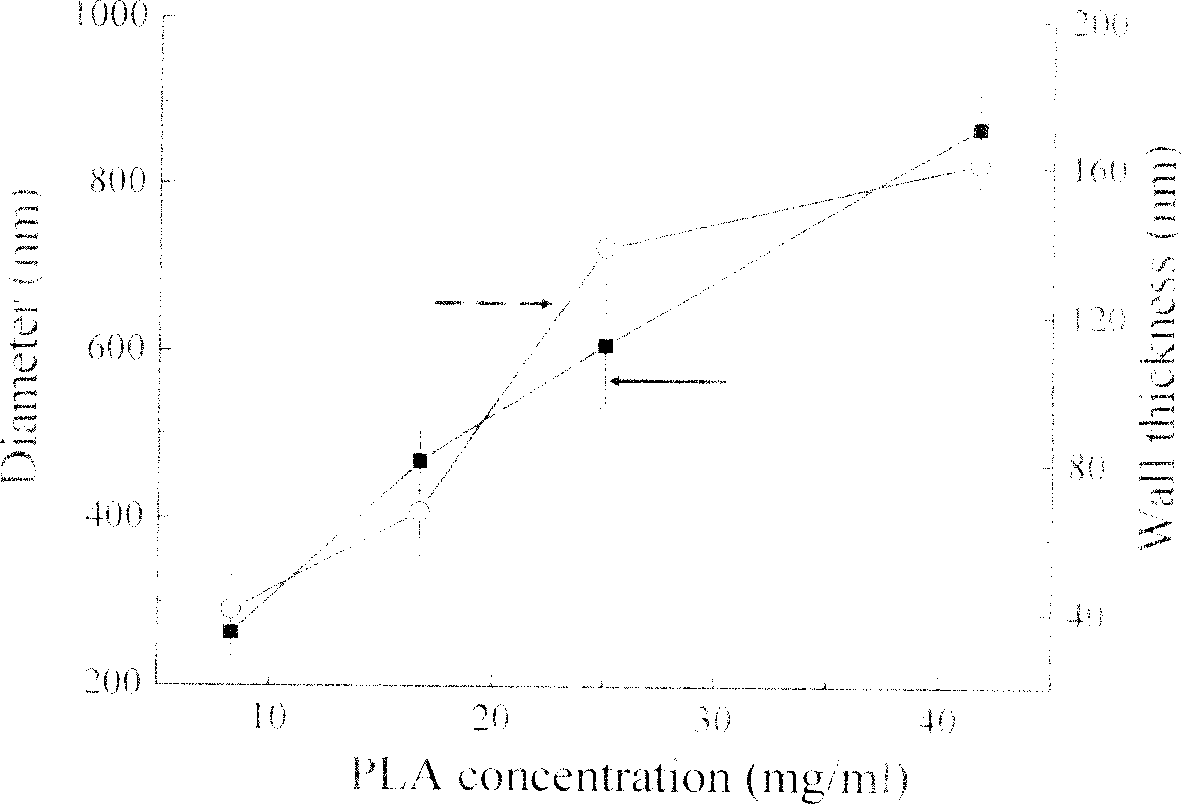
The present invention discloses the preparation process of microcapsule with included anticancer medicine. The process adopts colloid particle containing polyelectrolyte as template, assembles polyelectrolytes with dislike charges via layer-by-layer self-assembling to the surface of colloid particle, and dissolves or decomposes the colloid particle to obtain hollow polymer microcapsule containing polyelectrolyte. The microcapsule has wall micro structure and thickness capable of being regulated precisely in nanometer size, and the contained polyelectrolyte can interact with the medicine so as to include the medicine into the microcapsule. The included medicine has controllable release speed. Introducing polyelectrolyte with grafted polyglycol to the surface of the microcapsule can raise the biocompatibility of the microcapsule. The process of the present invention is simple, feasible and repeatable, and suitable for inclusion and controllable release of various kinds of water soluble anticancer medicine.
Owner:ZHEJIANG UNIV
A treating method for a nanometer suspension solidification process
ActiveCN105902496AInhibit aggregationGuaranteed Particle SizePowder deliverySulfonylurea active ingredientsPharmaceutical formulationSURFACTANT BLEND
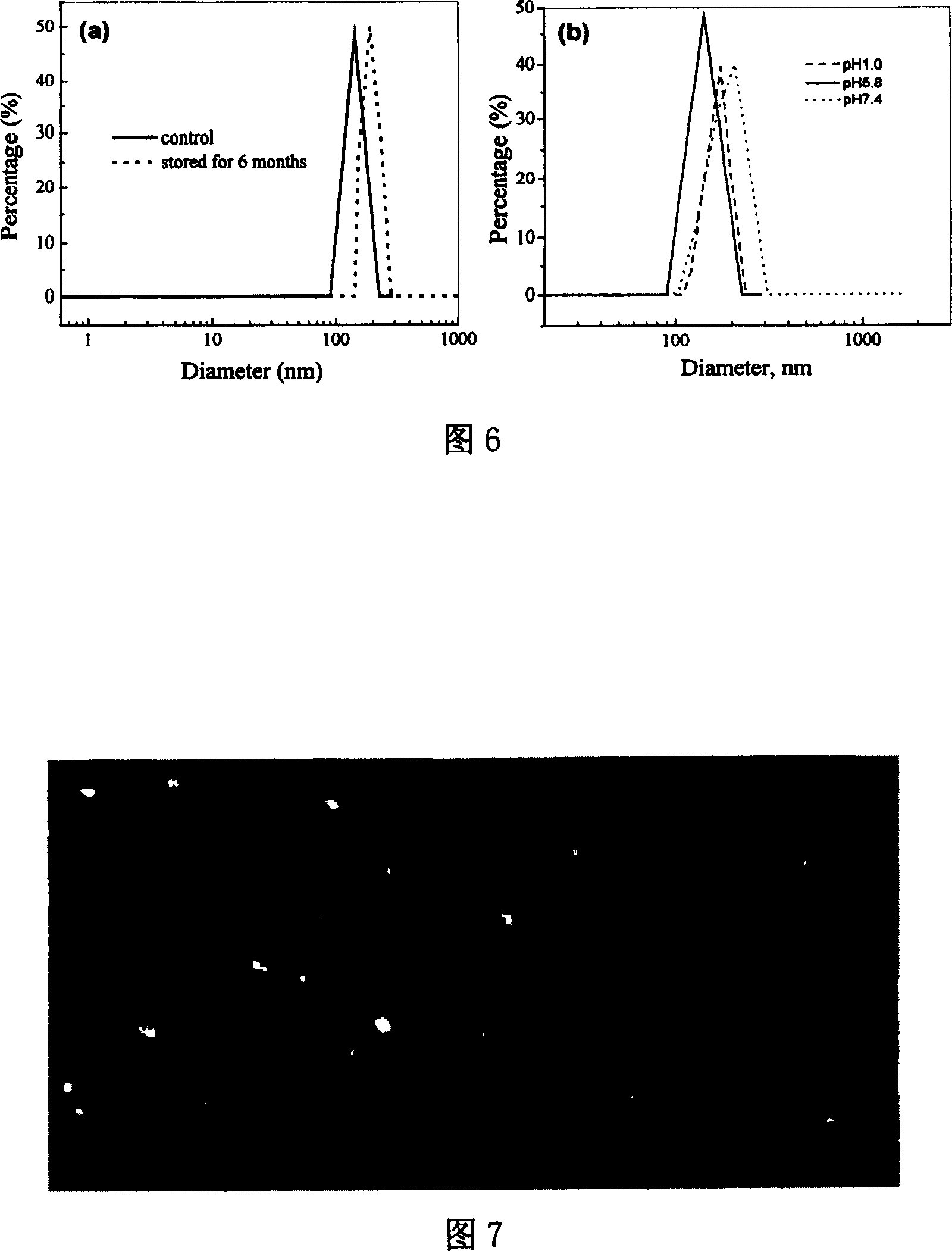
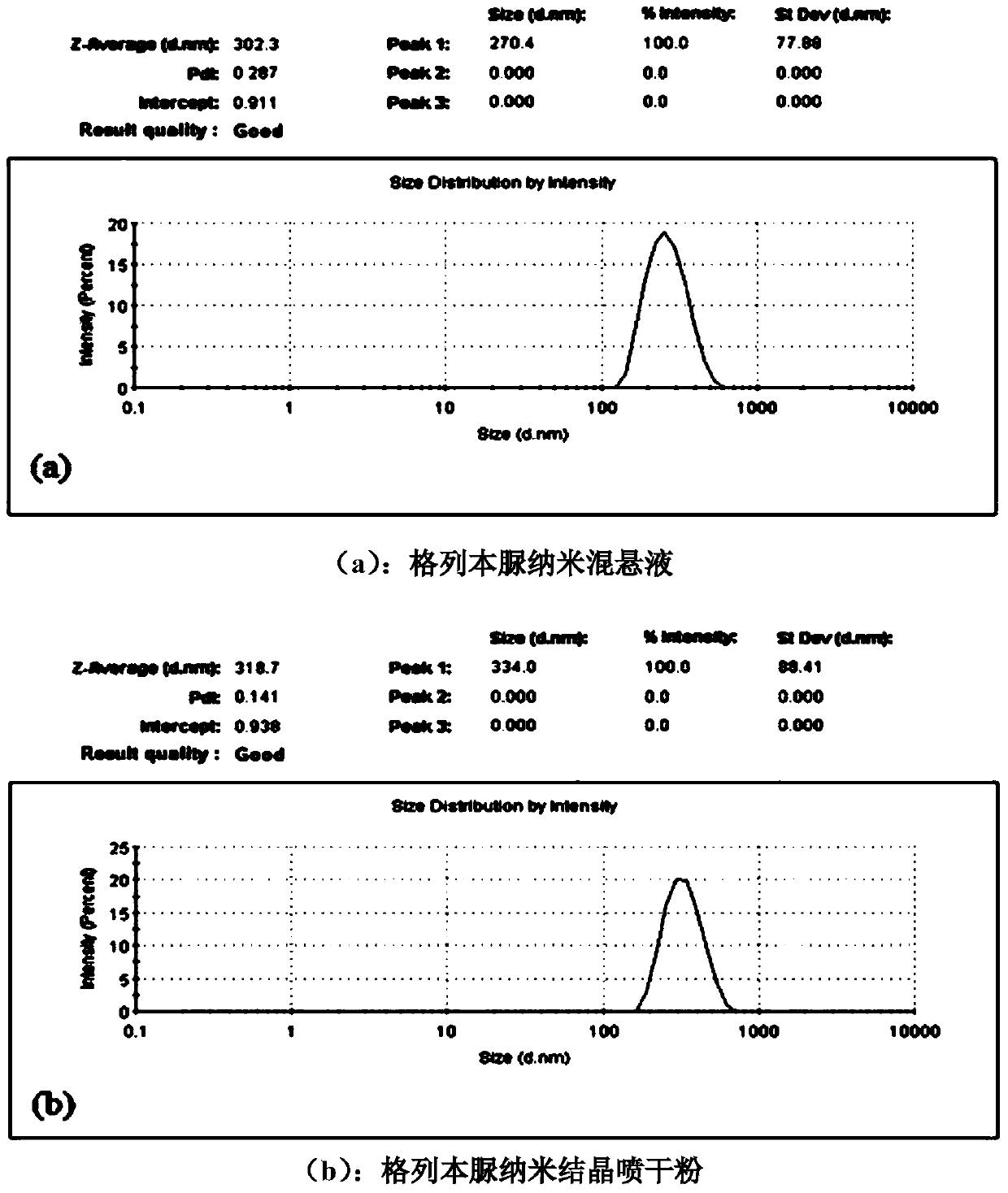
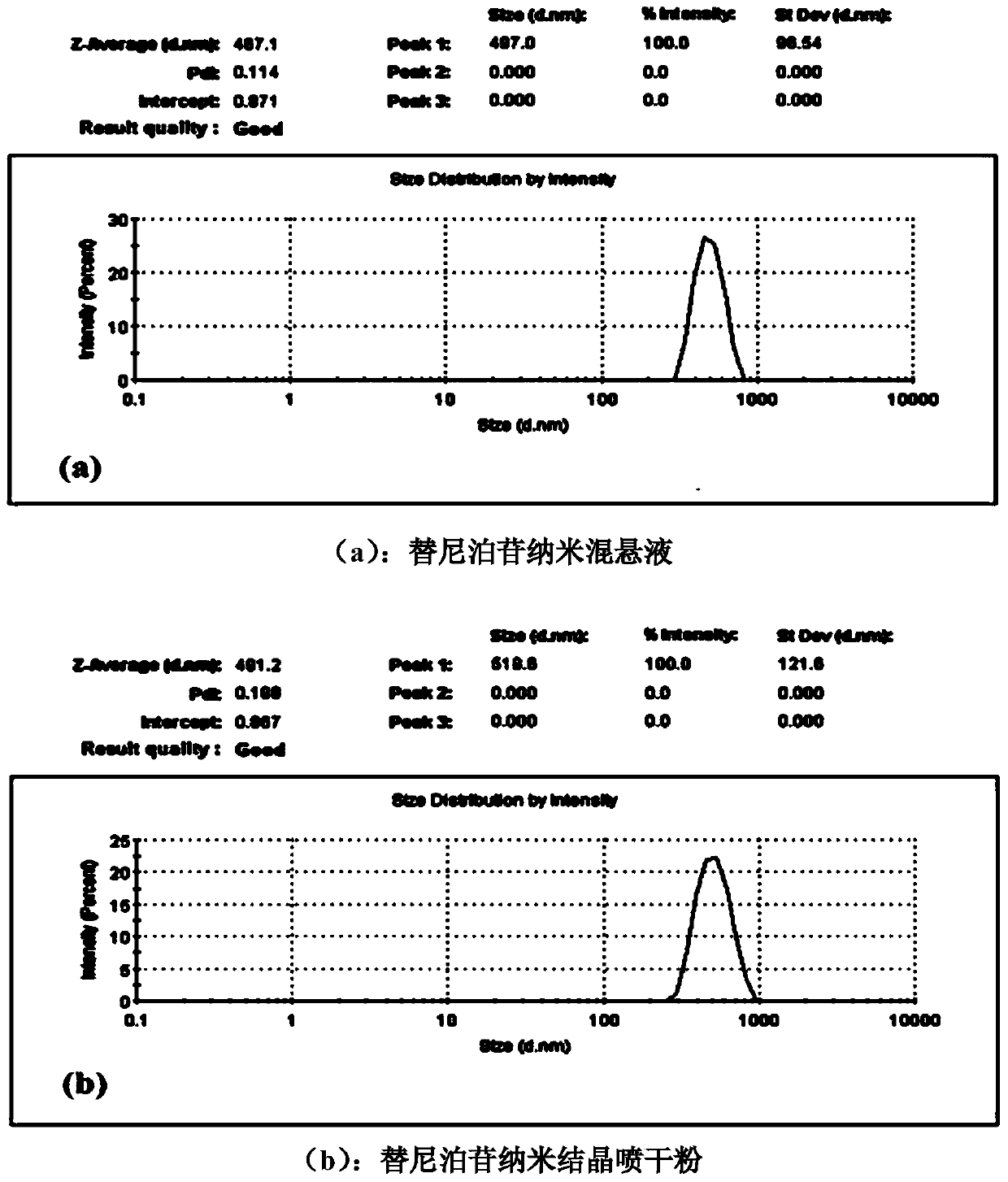
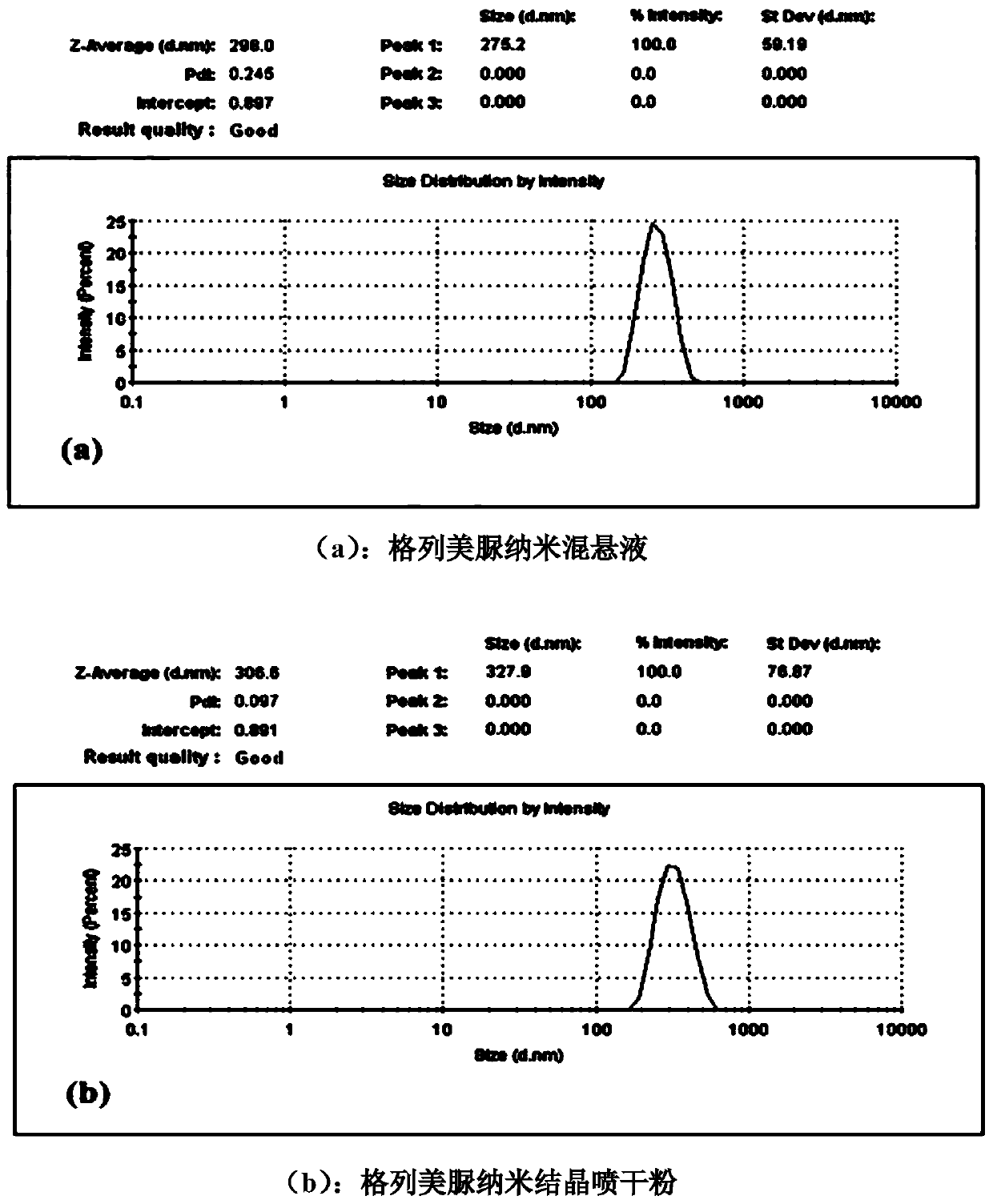
The invention discloses a treating method for a nanometer suspension solidification process, and belongs to the field of medicine preparations. The nanometer suspension comprises a medicine, a stabilizing agent and water. The method includes adding a surfactant into the medicine nanometer suspension before solidification, and solidifying. Before solidification of the nanometer suspension, 0.01-10% (w / v) of the surfactant is added to inhibit particle aggregation in the solidification process and to maintain the particle size, thus preparing nanometer crystal powder uniform in particle size distribution and easy in redispersion. The method reduces the using amount of a conventional solidification protective agent, and even can fully replace the conventional protective agent, thus increasing the medicine content in the nanometer crystal powder, reducing the cost of auxiliary materials and improving medicine taking compliance.
Owner:SHENYANG PHARMA UNIVERSITY
Method for preparing microcapsule having function of specific connecting with tumor cell
InactiveCN101099727AHigh degree of bindingHigh drug loadingPharmaceutical non-active ingredientsAntineoplastic agentsPolyethylene glycolMicroparticle
