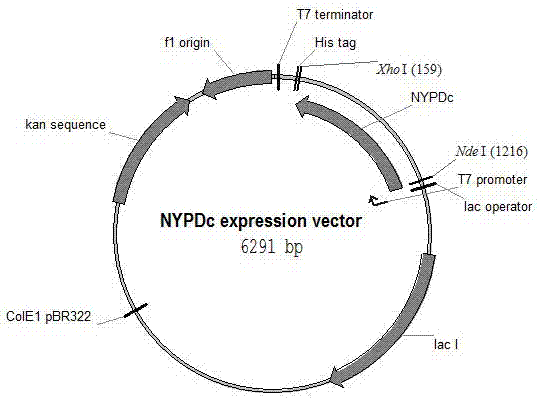
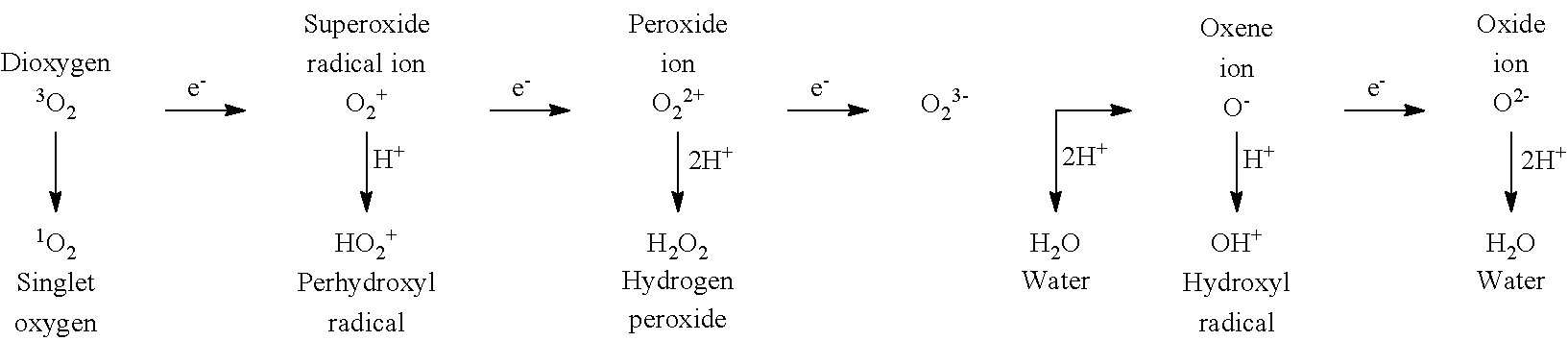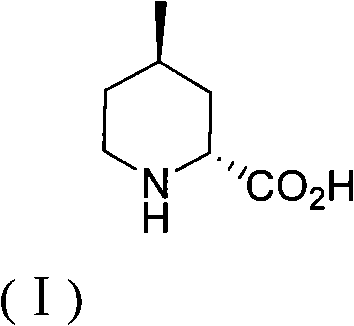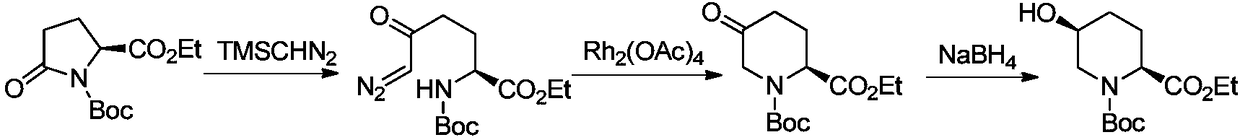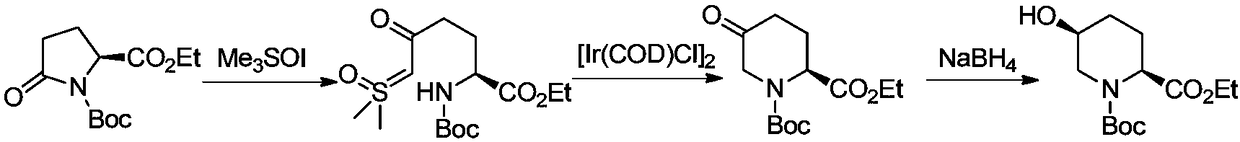Patents
Literature
50 results about "Pipecolic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pipecolic acid (piperidine-2-carboxylic acid) is a small organic molecule which accumulates in pipecolic acidemia. It is a carboxylic acid of piperidine.
