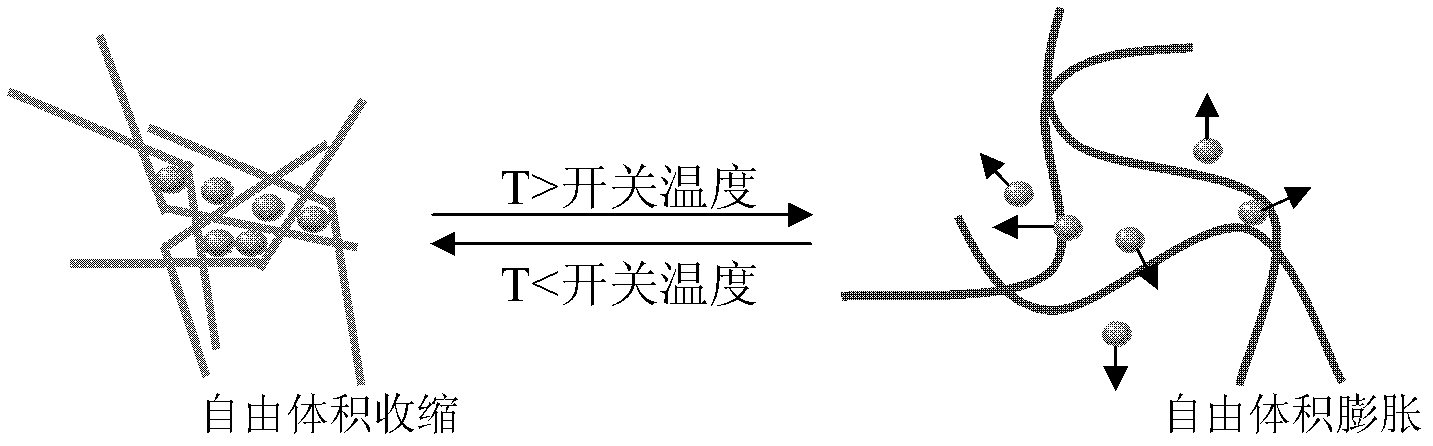
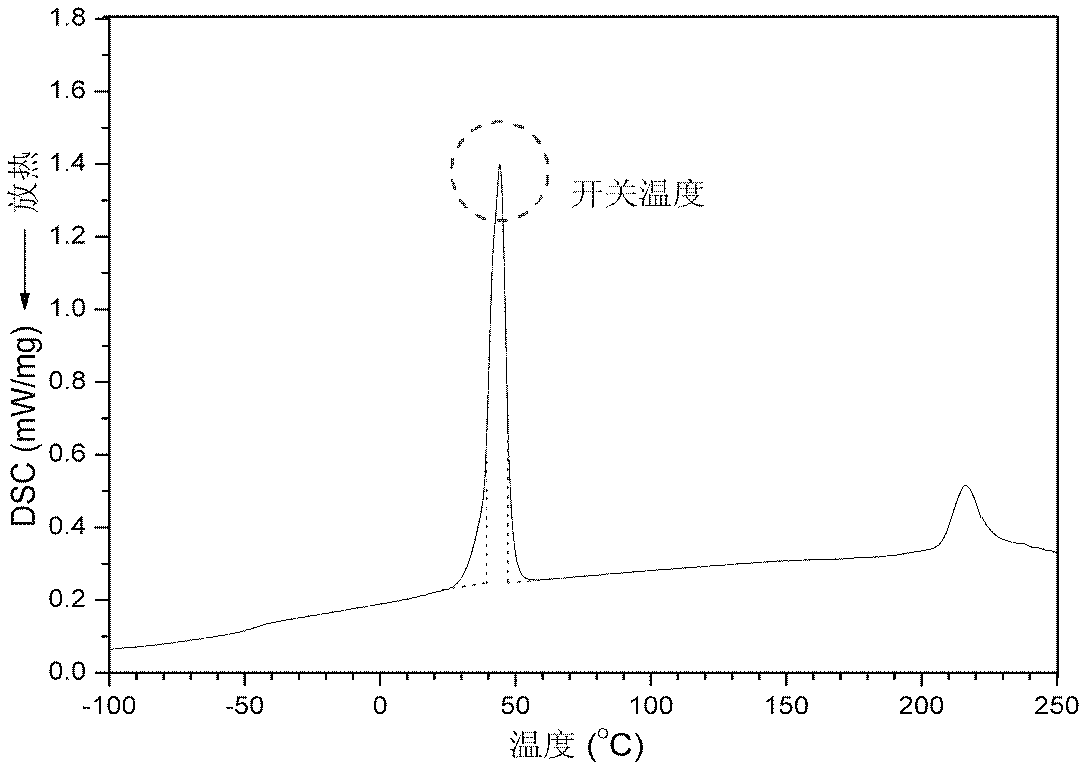
Polyurethane drug release controlling body with thermoswitch and preparation method thereof
A technology of drug controlled release and thermosensitive switch, which is applied in the direction of medical preparations of non-active ingredients, antipyretics, and drug combinations, etc. Insufficient sensitivity, comprehensive performance gap and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 100 parts of polycaprolactone diol with a number average molecular weight of 4000, 12.5 parts of 4,4'-diphenylmethane diisocyanate and 200 parts of N, N-dimethylformamide were added to the reaction vessel, under continuous stirring and nitrogen atmosphere Under protection, raise the temperature of the system to 80°C and react for 2.5 hours to prepare an NCO-terminated prepolymer; 13.2 parts of diol chain extender and 164 parts of dimethyl sulfoxide, and maintained at 80 ° C for 2.3 hours to continue the reaction to prepare a heat-sensitive polyurethane solution; 0.7 parts of paclitaxel was dissolved in N, N-dimethylformamide to Prepare a uniform drug solution; then blend it evenly with 80 parts of heat-sensitive polyurethane solution; after filtering and defoaming, pour the above-prepared mixture into a mold, and place it at 40°C for 48 hours until the solvent slowly volatilizes; Finally, continue to evaporate the residual solvent at 78° C. and a vacuum of 0.7 mmHg unti...
Embodiment 2
[0037] 20 parts of polyethylene adipate diol with a number average molecular weight of 2000, 60 parts of polycarbonate diol with a number average molecular weight of 10000, 8 parts of 4,4'-diphenylmethane diisocyanate and dimethyl Add 180 parts of sulfoxide into the reaction vessel, and