The present invention discloses a preparation method of microcapsule which can be specifically combined with tumor cell. Said preparation method includes the following steps: adopting removable colloidal particles as template, utilizing layer-by-layer self-assembly method to make polyelectrolyte with opposite charges be assembled on the surface of colloidal particles, then utilizing polyethylene glycol and tumor cell identification ligand to make modification, finally making the colloidal particles be dissolved or decomposed so as to obtain the invented polymer hollow microcapsule containing tumor cell identification ligand.
Owner:ZHEJIANG UNIV
Medical coating powder containing nano material
InactiveCN1616105AOvercome the defects that the quality is difficult to guarantee, etc.Simple and fast operationPharmaceutical non-active ingredientsDrageesUltimate tensile strengthMaterials science
The medicinal coating powder containing nanometer material consists of hydromellose 55-65 wt%, copolymer of vinyl pyrrolidone and vinyl acetate 8 wt%, glycerin 15 wt%, Span 8 wt%, coloring agent 2-4 wt%, and nanometer titania 2-10 wt%. The medicinal coating powder containing nanometer material has simple production process, wide application, high performance / cost ratio, no physiological toxicity and many other advantages, and may meet the requirement of coating various solid Chinese medicine preparation.
Owner:GUANGDONG GUOFANG MEDICAL TECH
Method of producing hepatic targeting drug microcapsule
InactiveCN101129342AHigh selectivityLess by-productsPharmaceutical non-active ingredientsMicroballoon preparationDrug capsuleVinyl ester
The invention discloses a method for preparing a medicine with liver target taxis for slow-releasing the nanometer microcapsule. The method comprises the following steps: proceeding with enzymatic reaction of liver target taxis gene with sugar monomer of cerebrose residue and ethylated carboxylate; copolymerizing vinyl ester with glycosyl and unsaturated cationic or anion; getting liver target taxis polyelectrolyte; getting the medicinal microcapsule with liver target taxis by multilayer packaging polyelectrolyte on the surface of medicine by electrostatic action, wherein the deactivation speed of microcapsule medicine can be controlled. The polymerization method is simple, high effective and non-toxicity. The coating method has the high efficient, the simple operation and the mild technology, which can cycle several times. The preparing medicine can save for a long time, which can release step by step, can accumulate in the liver, improves the medicinal effect of disease portion, reduces the toxic effect of the other healthy organ, and has the good application prospect.