Increasing abiotic stress tolerance in plants
InactiveUS20160037772A1Reduced fruit sizeReduce yieldBiocideDead plant preservationPlant hormoneVegetable oil
Methods for treating a plant comprise contacting a plant or a part of a plant with one or more of 9-oxononanoic acid, arachidonic acid, or a salt or ester thereof; wherein the amount is effective to increase tolerance to abiotic stress in the plant or to reduce a consequence of abiotic stress in the plant. Additional ingredients can be included such as dicarboxylic acids, pipecolic acid, or salicylic acid, or salts / esters thereof; sunblocks such as kaolin or calcium carbonate; carriers such as inert powders or liquids; and co-treatment materials such as fertilizers, plant nutrients, biostimulants, micronutrients, amino acids, plant hormones, pesticides, fungicides, insecticides, nematicide, stearic acid, vegetable oil, or phospholipid.
Owner:GUERRERO MENDEZ MARIO MIGUEL
Hiv Prodrugs Cleavable by Cd26
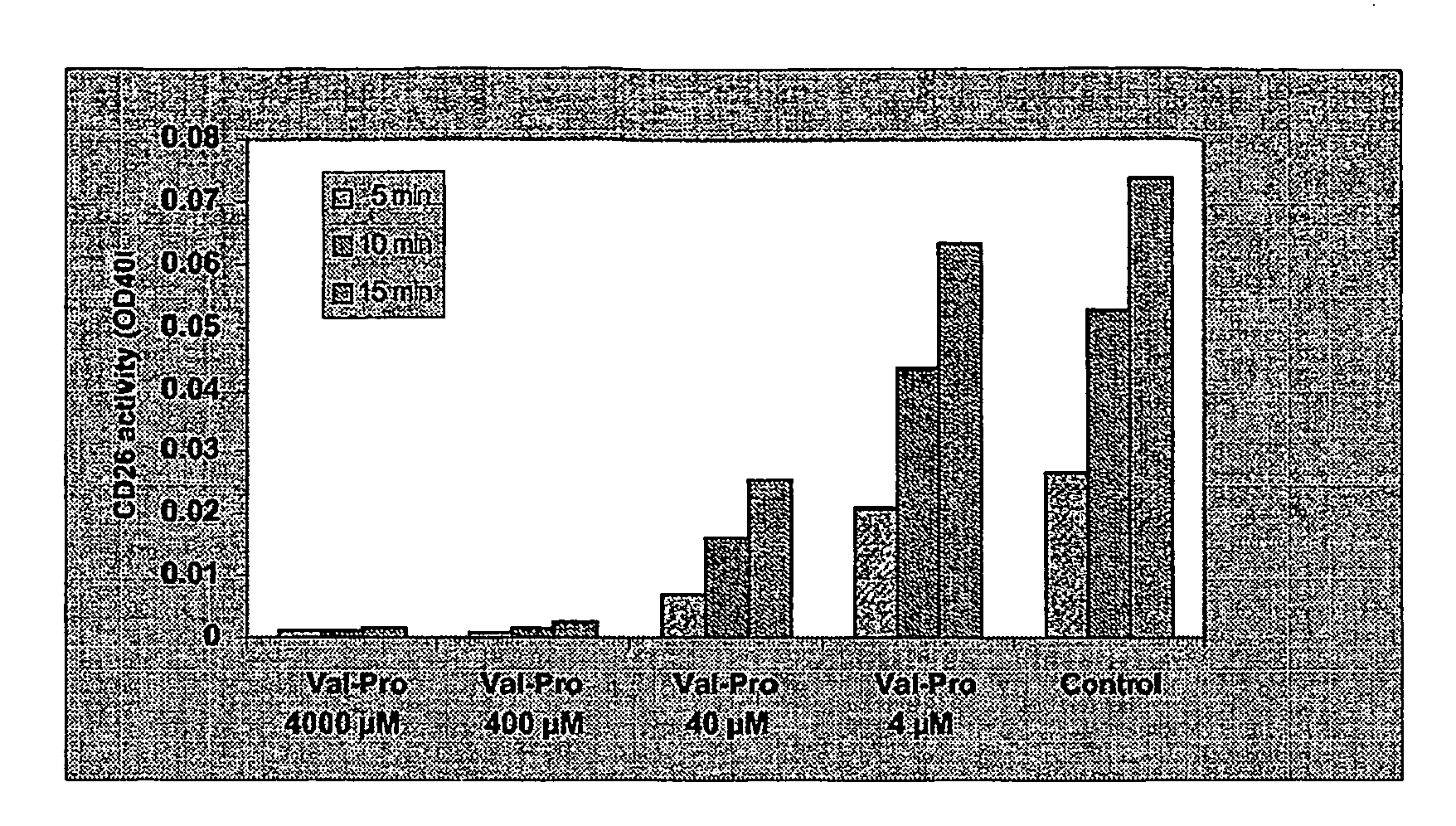
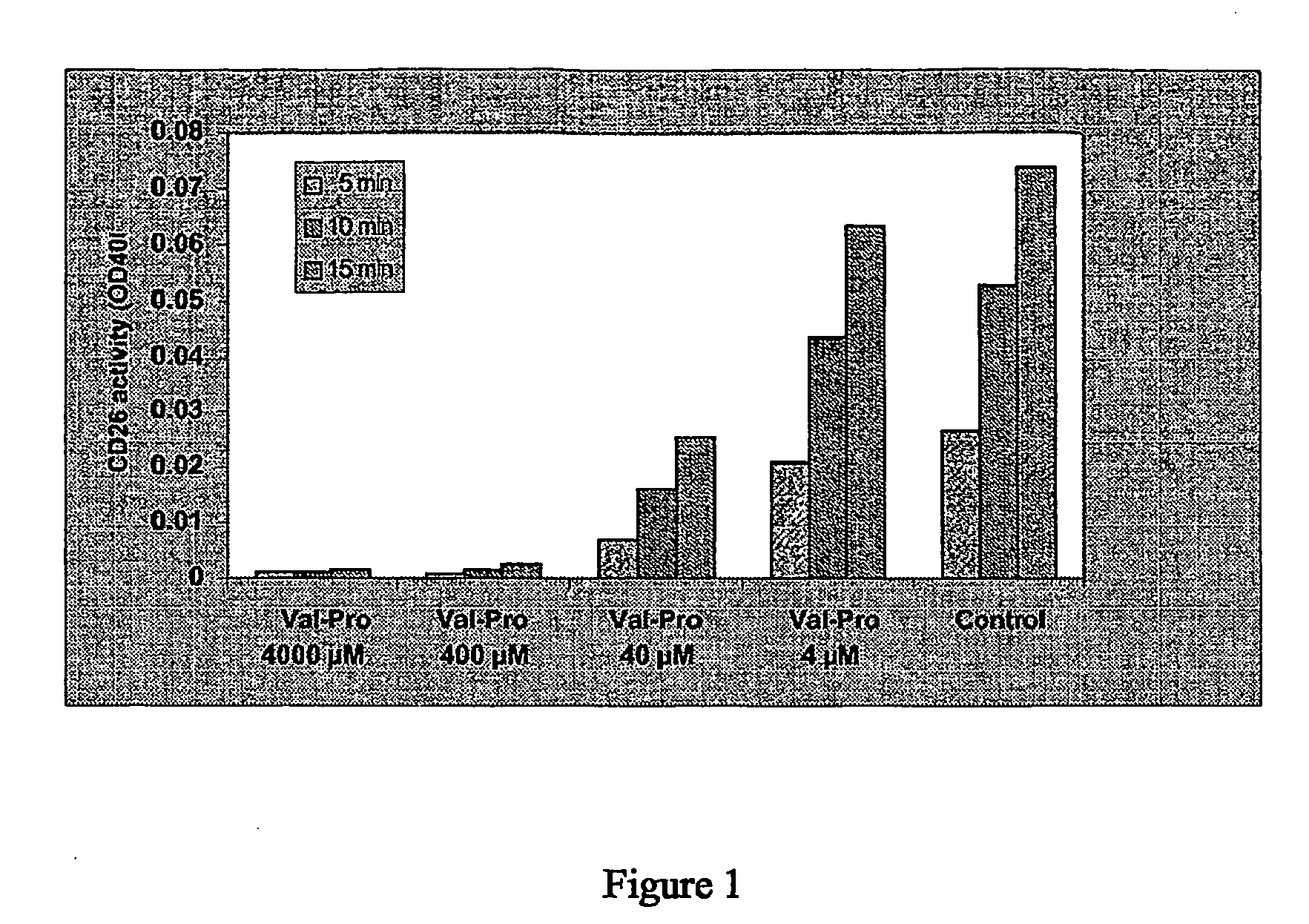
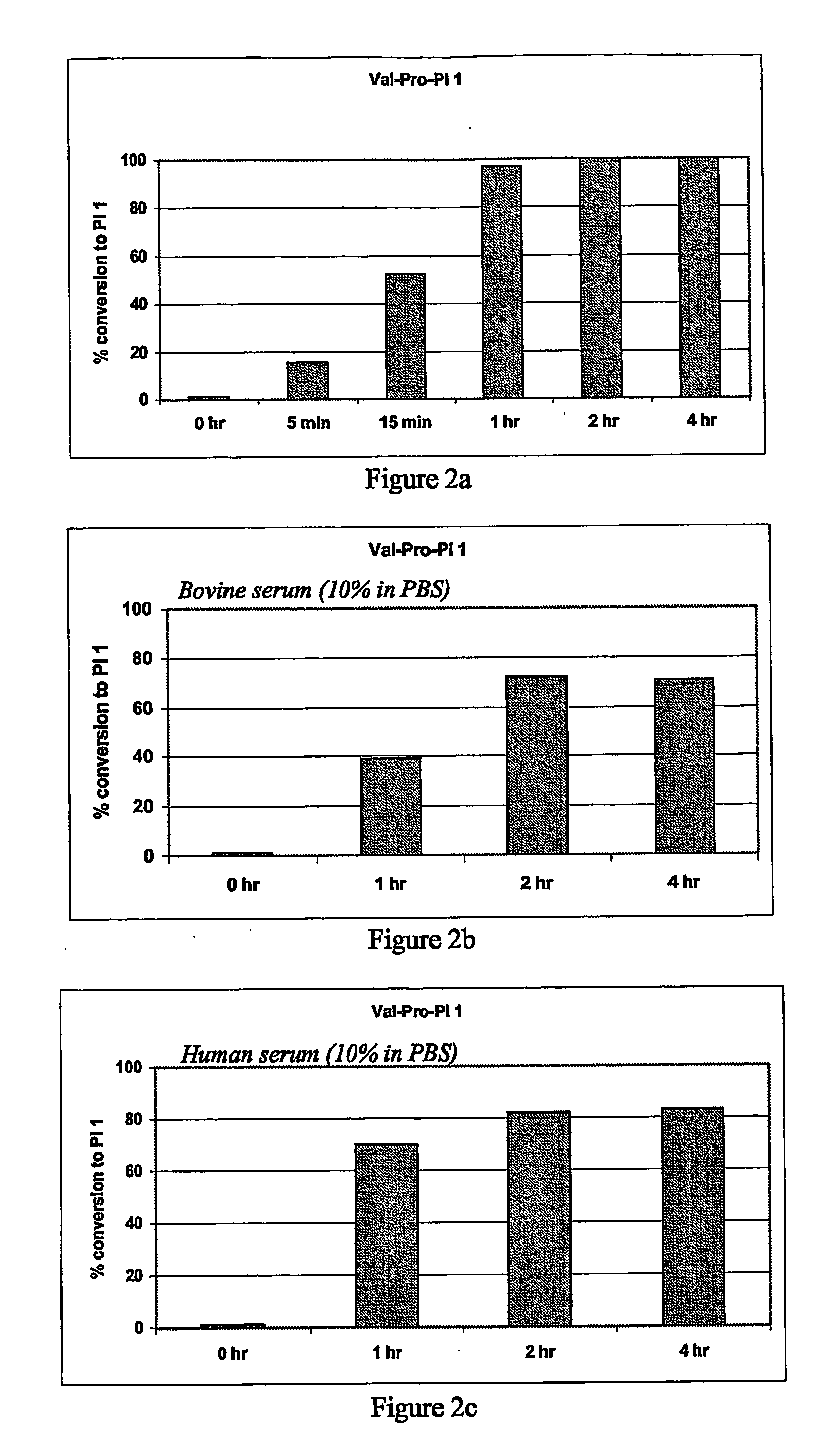
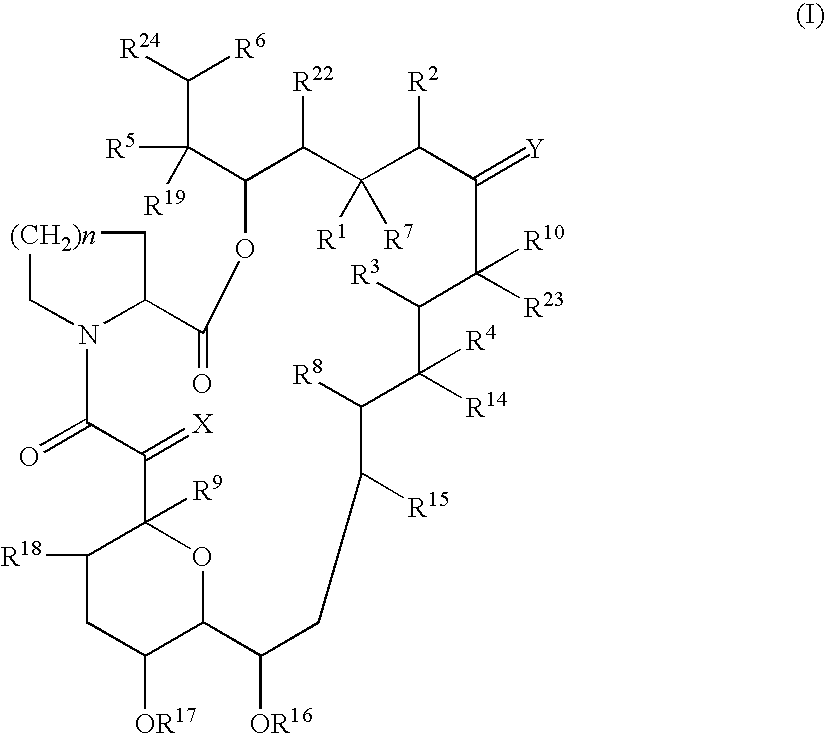