under the protection of continuous stirring and nitrogen atmosphere, raise the temperature of the system to 70°C and react for 2.3 hours to prepare the NCO-terminated prepolymer; after the first step of reaction is completed, add 4,4' -29.0 parts of diphenylmethane diisocyanate, 10.0 parts of 1,3-propanediol chain extender and 36 parts of N,N-dimethylformamide, and maintained at 74 ° C for 2.5 hours to prepare a thermosensitive polyurethane solution; Dissolve 0.5 parts of sulfamethoxazole in dimethyl sulfoxide to prepare a uniform drug solution; then blend it with 75 parts of heat-sensitive polyurethane solution; after filtering and defoaming, pour the above-prepared mixture into a mold and pla...
Embodiment 3
[0040] 25 parts of polybutylene adipate diol with a number average molecular weight of 6000, 35 parts of polycaprolactone diol with a number average molecular weight of 8000, 3 parts of 2,4-toluene diisocyanate and N, N-di Add 150 parts of methylformamide into the reaction vessel, and under continuous stirring and under the protection of nitrogen atmosphere, raise the temperature of the system to 77°C for 2 hours to prepare the NCO-terminated prepolymer; after the first step of reaction is completed, add 2, 16.9 parts of 4-toluene diisocyanate, 12.4 parts of 1,6-hexanediol chain extender, and 65 parts of N,N-dimethylformamide were maintained at 70°C for 2 hours to prepare a heat-sensitive polyurethane solution; Dissolve 0.1 part of lidocaine in N,N-dimethylacetamide to prepare a homogeneous drug solution; then blend it with 50 parts of heat-sensitive polyurethane solution; filter and defoam, the above prepared The mixture was poured into a mold, and placed at 30°C for 24 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com