Owner:ZHEJIANG UNIV
Different types of chitosan methacrylate and preparation method thereof
The invention relates to chitosan methacrylate with bioactivity, which is prepared by adopting the steps of: dissolving chitosan with different deacetylating degrees and different molecular weights into an acetic acid solution; adding a cellulase into the solution, uniformly mixing and then vibrating at constant temperature for enzymolysis; measuring the viscosity of the solution by using a Ubbelohde viscosity meter, calculating the molecular weight of the chitosan; after the enzymolysis, inactivating cellulase; adding methacrylic acid into the solution, uniformly mixing and then vibrating at constant temperature for substitution reaction; characterizing a grafted copolymer by using an infrared spectrum, calculating the substitution ratio of the methacrylic acid; settling chitosan methacrylate generated by the reaction by using alcohol, cooling and air-drying; and dissolving the chitosan methacrylate into different pH values of water solutions when in use, adding a crosslinking agent and placing at constant temperature to ensure that the chitosan methacrylate is polymerized into gel. The chitosan methacrylate can be used for manufacturing tissue engineering support materials, medicine slow release materials and enzyme fixing agents.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
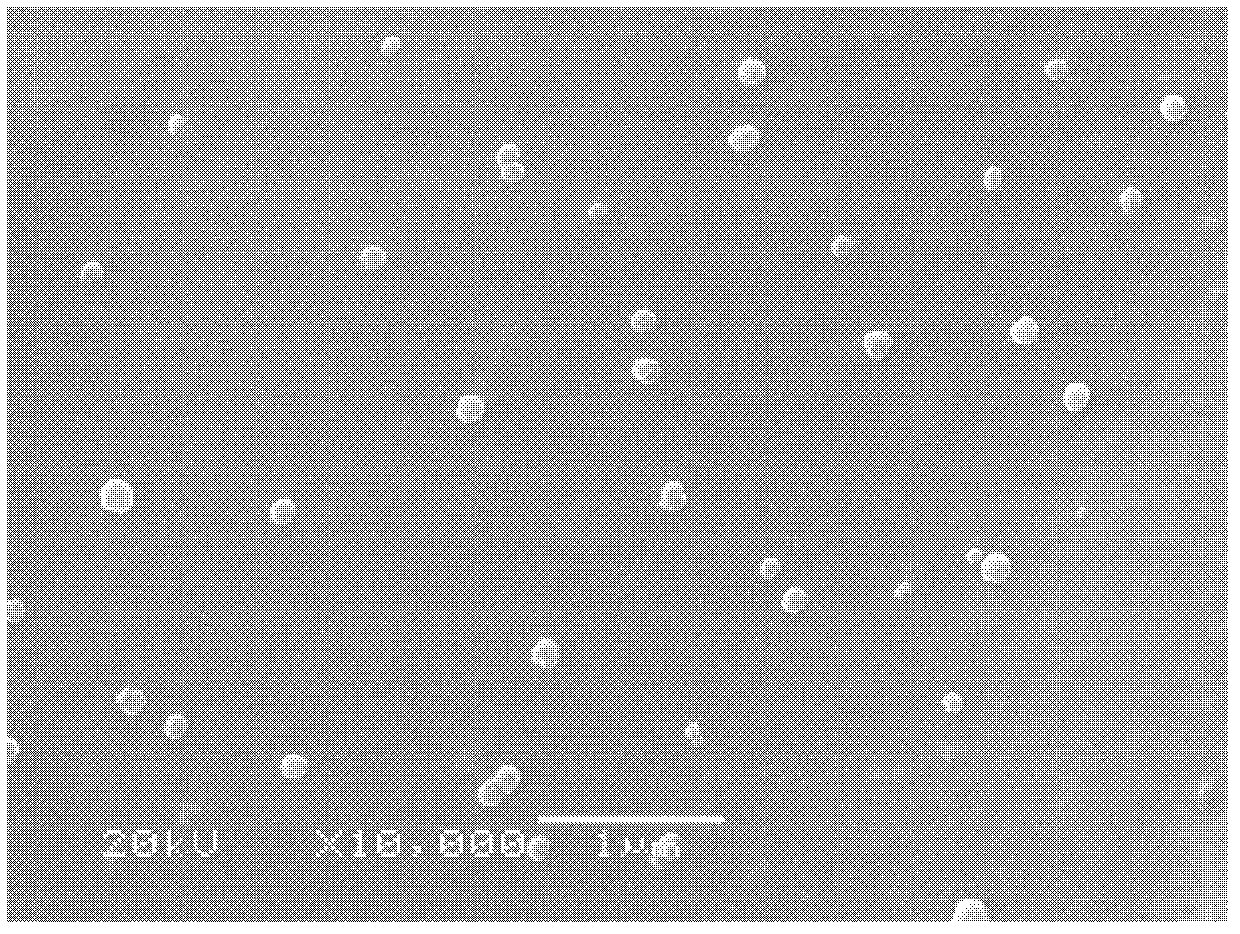
Asymmetrical polyurethane/nano TiO2 thin film wound dressing and preparation method thereof
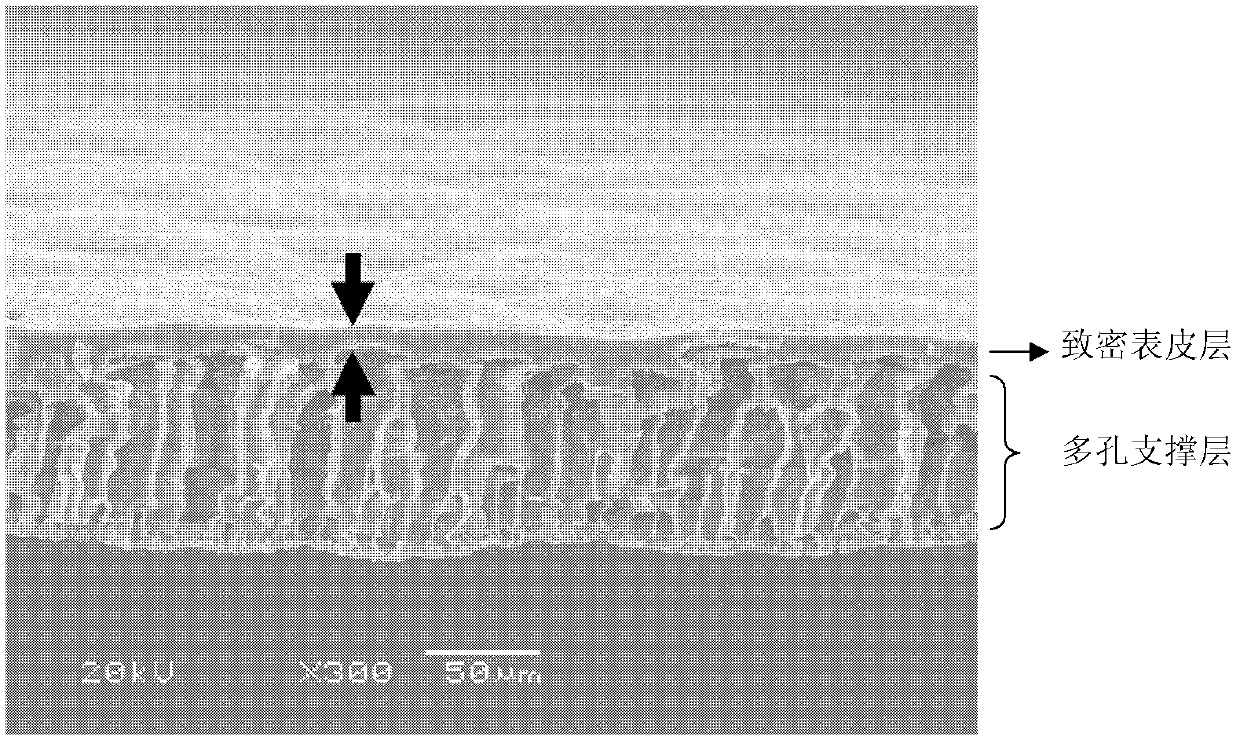
InactiveCN102430145ASpecial structureStrong water absorptionAdhesive dressingsAbsorbent padsSurface layerWound dressing
The invention discloses an asymmetrical polyurethane / nano TiO2 thin film wound dressing, which is characterized by being composed of a dense surface layer and a porous support layer, wherein the dense surface layer is integrally shaped with the porous support layer, the dense surface layer has the thickness of 7-15 mu m, the porous support layer has the total pore volume of 4.80-5.24 cm<3> / g, and in-situ generated nano TiO2 particles are uniformly dispersed in an entire polyurethane thin film and have the particle size of 30-150 nm. The invention also discloses a preparation method of the asymmetrical polyurethane / nano TiO2 thin film wound dressing. In the technical scheme disclosed by the invention, a solvent evaporation film-forming method, a wet process phase inversion film-forming method and a nano particle in-situ generation technique are felicitously organically combined, the structure of the polyurethane thin film wound dressing is reconstructed, the defects of the existing performance of the polyurethane thin film wound dressing are compensated, the comprehensive and clinical application value of the product is greatly improved, and a new method is provided for preparation of the asymmetrical polyurethane / nano TiO2 thin film wound dressing.
Owner:SICHUAN UNIV
Dragon blood nano medicament crystallized preparation and preparation method thereof
InactiveCN102579737AImprove utilizationGood treatment effectPowder deliveryAntipyreticFreeze-dryingSuspending Agents
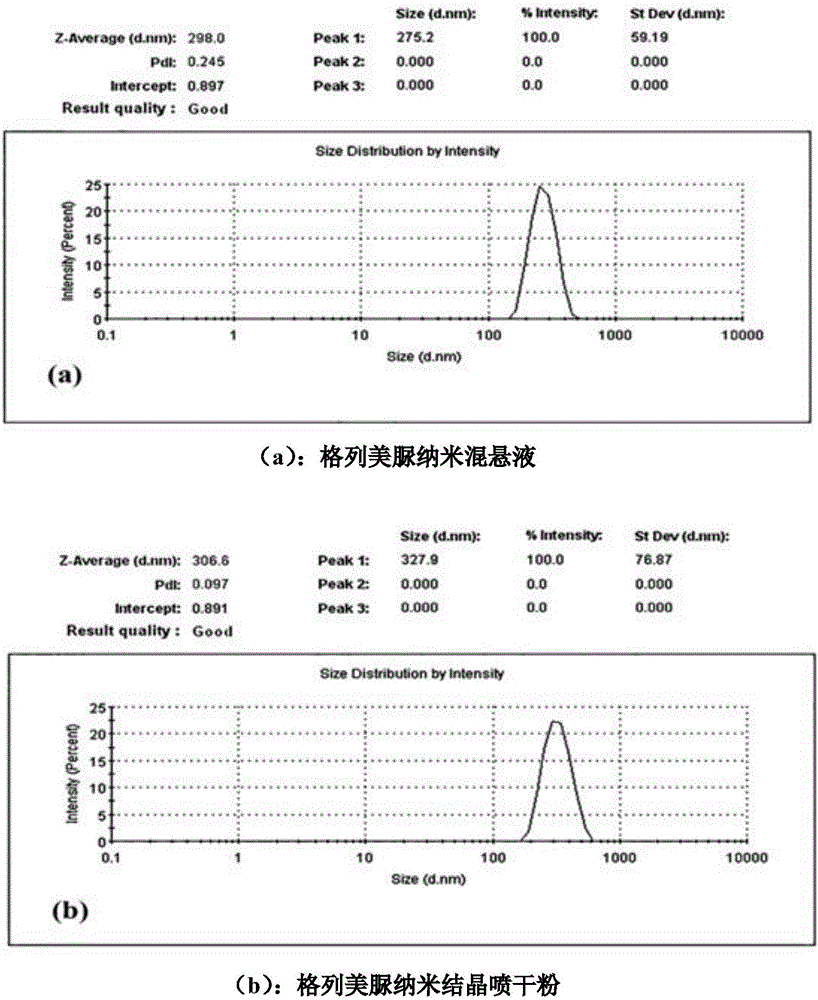
The invention discloses a dragon blood nano medicament crystallized preparation and a preparation method thereof, and relates to the field of medicinal preparation, and contains a dragon blood, a stabilizing agent and a mixed solvent, wherein the stabilizing agent contains a surfactant and a suspending agent, and the weight ratio of each component is as follows: the dragon blood 0.01-5.0g; the surfactant 0.01-2.0 g; the suspending agent 0.002-1.0 g; the mixed solvent 0.01-5.0g. The preparation technology of the dragon blood nano medicament crystallized preparation has the advantages of easy enlargement, simple prescription, improved medicament stability after freeze drying and reduced administration volume; the medication is prepared into an oral nanometer crystallized suspension for improving bioavailability, reducing administration dosage and saving resource effectively.
