Dendritic fluorine-containing surfactant as well as preparation method and application thereof
A surfactant and solvent technology, applied in the field of polymer surfactants, can solve the problems of complicated post-processing steps, limited environmental humidity synthesis technology, etc., and achieve the effects of reducing production costs, improving experimental safety, and being widely used.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
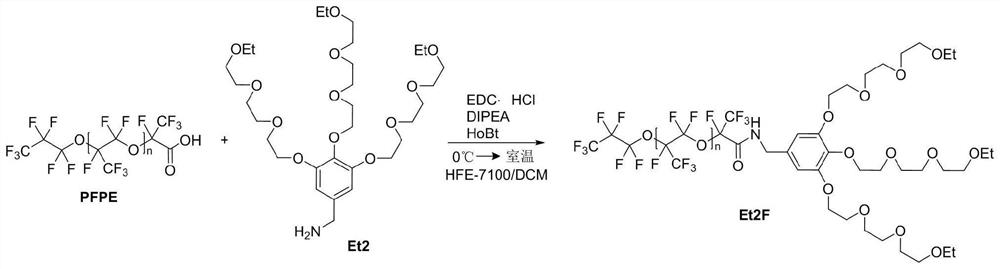
[0041] In the following examples of the present invention, the preparation method specifically includes: providing alkoxy ethers containing perfluoropolyether carboxylic acid (PFPE), terminal amino groups, 1-hydroxybenzotriazole (HoBt), N, N - A mixed reaction system of diisopropylethylamine (DIPEA), 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) and a solvent. A slight excess of alkoxy ether to perfluoropolyether carboxylic acid is used to completely react the carboxylic acid, and the reaction system is mixed in an ice-salt bath, and reacted at room temperature for 12-36 hours, and fully reacted to obtain the fluorine-containing surfactant.
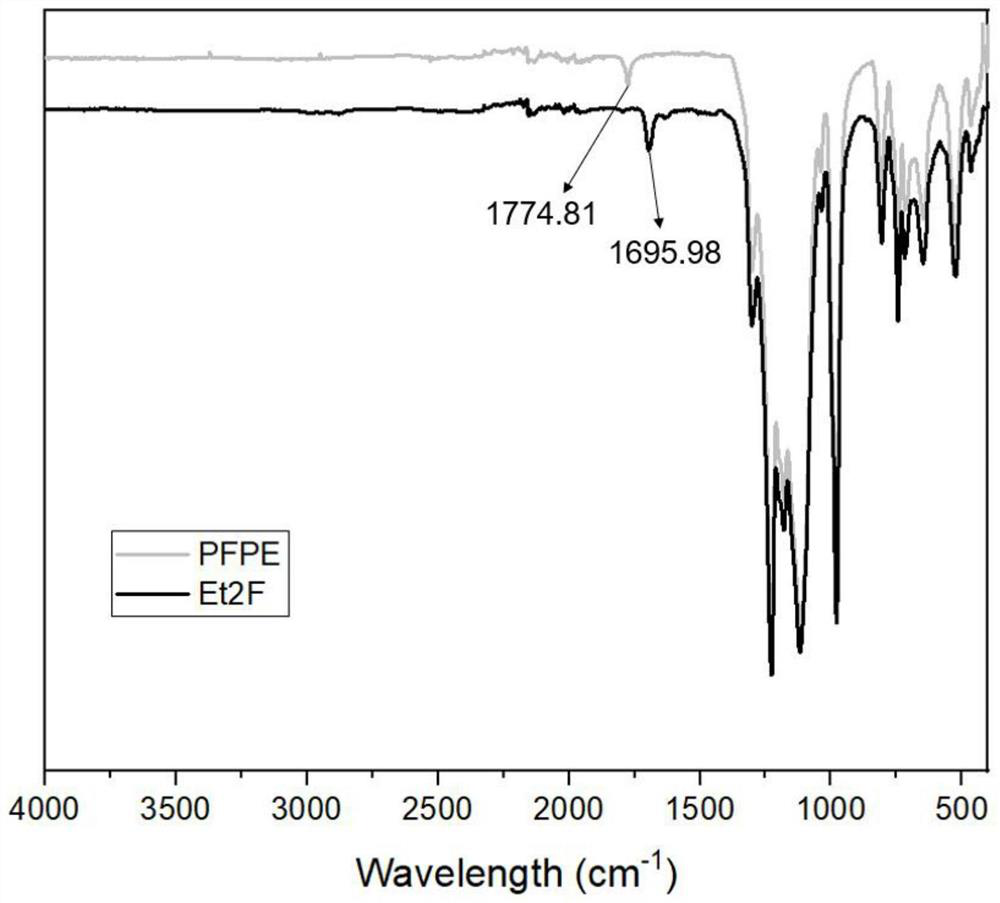
[0042] In the following embodiments of the present invention, the preparation method specifically includes: after mixing all the reactants, take a drop of the mixed solution on the pH test paper, after the solvent evaporates, take an appropriate amount of water to moisten, observe the color range of the pH test paper...