The present invention provides new prodrugs which are conjugates of a therapeutic compound and a peptide wherein the conjugate is cleavable by dipeptidyl-peptidases, more preferably by CD26, also known as DPPIV (dipeptidyl aminodipeptidase IV). The present prodrugs have the formulathe stereoisomeric forms and salts thereof, wherein n is 1 to 5; Y is proline, alanine, hydroxyproline, dihydroxyproline, thiazolidinecarboxylic acid (thioproline), dehydroproline, pipecolic acid (L-homoproline), azetidinecarboxylic acid, aziridinecarboxylic acid, glycine, serine, valine, leucine, isoleucine and threonine; X is selected from any amino acid in the D- or L-configuration; X and Y in each repeat of [Y-X] are chosen independently from one another and independently from other repeats; Z is a direct bond or a bivalent straight or branched saturated hydrocarbon group having from 1 to 4 carbon atoms; R1 is an aryl, heteroaryl, aryloxy, heteroaryloxy, aryloxyC1-4alkyl, heterocycloalkyloxy, heterocycloalkylC1-4alkyloxy, heteroaryloxyC1-4alkyl, heteroarylC1-4alkyloxy; R2 is arylC1-4alkyl; R3 is C1-10alkyl, C2-6alkenyl or C3-7cycloalkylC1-4alkyl; R4 is hydrogen or C1-4alkyl. The present invention furthermore provides the use of said prodrugs as medicines as well as a method of producing said prodrugs.