Owner:TAISHAN MEDICAL UNIV
Oil-soluble medicine degradable polymer microcapsule injecta and preparing method
InactiveCN1742707ANo physiological toxicityNo toxicityPharmaceutical non-active ingredientsMicrocapsulesEmulsion polymerizationDouble bond
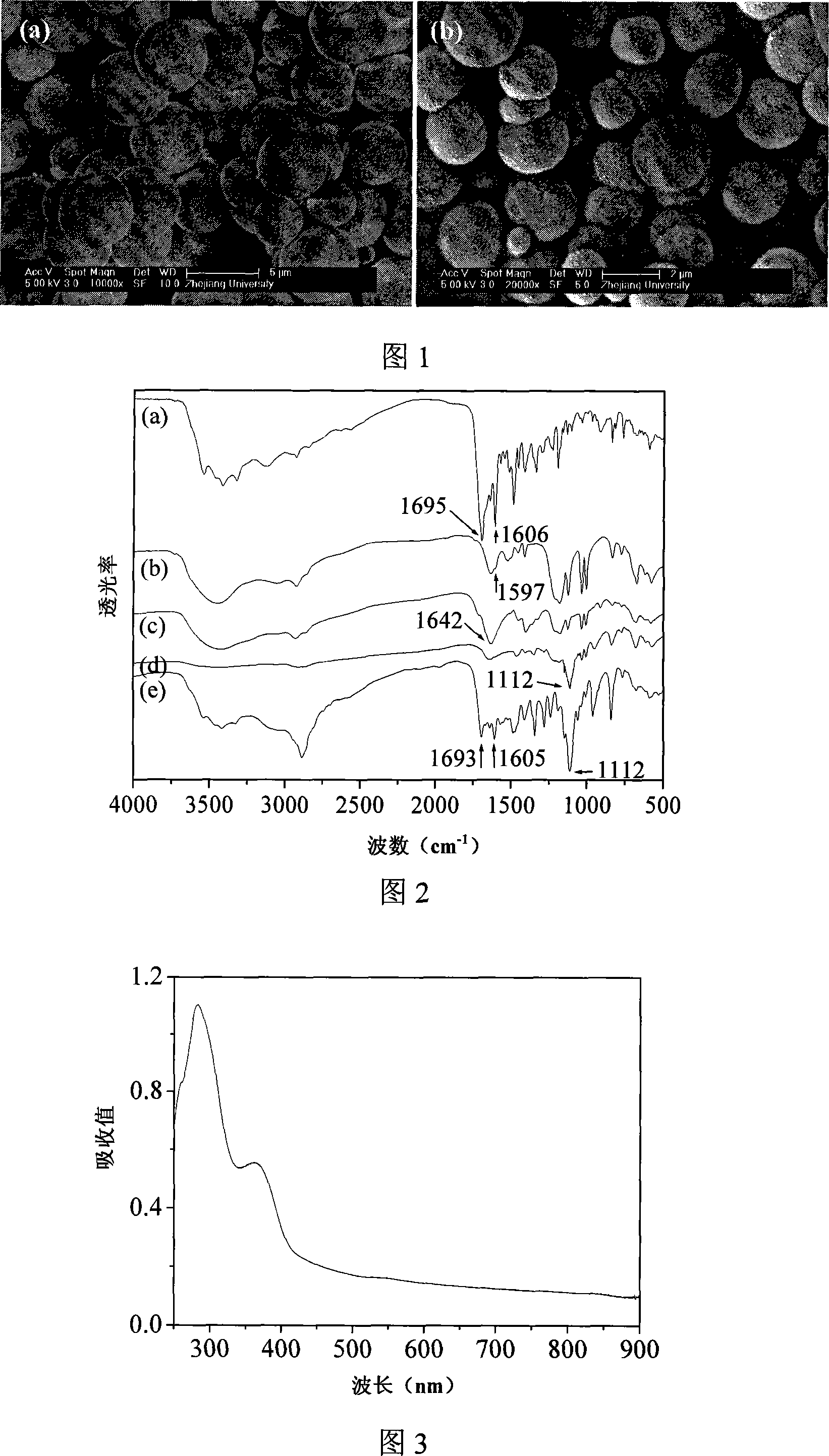
The present invention discloses a degradable polymer microcapsule injection preparation of oil-soluble medicine and its preparation method. It uses polysaccharide containing double-bond and polylactic acid which have good biological compatibity and biological degradability as capsule shell material, adopts O / W emulsion polymerization technique and controls the ratio of mixing polysaccharide and polylactic acid to obtain the invented medicine microcapsule injection preparation whose grain size is 100-3000 nm.
Owner:ZHEJIANG UNIV
Insulating paint and preparation method thereof
ActiveCN105778753AHigh hardnessIncrease the areaAntifouling/underwater paintsPaints with biocidesEpoxyHardness
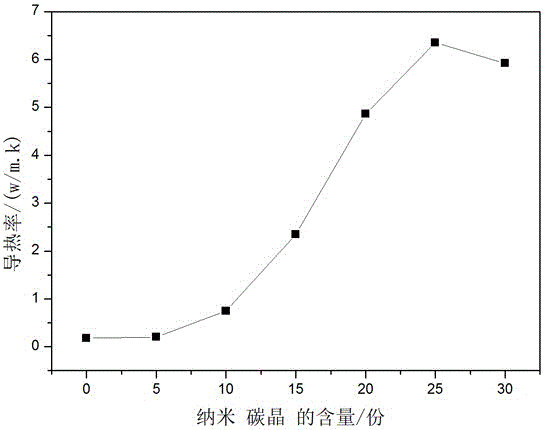
The invention discloses insulating paint and belongs to the technical field of paint. The insulating paint is prepared from, by weight, 5-20 parts of nano carbon crystal, 25-40 parts of polyimide, 30-50 parts of epoxy resin, 0.1-1 part of a dispersant, 0.1-2 parts of flatting agent, 0.2-1.0 part of a corrosion inhibitor, 0.1-2 parts of a defoamer and 0.1-3 parts of lysozyme. A preparation method of the insulating paint includes: mixing polyimide with the epoxy resin at 65-80 DEG C; adding nano carbon crystal, the dispersant, the flatting agent, the corrosion inhibitor, the defoamer and lysozyme; stirring. Raw materials are reasonably combined to form the waterborne insulating paint, and the insulating paint has good insulativity, adhesiveness and cohesiveness, a paint film is high in hardness and fullness, the surface of the paint film is uniform, compact, smooth and micropore-free, and the insulating paint has good mechanical performance, wear resistance and scratch resistance.