Embodiment 1
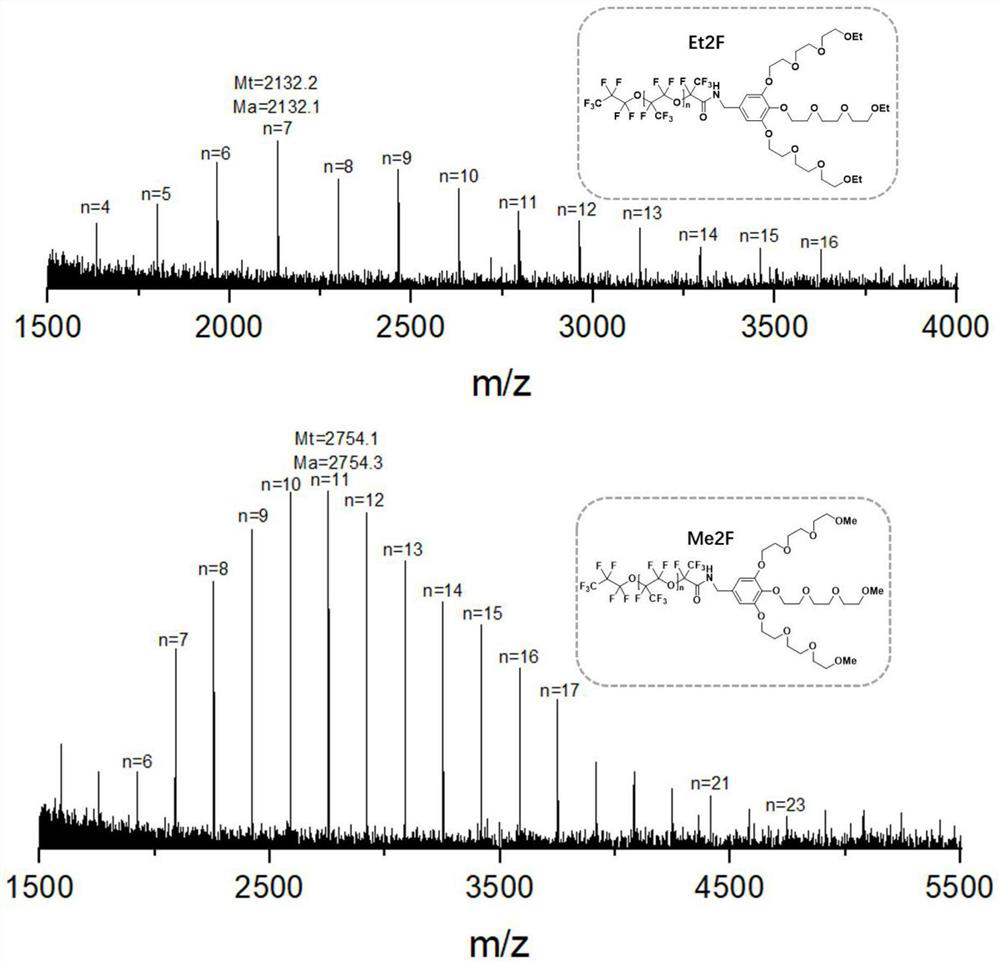
[0055] In this example, 6 g of perfluoropolyether carboxylic acid (PFPE) was weighed in a 250 mL round bottom flask, and 60 mL of methyl nonafluorobutyl ether (HFE-7100) was dissolved to form a solution. Then, 0.2 g of 1-hydroxybenzotriazole (HoBt) was added under an ice-salt bath, followed by stirring. Weighed 2.54g of Et9 and 0.2g of N,N-diisopropylethylamine (DIPEA) successively, dissolved them in 10mL of dichloromethane, and added them dropwise to the round bottom flask. 0.3 g of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl) was added. After stirring evenly, use a glass rod to take a drop of the reaction solution on the pH test paper. After the solvent evaporates, take a few drops of water to moisten it. The color of the pH test paper indicates that it is in the alkaline range. After the bottle was corked and stirred for a period of time, the ice-salt bath was removed, and the reaction was carried out at room temperature for 36 hours. The perfluorop...
Embodiment 2
[0057] This embodiment is basically the same as Embodiment 1, and the special features are:
[0058] In this example, 5 g of perfluoropolyether carboxylic acid (PFPE) was weighed in a 250 mL round bottom flask, and 60 mL of methyl nonafluorobutyl ether (HFE-7100) was dissolved to form a solution. Then, 0.25 g of 1-hydroxybenzotriazole (HoBt) was added under an ice-salt bath, followed by stirring. Weighed 2.48g Me9 and 0.24g N,N-diisopropylethylamine (DIPEA) successively, dissolved them in 10mL of dichloromethane, and added them dropwise to the round bottom flask. 0.36 g of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl) was added. After stirring evenly, use a glass rod to take a drop of the reaction solution on the pH test paper. After the solvent evaporates, take a few drops of water to moisten it. The color of the pH test paper indicates that it is in the alkaline range. After the bottle was corked and stirred for a period of time, the ice-salt bath was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com