Cell-penetrating peptide and preparation method and application thereof
A technology for solid-phase synthesis of membrane-penetrating peptides and polypeptides, applied in the biological field, can solve the problems of erythromycin being difficult to penetrate, and achieve the effects of easy production and promotion, mature technology, and expanded application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Synthesis of Penetrating Peptides
[0018] 1) Activation of the resin: Weigh 1500mg of Fmoc-Pro wang Resin, add 25mL of DMF and soak for 10min to make it fully swell.
[0019] 2) Deprotection: Remove the DMF soaking the resin by suction filtration, add 25mL DMF solution containing 20% piperidine, blow and boil with nitrogen for 25min, then remove by suction filtration, wash the resin three times alternately with 25mL isopropanol and DMF, and then use ninhydrin The resin should be black or purple when detected by the method.
[0020] 3) Condensation reaction: connect the next amino acid, weigh 1.4mmol / g resin Fmoc-amino acid, use 910mgTBTU, 0.45g HOBt and 0.52ml DIEA in 25mL DMF as the reaction solution, and react with nitrogen blowing at room temperature for 3h. After the reaction was completed, the resin was alternately washed three times with 25 mL of isopropanol and DMF. Detect amino groups.
[0021] 4) Repeat steps 2)-3) process: extend the polypepti...
Embodiment 2
[0024] Embodiment 2: Cytotoxicity experiment
[0025] 1) Take a 96-well plate and add 7×10 3 Cervical cancer Hela cell culture medium, 37 ° C, 5% carbon dioxide incubator for 36 hours, so that the cells adhere to the wall.
[0026] 2) A medium containing a penetrating peptide at a concentration of 1 mM was prepared, and a medium without a penetrating peptide was used as a negative control well (Control), and cultured at 37° C. with 5% carbon dioxide for 48 hours.
[0027] 3) Add 20 μl MTT to each well of adherent cells, discard the culture medium after continuing to incubate for 2 hours, add 150 μl DMSO to each well, and shake for 15 minutes.
[0028] 4) Select a wavelength of 490nm, and calculate the cell survival rate by the light absorption value on the microplate reader immunodetector.
[0029] Table 1. Penetrating peptide toxicity test
[0030]
[0031] It can be seen from Table 1 that the membrane-penetrating peptide of the present invention has no obvious damage t...
Embodiment 3
[0032] Implementation Example 3: Mediated erythromycin delivery experiment
[0033] 1) Preparation of erythromycin complex: adding 5 μg / ml erythromycin and 0.1 mM penetrating peptide in physiological saline and co-incubating for 30 min;
[0034] 2) verify the performance of the resulting erythromycin complex:
[0035] Experimental group: Inject 0.1 ml of the erythromycin complex obtained in step 1) into the mouse tail vein
[0036] Control group: Inject 0.1 ml of normal saline containing only 5 μg / ml erythromycin into the tail vein of mice.
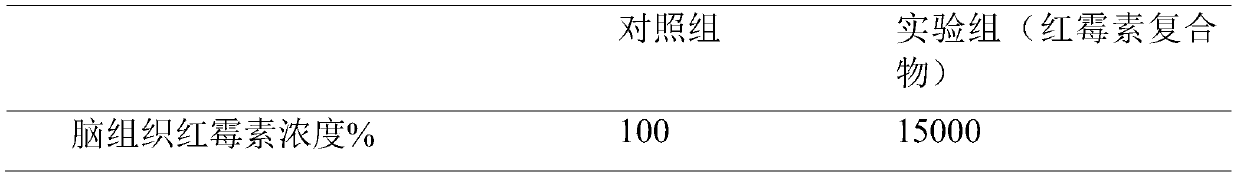
[0037] 3) After 5 minutes of injection, the mice were killed to take brain tissue, the brain tissue was frozen and homogenized, and the erythromycin in the brain tissue was extracted with methanol.
[0038] 4) The concentration of erythromycin in the brain tissue extract was determined by HPLC. Take the content of the control group as a reference (100%)
[0039] Table 2. Penetrating peptide-mediated blood-brain barrier test
[0040] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com