Owner:HENAN YUXING MICRON DIAMOND CO LTD
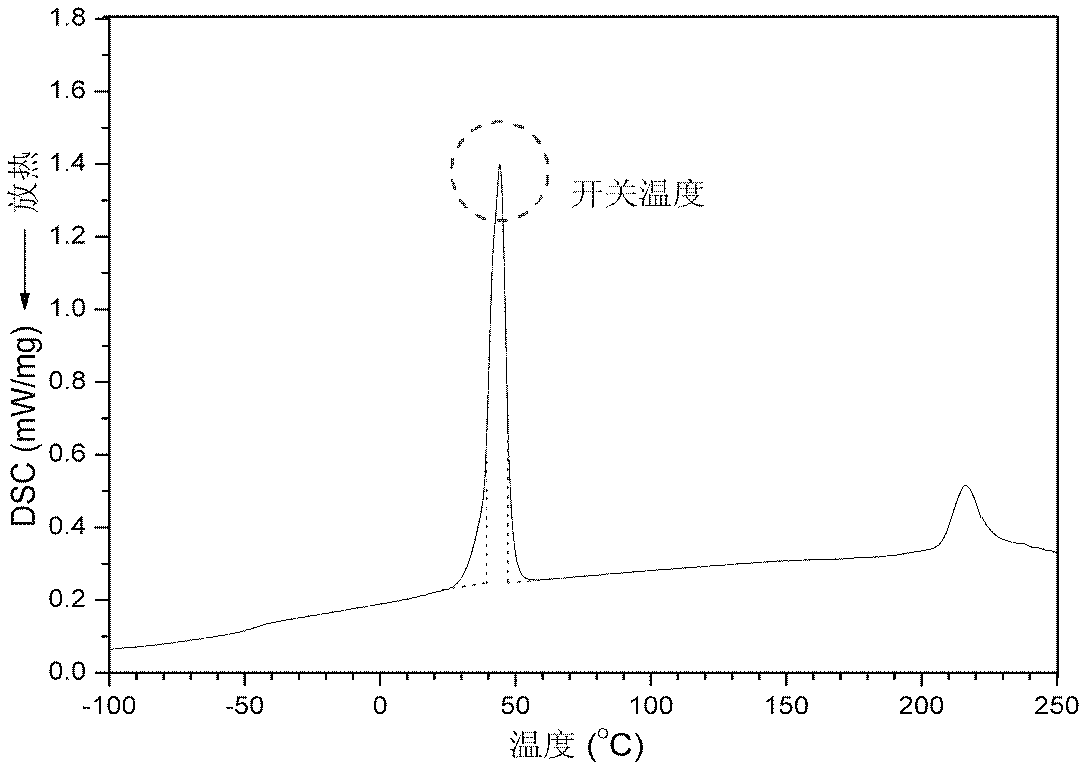
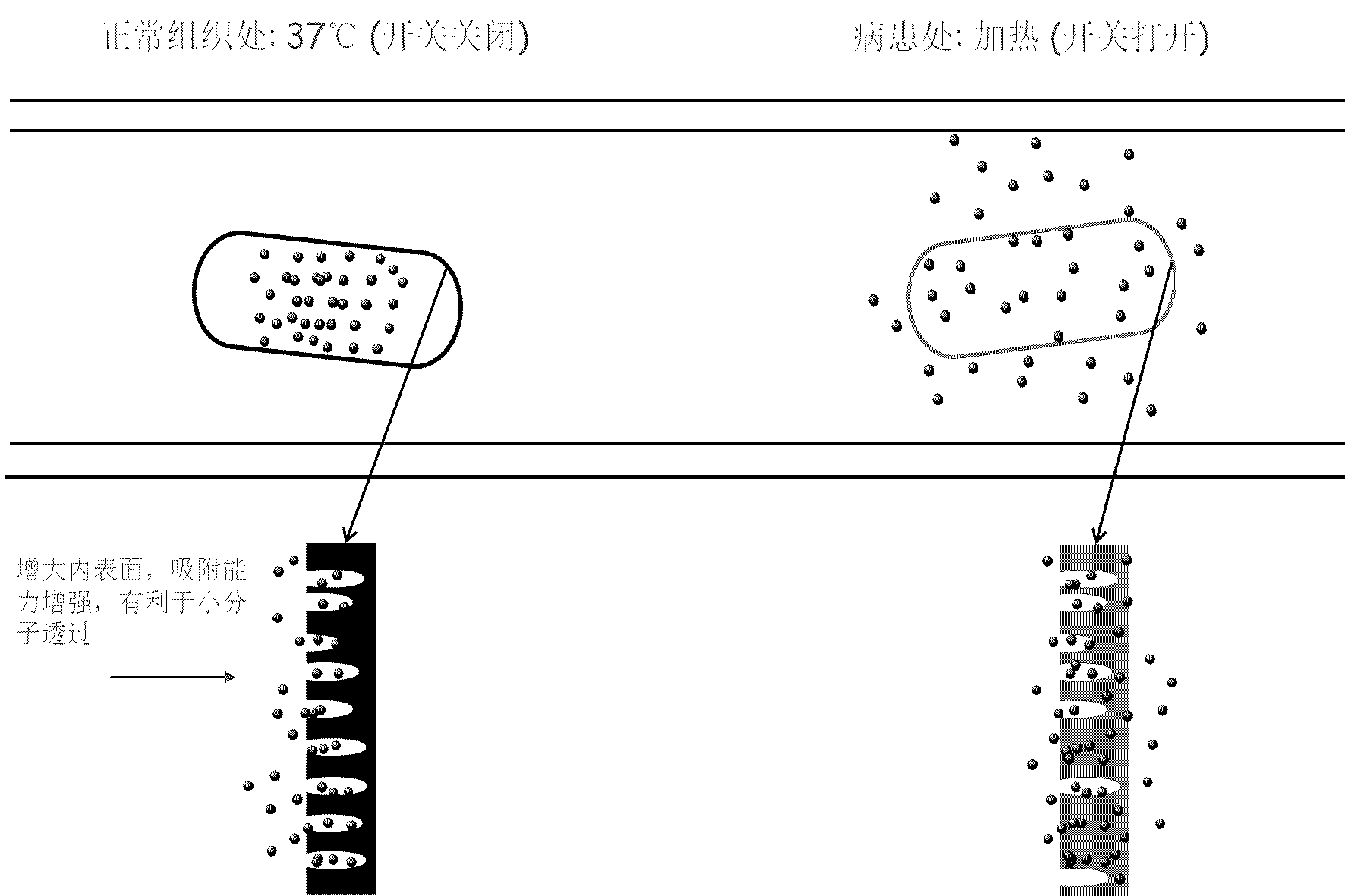
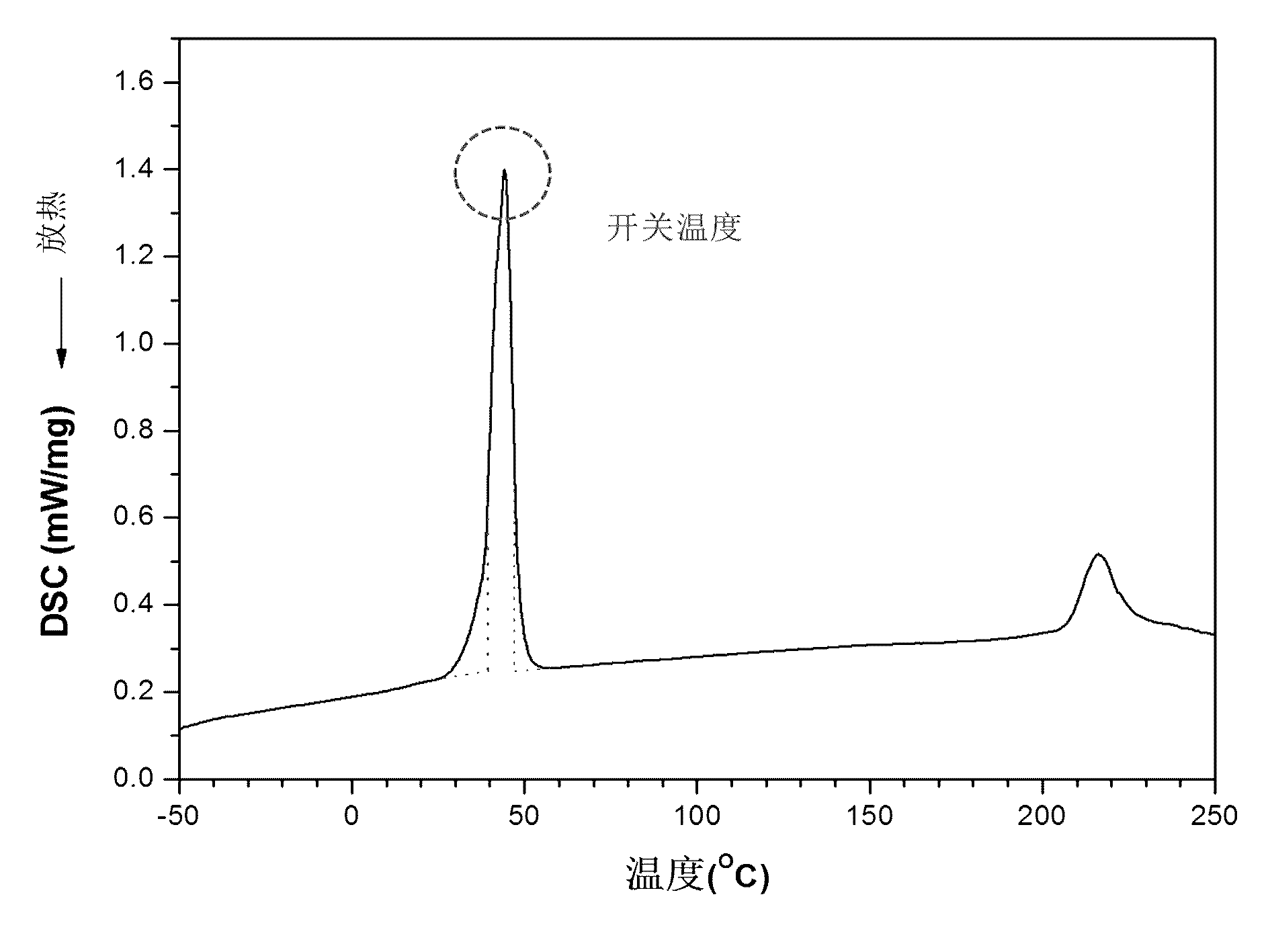
Polyurethane drug release controlling body with thermoswitch and preparation method thereof
InactiveCN102580102ARelease on demandQuick responseAntibacterial agentsAntipyreticToxicityPolymer chemistry
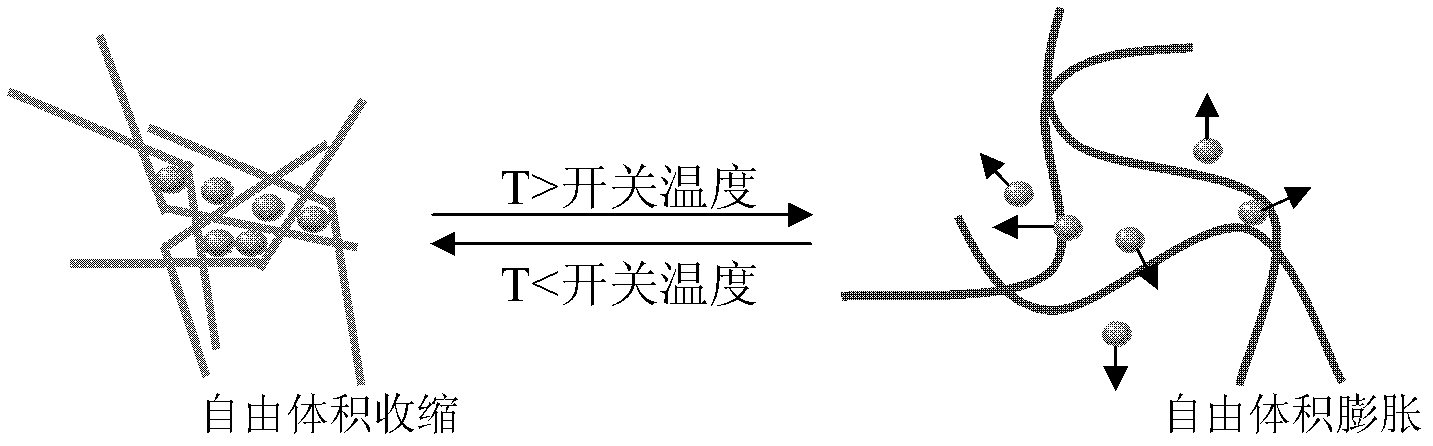
The invention discloses a polyurethane drug release controlling body with a thermoswitch, which is prepared by compounding thermosensitive polyurethane with drug micromolecules. The free volume of the thermosensitive polyurethane is subjected to reversible contraction-expansion mutation at a certain temperature from 39 to 45 DEG C so as to have an on-off effect, thus the drug micromolecules loaded in the thermosensitive polyurethane have the characteristic of releasing in response to the temperature, wherein below the on-off temperature, the releasing rate of the drug micromolecules is extremely low; and when the temperature rises to above the on-off temperature, the releasing rate of the drug micromolecules rises dramatically. The invention also discloses the preparation method of the polyurethane drug release controlling body. The polyurethane drug release controlling body can conveniently control the response release of the drug micromolecules through temperature change and is highin response speed and free of physiological toxicity, the repeatability of the release switch is good, and the drug release amount can be adjusted freely, thus the polyurethane drug release controlling body has wide application prospect in treatment on postoperation pain, organ infection, local tumor and other diseases; and the preparation method is simple and mature and is easy to grasp.
Owner:SICHUAN UNIV
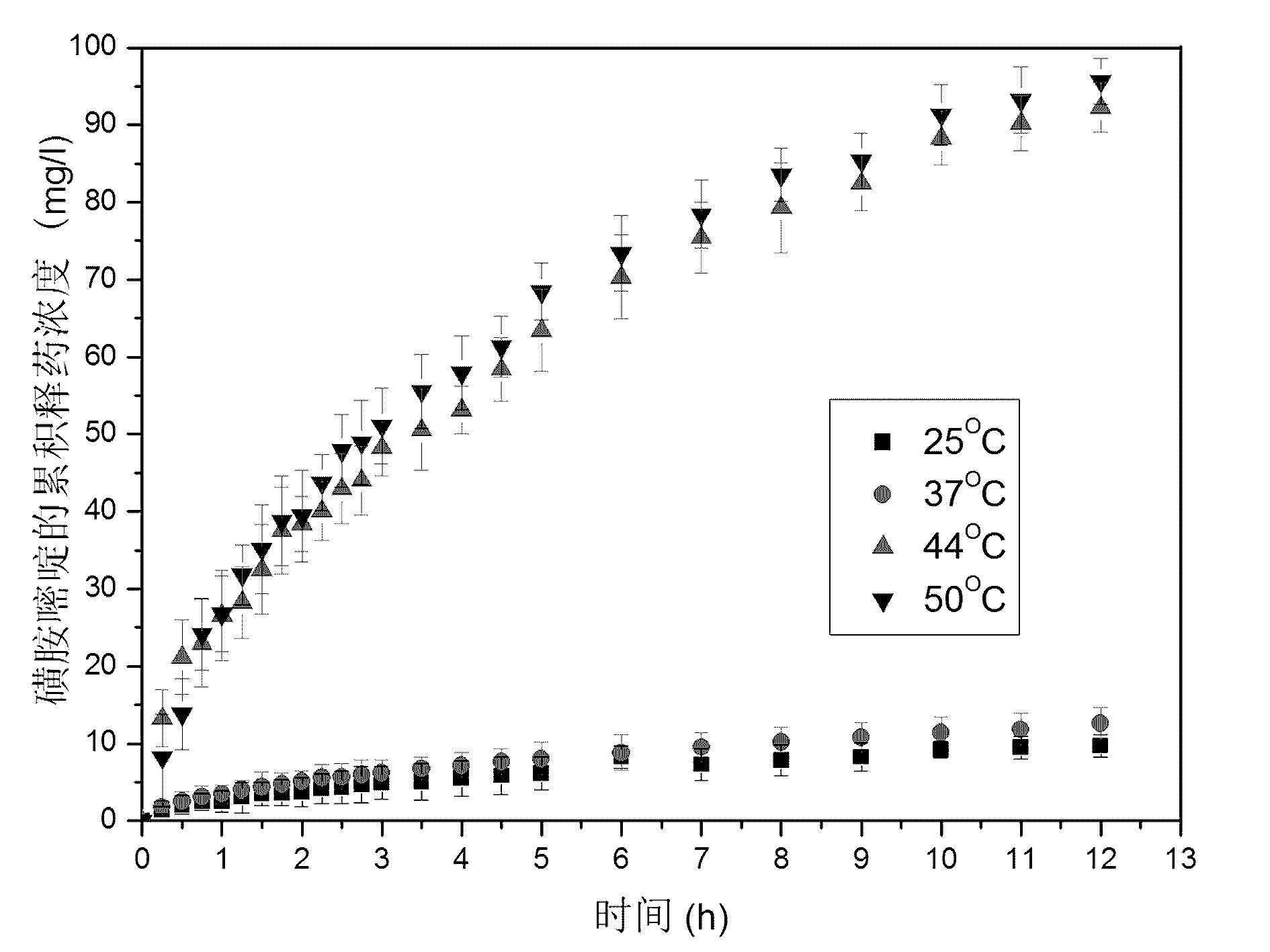
Capsule polyurethane drug controlled release body with temperature control switch, and preparation method thereof
InactiveCN103223173AAchieve releaseAchieve closurePharmaceutical non-active ingredientsPharmaceutical active ingredientsControl releaseMedicine
The invention discloses a capsule polyurethane drug controlled release body with a temperature control switch. The capsule polyurethane drug controlled release body is prepared by loading the drug by a temperature-sensitive polyurethane capsule pipe. In the application of the capsule polyurethane drug controlled release body, a drug release characteristic with temperature response is expressed; and an 'open-close' effect is present. The capsule wall polyurethane molecules are in a rigid crystalline state below the switch temperature, so that diffusion and release of the drug in the controlled release body are blocked; when the temperature rises to higher than the switch temperature, the polyurethane molecules are in a molten state, so that the drug in the body migrates to the capsule wall and the capsule wall drug release outward continuously, with the concentration as a mass ladder force. Besides, the special design of the capsule wall can realize ideal drug diffusion capacity. The invention also discloses a preparation method of the capsule polyurethane drug controlled release body. The temperature-sensitive polyurethane capsule pipe of the drug controlled release body and unsymmetrical structure of the capsule wall design makes the drug controlled release body have fast temperature response speed, good repeatability of the drug release switch, freely adjustable drug release amount, capacity of uniform drug release, no physiological toxicity, so that the drug controlled release body has wide application prospects in clinic local drug treatment.
Owner:SICHUAN UNIV
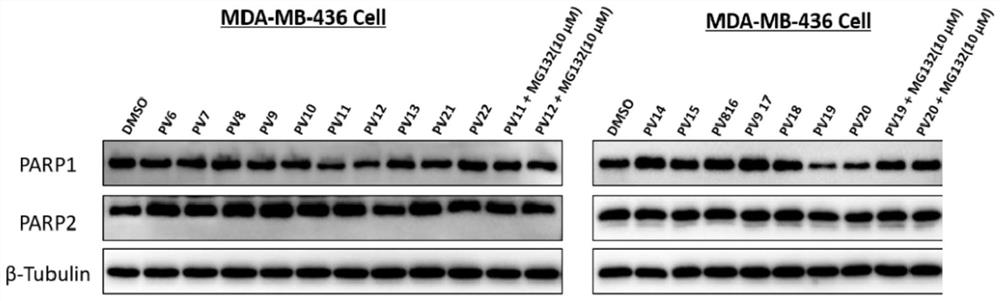
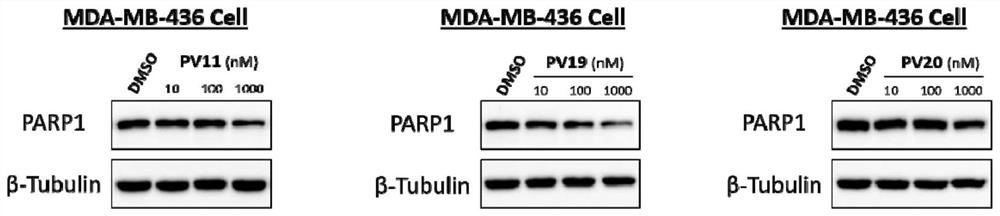
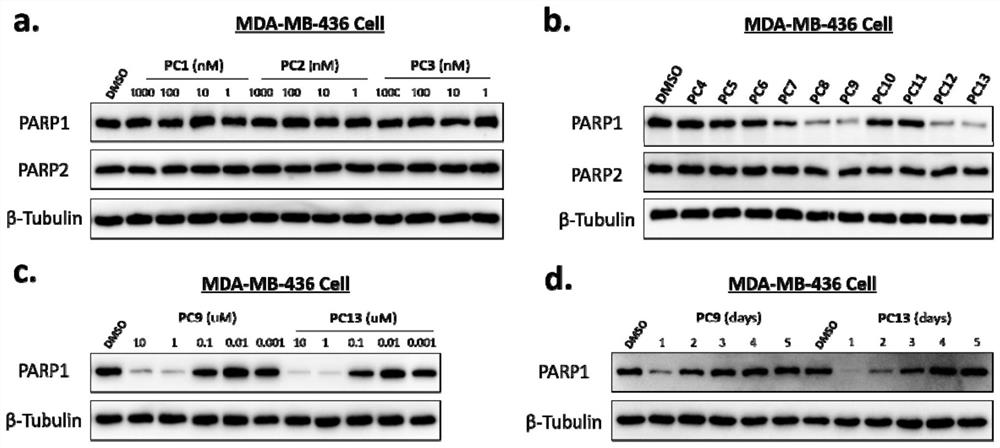
PARP1 protein degradation agent and application thereof in tumor resistance
ActiveCN111606969AEfficient degradationPrevent proliferationOrganic active ingredientsHeavy metal active ingredientsUbiquitin ligaseEfficacy
The invention discloses a PARP1 protein degradation agent and application of the PARP1 protein degradation agent in tumor resistance. The degradation agent comprises a compound with a structural formula shown in the specification, wherein L is a hydrophobic connection unit; and B is an E3 ubiquitin ligase ligand. The PARP1 protein degradation agent prepared by the invention can effectively degradePARP1 protein, inhibit cell proliferation and induce tumor cell apoptosis. Meanwhile, when the PARP1 protein degradation agent is combined with chemotherapeutic drugs, the effect of enhancing the chemotherapeutic efficacy is achieved, and almost no physiological toxicity exists; and the compound is expected to provide an ideal way for treatment of various diseases caused by PARP1 excessive activation.
Owner:SICHUAN UNIV
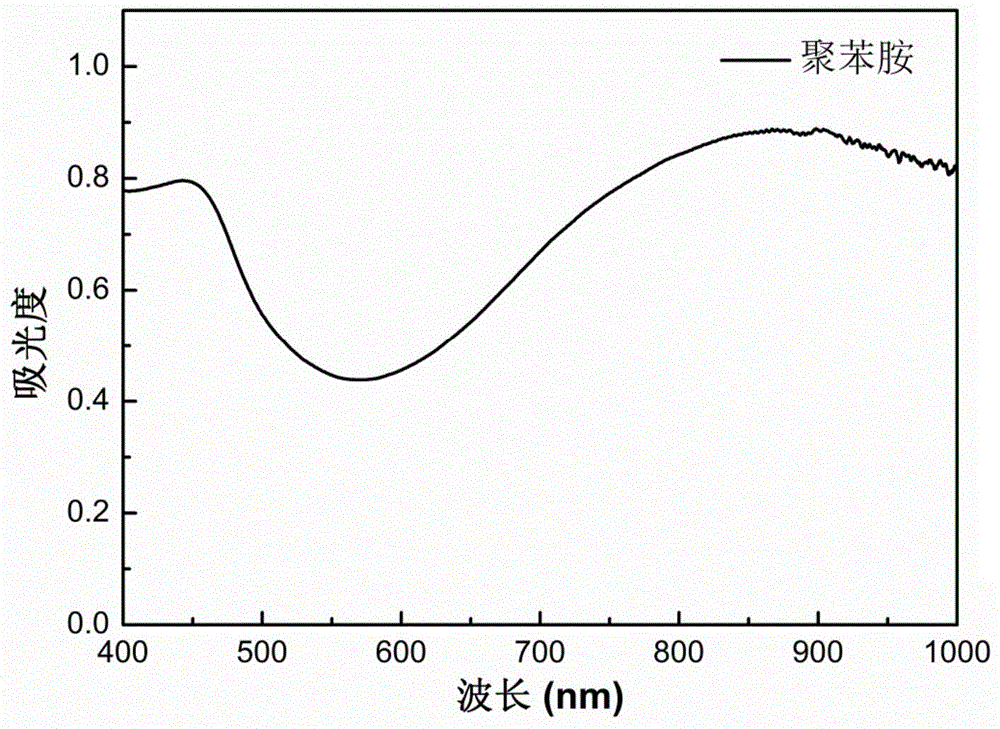
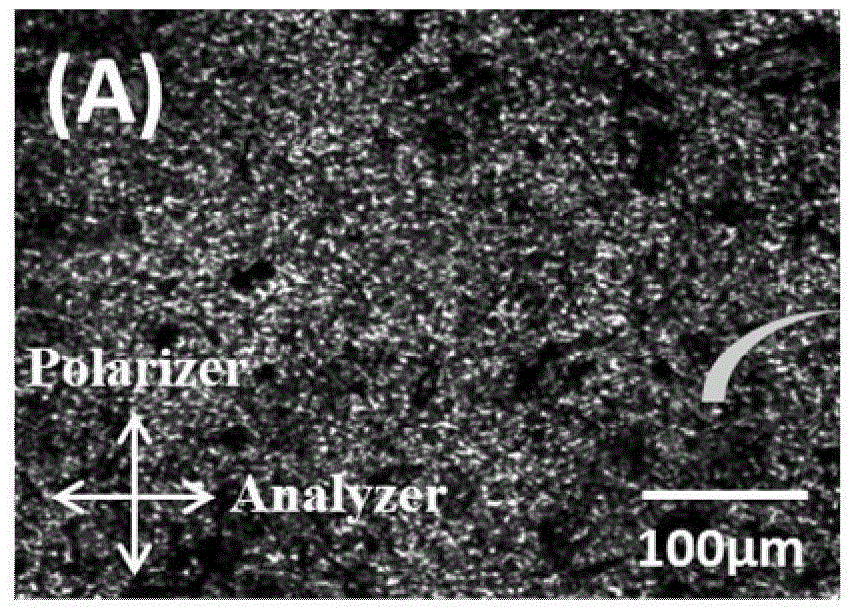
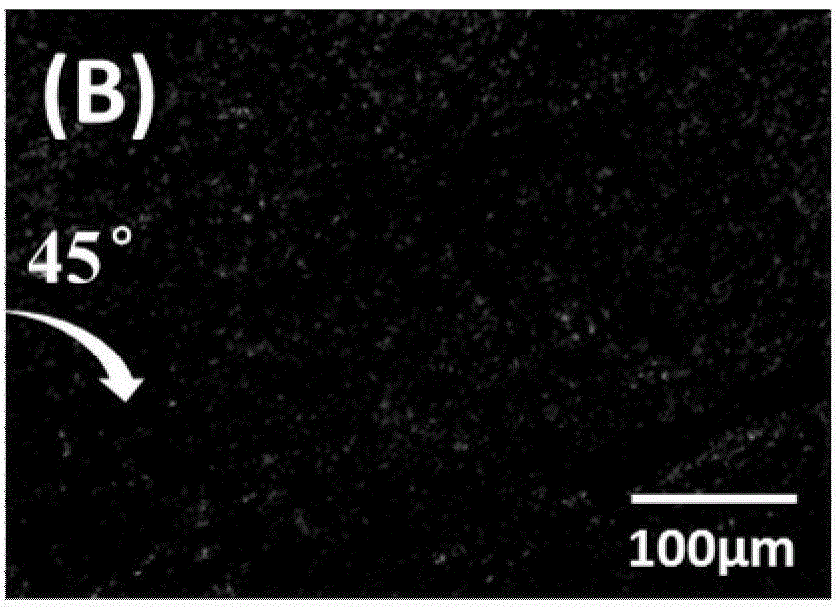
Polyaniline/liquid crystal elastomer composite membrane material and preparation method thereof
The invention discloses a polyaniline / liquid crystal elastomer composite membrane material and a preparation method thereof. The polyaniline / liquid crystal elastomer composite membrane material is a conjugated polyaniline doped liquid crystal elastomer polymer, polysiloxane is taken as a main chain in the liquid crystal elastomer polymer, a certain amount of a catalyst is added into a monomer with an ethylenic bond at a single end and a cross-linking agent with ethylenic bonds at two ends so as to prepare the liquid crystal elastomer composite membrane material in a two-step cross-linking manner under a heating condition, and conjugated polyaniline is doped in different ratios in the preparation process. Conjugated polyaniline is a photo-thermal conjugated polymer which is high in photo-thermal conversion efficiency, good in stability and free of biotoxicity, has an intensive absorbing peak 800 nm near a near-infrared region, and can very intensively absorb near-infrared light. The polyaniline / liquid crystal elastomer composite membrane material disclosed by the invention can be applied to fields such as solar photo-thermal conversion, shape memory materials, biosensors and medicines.
Owner:SOUTHEAST UNIV
Power relay equipment for insulating building
InactiveCN106519874APlay the role of positioningImprove insulation performanceInsulatorsElectromagnetic relay detailsEngineeringElectrical impulse
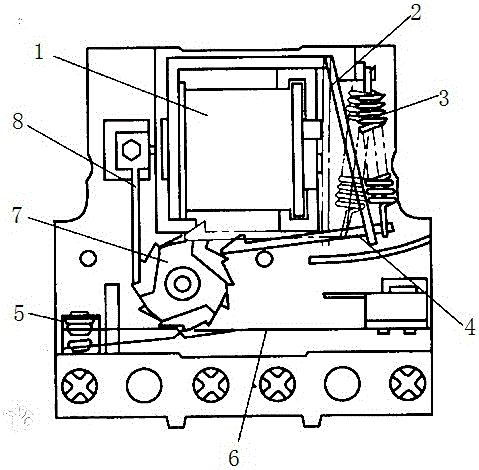
The invention discloses power relay equipment for an insulating building. The power relay equipment comprises a base, a coil, an armature, a restoring spring, a push rod, contacts, a lower plate spring, a middle ratchet wheel and an upper plate spring, wherein an insulating shell is arranged above the base; an iron core is arranged in the insulating shell through a bracket; the coil is arranged on the iron core; the bracket is connected with the restoring spring. The power relay equipment for the insulating building, disclosed by the invention, is simple in structure and convenient to operate; the armature attracts the iron core under the action of electromagnetic attraction to drive the push rod to rotate the middle ratchet wheel anticlockwise; the plate springs move, so that a contact state of upper and lower layers of the contacts is changed; when driving electric pulse disappears, the armature returns to an initial state under the action of the restoring spring, a ratchet abuts against a tooth surface on a seedling wheel and the ratchet wheel does not rotate, so that the contacts can keep the state unchanged; only if the quantity of teeth and tooth shapes of upper and lower layers of ratchet wheels are changed, 2 to 4 steps form one cycle of 1 to 2 contacts, and different control functions are realized.
Owner:芜湖浩权建筑工程有限公司
Method for preparing amorphous layer of bulk material on surface of magnesium alloy
The invention discloses a method for preparing an amorphous layer of a bulk material on the surface of magnesium alloy. After the surface of the magnesium alloy is polished, the amorphous layer is formed on the surface of the magnesium alloy by laser modification. A coating obtained by the method has an amorphous structure, and can effectively solve the problem of excessive degradation rate of themagnesium alloy. The amorphous layer is derived from the bulk material, and can ensure that the coating is not physiologically toxic. The coating is tightly bonded to a magnesium alloy matrix, and has good biocompatibility and corrosion resistance.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
Process for producing chloro-fatty acid methyl ester by utilizing biodiesel
InactiveCN110317135ALow priceHigh flash pointOrganic compound preparationCarboxylic acid esters preparationPlasticizerNitrogen
The invention discloses a process for producing chloro-fatty acid methyl ester by utilizing biodiesel. The process for producing the chloro-fatty acid methyl ester by utilizing the biodiesel comprisesthe synthetic steps that step 1-6, and in the reaction preparation for the producing the chloro-fatty acid methyl ester by utilizing the biodiesel, the preparation of a preparation reaction is carried out through step 2-5, so that the cost of the preparation through multiple steps is reduced, the cost of capital input is reduced, and preparation equipment is reduced, so that the labor cost is effectively reduced, and the preparation efficiency is effectively improved; in the preparation, protection is carried out through nitrogen, so that a reaction with an air substance is avoided, and the reaction is more efficient and sufficient; the chloro-fatty acid methyl ester prepared through the four steps has good advantages of low cost, high flashing point, low condensation point, no physiological toxicity and the like, has outstanding comprehensive performance, and has more market competition compared with a common plasticizer DOP.
Owner:HEBEI JINGU RECYCLING RESOURCES DEV
Doxorubicin hydrochloride self-assembled polymer nanoparticle and preparation method thereof
ActiveCN107126426AThe prescription process is simpleImprove securityOrganic active ingredientsPharmaceutical non-active ingredientsRelease timeTherapeutic effect
The invention discloses a doxorubicin hydrochloride self-assembled polymer nanoparticle which is prepared from doxorubicin hydrochloride and an amphipathic high-molecular polymer PLGA (Polylactic-co-Glycolic Acid), wherein the PLGA is formed by two monomers, namely lactic acid (LA) and glycolic acid (GA). The preparation method comprises the following steps: 1) dissolving a drug doxorubicin hydrochloride into a polar organic solvent A so as to obtain a doxorubicin hydrochloride solution; 2) dissolving the PLGA into a polar organic solvent B so as to obtain a PLGA solution; 3) uniformly mixing the doxorubicin hydrochloride solution with the PLGA solution so as to obtain an eutectic solution, dripping the eutectic solution into an aqueous phase under the stirring condition, continuously stirring, and volatilizing the organic solvent; and 4) centrifuging the completely volatilized solution at a high speed by using a centrifugal machine, and collecting the precipitate, thereby obtaining the doxorubicin hydrochloride self-assembled polymer nanoparticle. The prepared product can achieve the effects of reducing the drug toxicity, reducing the administration frequency, improving the drug stability, delaying in-vivo release time of the drug, reducing adverse reactions of the drug, improving the bioavailability and efficacy of the drug and achieving excellent treatment effects.
Owner:LIAONING UNIVERSITY
High-flash-point electric spark working fluid
The invention relates to a high-flash-point electric spark working fluid. The invention is characterized in that the working fluid is prepared from the following steps: (1) dissolving 1 part of trisodium phosphate, 5 parts of ammonium borate, 3 parts of magnesium oxide, 1 part of sodium metasilicate, 1 part of polypropylene glycol and 4 parts of lauric acid in deionized water; (2) uniformly stirring 23 parts of coconut oil fatty acid, adding into the solution in the step (1) while stirring, and stirring uniformly; (3) adding 2 parts of N-amidoamino acid into the solution in the step (2), continuing stirring for 20 minutes, finally adding 0.5 part of polyoxyethylene polyoxypropylene pentaerythritol ether, and inspecting to obtain the qualified electric spark wire working fluid. The working fluid has the function of environment protection, can not cause the problem of environmental pollution, does not have physiological toxicity, and is an ideal environment-pollution-free novel complete-synthesis electric spark wire cutting metal working fluid.
Owner:天津中澳星环保科技发展有限公司
Preparation method of moisture-proof traditional Chinese medicine coated film
InactiveCN104587481AChemically stableDry fastPharmaceutical delivery mechanismOil/fats/waxes non-active ingredientsSugar Coated TabletMoisture resistance
The invention discloses a preparation method of a moisture-proof traditional Chinese medicine coated film, which relates to a coating technology of the traditional Chinese medicine and belongs to the medicinal preparation field. The moisture-proof traditional Chinese medicine coated film comprises the following components: 30-40 parts of hydroxypropyl methyl cellulose, 5-8 parts of triethyl citrate, 6-10 parts of xylitol, 4-8 parts of castor oil, 4-8 parts of tween-80, 14-18 parts of dextrin, 6-10 parts of aerosol, 6-10 parts of zein, 5-10 parts of 1% of artificial food coloring solution, 350-420 parts of distilled water (40-50 DEG C) and balance of ethanol with 95% of concentration; wherein weight unit of solid is gram, and volume unit of liquid is milliliter. The moisture-proof traditional Chinese medicine coated film has the beneficial effect of stable chemical property of a film forming substance, inertia to an enzyme system, no physiological toxicity and no usage of cane sugar; the coated film has the advantages of moisture resistance, heat resistance, cold resistance and abrasion resistance which are better than that of the sugar coated tablets, so that the stability of the coated film is increased, and storage period is prolonged.
Owner:刘桐言
Water-dispersive nano-grade ibuprofen injecta and preparing method
InactiveCN1742714ANo hidden danger of pollutionNo physiological toxicityOrganic active ingredientsPowder deliveryWater dispersibleDouble bond
The present invention uses polysaccharide and polysaccharide containing double bond as covering material of medicine, controls the ratio of medicine and polysaccharide and adopts co-condensation-polymerization technique to obtain the water-dispersible brufen injection preparation whose grain-size is 20-1000nm.
Owner:ZHEJIANG UNIV
Method for preparing calcium lysine chelate with shell of Argopecten irradias as calcium source
ActiveCN106631848AConsiderable yieldAppreciable calcium contentMetabolism disorderOrganic compound preparationAlcoholCentrifugation
The invention discloses a method for preparing a calcium lysine chelate with the shell of Argopecten irradias as a calcium source. The method comprises the following steps: treatment of the shell of Argopecten irradias: successively subjecting the shell of Argopecten irradias to conventional cleaning, air drying, crushing, charing and ashing; ash content treatment: treating the ash content of the shell with a slightly excess HCl solution so as to obtain a CaCl2 solution; mixing reaction: heating and mixing the CaCl2 solution and lysine under stirring so as to realize chelating; centrifugation: carrying out centrifugation and retaining a supernatant; evaporative concentration: subjecting a reaction solution to slow heating for evaporative concentration; alcohol precipitation: purifying a calcium lysine chelate obtained in the previous step by using absolute ethyl alcohol; and drying: collecting a gel precipitate, repeatedly washing the gel precipitate with absolute ethyl alcohol and then carrying out drying at a constant temperature of 60 to 80 DEG C so as to obtain the calcium lysine chelate. The preparation method for the calcium lysine chelate in the invention uses processing waste of Argopecten irradias, i.e., the shell, and the essential amino acid lysine as raw materials and prepares the calcium lysine chelate through water-phase synthesis; and the method is low in cost, good in chelation yield and suitable for large-scale batch production.
Owner:HEBEI AGRICULTURAL UNIV.
Cell-penetrating peptide and preparation method and application thereof
ActiveCN109734780AMature technologyNo physiological toxicityOrganic active ingredientsNervous disorderAmino acidToxicity
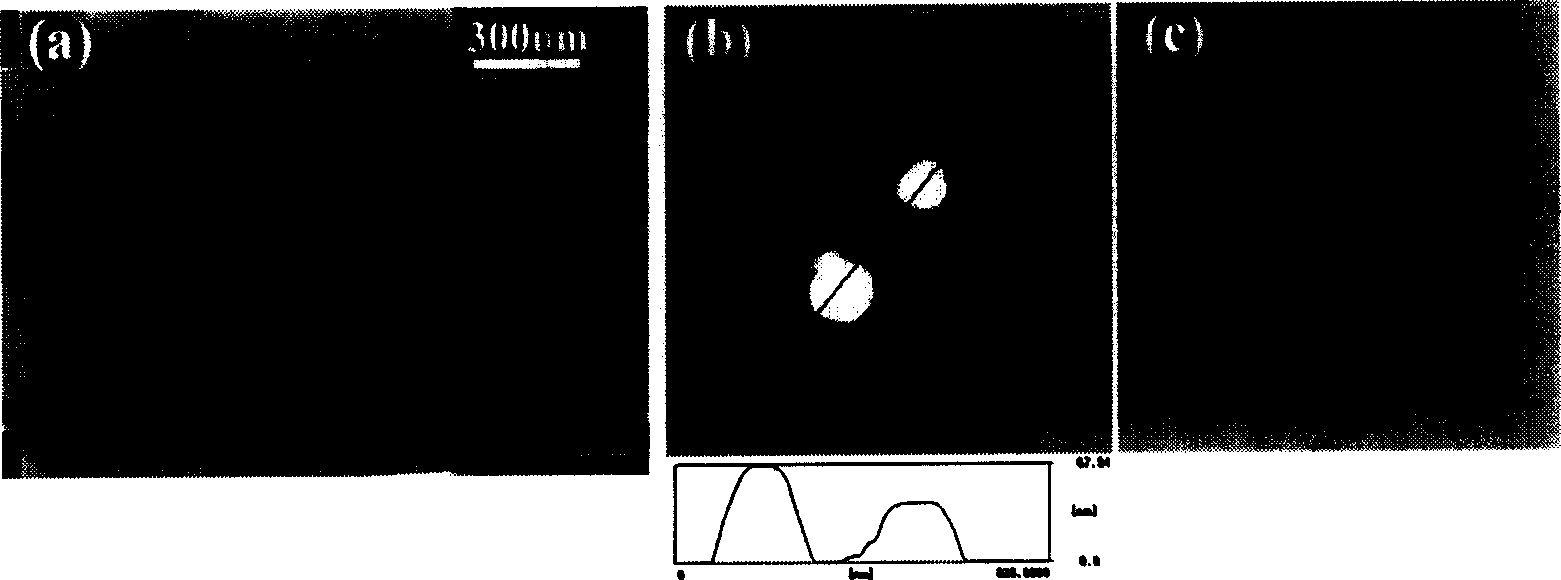
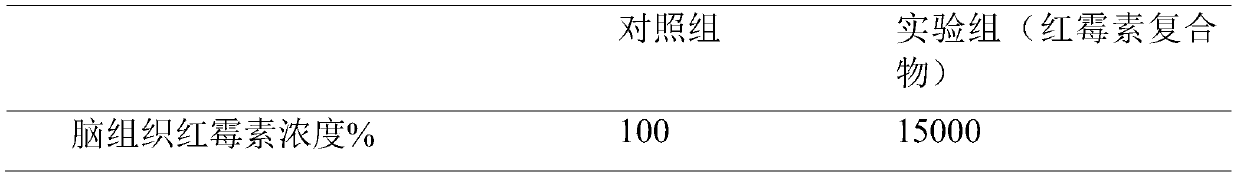
The invention relates to the technical field of biology, in particular to a cell-penetrating peptide and a preparation method and application thereof. The amino acid sequence of the cell-penetrating peptide is Arg-Arg-Leu-Ser-Tyr-Thr-Lys-Lys-Lys-Trp-Trp-Pro. The cell-penetrating peptide is free of physiological toxicity and can be combined with erythrocin under the non-covalence effect, and the erythrocin is effectively mediated for penetrating a blood brain barrier.
Owner:NANYANG NORMAL UNIV
Mechanical electro-spark wire-electrode cutting fluid and preparation method thereof
InactiveCN106675746ANo physiological toxicityDoes not cause pollution problemsLubricant compositionSodium metasilicateStearic acid
The invention discloses a mechanical electro-spark wire-electrode cutting fluid and a preparation method thereof. The mechanical electro-spark wire-electrode cutting fluid comprises the following raw materials in parts by weight: a spark discharge modifier, an anti-rust agent, sodium carbonate, calcium chloride, dodecanoic acid, hydroxypropyl methyl cellulose, stearic acid, a chip clearing agent, sodium tetraborate, sodium metasilicate, petroleum sodium sulfonate, ricinolein, sulfonated oil, oxalic tetraethylammonium, deionized water and polypropylene glycol. The spark discharge modifier in the mechanical electro-spark wire-electrode cutting fluid can control the spark discharge under large current; the arc extinguishing effect is good; the components of the mechanical electro-spark wire-electrode cutting fluid are not sintered under an instant high temperature; the mechanical electro-spark wire-electrode cutting fluid has excellent cooling property, lubricating property, anti-rust property and chip removal property; the mechanical electro-spark wire-electrode cutting fluid has a function of environmental protection, will not result in environmental pollution problem and is free from physiological toxicity.
Owner:HARBIN UNIV OF SCI & TECH
Method for preparing electrical discharge machining liquid
InactiveCN105567391ANo physiological toxicityEnvironmental performanceLubricant compositionStearic acidPollution
The invention relates to a method for preparing electrical discharge machining liquid. The method includes steps: (1) dissolving 1 part of ammonium borate, 2 parts of sodium carbonate, 1 part of calcium chloride, 4 parts of lauric acid and 1 part of hydroxypropyl methyl cellulose in water; (2) well stirring 22 parts of polypropylene glycol compatibly, and adding into a solution obtained at the step (1); (3) after 30 minutes, adding 2 parts of stearic acid into a solution obtained at the step (2), stirring for 20 minutes, and finally adding 0.1 part of silicone emulsion to obtain the electrical discharge machining liquid after quality inspection is passed. All additives of the novel completely-synthesized wire cut electrical discharge machining liquid are additives high in biodegradation speed, so that the machining liquid is environment friendly and avoids the problem of environment pollution. In addition, the machining liquid is free of physiological toxicity, thereby being novel completely-synthesized wire cut electrical discharge machining liquid which is favorable and free of environment pollution.
Owner:天津中澳星环保科技发展有限公司
Electric spark machining liquid and preparation method of electric spark machining liquid
InactiveCN106753714ADoes not cause pollution problemsEnvironmental performanceLubricant compositionSodium metasilicateSolvent
The invention discloses electric spark machining liquid and a preparation method of the electric spark machining liquid. The electric spark machining liquid is prepared from the following formula components in parts by mass: 6 to 8 parts of ammonium borate, 1 to 2 parts of an additive, 6 to 8 parts of sodium tetraborate, 4 to 8 parts of sodium carbonate, 6 to 8 parts of calcium chloride, 7 to 9 parts of coconut oil fatty acid, 5 to 7 parts of alkylol amine, 6 to 8 parts of dodecanoic acid, 4 to 6 parts of C14, 7 to 9 parts of oleic acid, 3 to 5 parts of hydroxyl propyl methyl cellulose, 1 to 3 parts of solvent oil, 3 to 5 parts of polypropylene glycol and 4 to 6 parts of sodium metasilicate. The electric spark machining liquid has environment-friendly effect and does not cause the environment pollution problem; the machining liquid has no physiological toxicity and is relatively ideal novel fully synthetic electric spark incision metal machining liquid which has no pollution to the environment.
Owner:QINZHOU UNIV
Copper-titanium corrosive liquid for integrated circuit and production process of copper-titanium corrosive liquid
The invention discloses a copper-titanium corrosive liquid for an integrated circuit, and belongs to the technical field of microelectronic chemical reagents. The copper-titanium corrosive liquid comprises, by mass, 10-19% of sulfuric acid, 5-8% of nitric acid, 28-33% of acetic acid, 2-5% of potassium persulfate, 2-4% of hydrogen peroxide, 0.05-0.1% of adsorbent and the balance pure water. The invention also discloses a production process of the corrosive liquid. The corrosion liquid is uniform in corrosion to a copper-titanium composite metal layer and stable in performance, due to the fact that the adsorbent is added, the corrosive liquid can be purified, the generation of waste corrosive liquid is reduced, the service life of the corrosive liquid is prolonged, the corrosive liquid does not contain fluorine, a silicon base material, silicon nitride and non-crystalline silicon cannot be corroded, meanwhile, the product yield, safety and environmental friendliness are considered, the risk of defects after reworking is reduced, and the production process can be widely applied to the fields of the preparation of integrated circuit industry, flat panel displays, color filters, touch panels, organic light-emitting diodes and the like.
Owner:JIANGYIN RUNMA ELECTRONICS MATERIAL
Metal machining liquid
InactiveCN103013641AFast biodegradationThe rate of biodegradation hasLubricant compositionEnvironmental resistanceCalcium magnesium
The invention relates to a metal machining liquid which comprises the following elements in parts by weight: 65 parts of deionized water, 25 parts of a lubricant additive, 1 part of an antirust agent, 1 part of a calcium magnesium ion softener, 5 parts of lauric acid, 2 parts of polyvinyl ether, 0.1 parts of high alcohol and 2 parts of a surfactant. All the additives in the prescription of the metal machining liquid provided by the invention are the additives with rather fast biological degradation speed, so the machining liquid is environment-friendly and does not cause environmental pollution; besides, the machining liquid has no physiological toxicity, so the machining liquid is a rather ideal metal machining liquid friendly to the environment.
Owner:HAO YU ADDITION AGENT
Water-dispersive nano-grade ibuprofen injecta and preparing method
InactiveCN100348181CNo hidden danger of pollutionNo physiological toxicityOrganic active ingredientsPowder deliveryWater dispersibleDouble bond
The present invention uses polysaccharide and polysaccharide containing double bond as covering material of medicine, controls the ratio of medicine and polysaccharide and adopts co-condensation-polymerization technique to obtain the water-dispersible brufen injection preparation whose grain-size is 20-1000nm.
Owner:ZHEJIANG UNIV
A treatment method for the solidification process of nano-suspension
ActiveCN105902496BInhibit aggregationGuaranteed Particle SizePowder deliverySulfonylurea active ingredientsPharmaceutical formulationSURFACTANT BLEND
Owner:SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com