Owner:DE KOCK HERMAN AUGUSTINUS +2
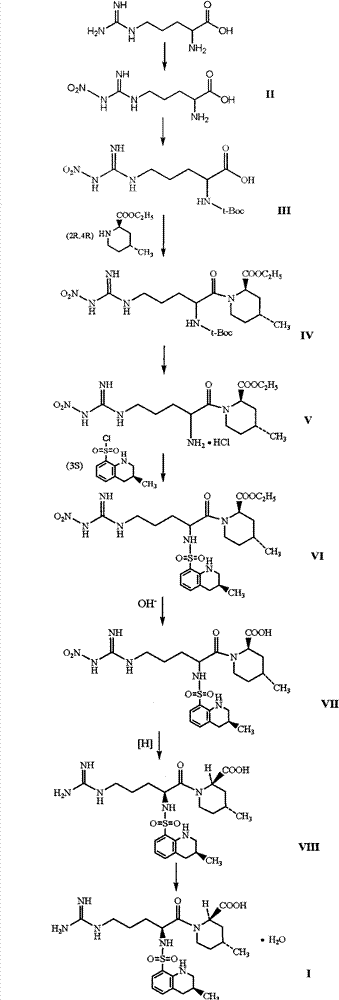
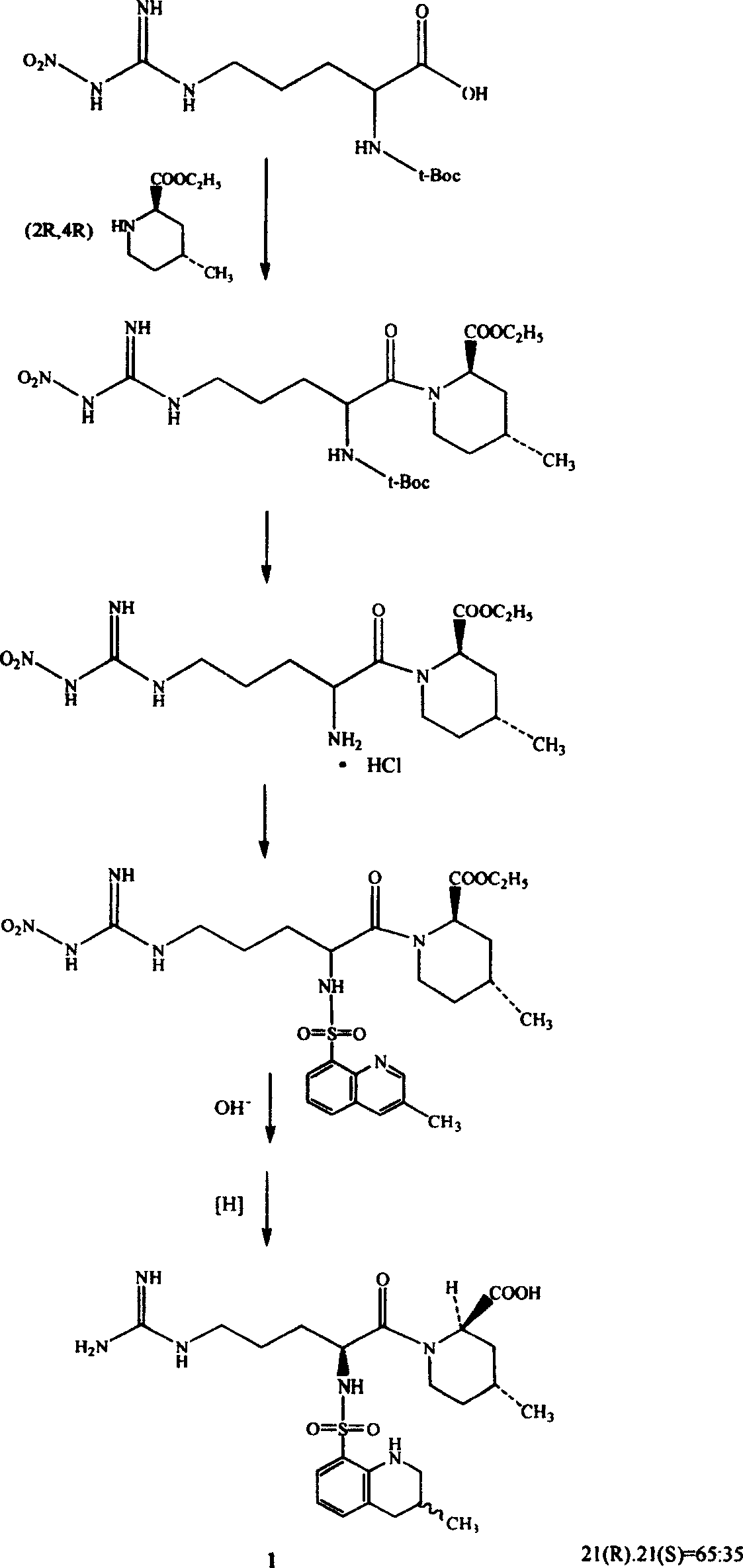
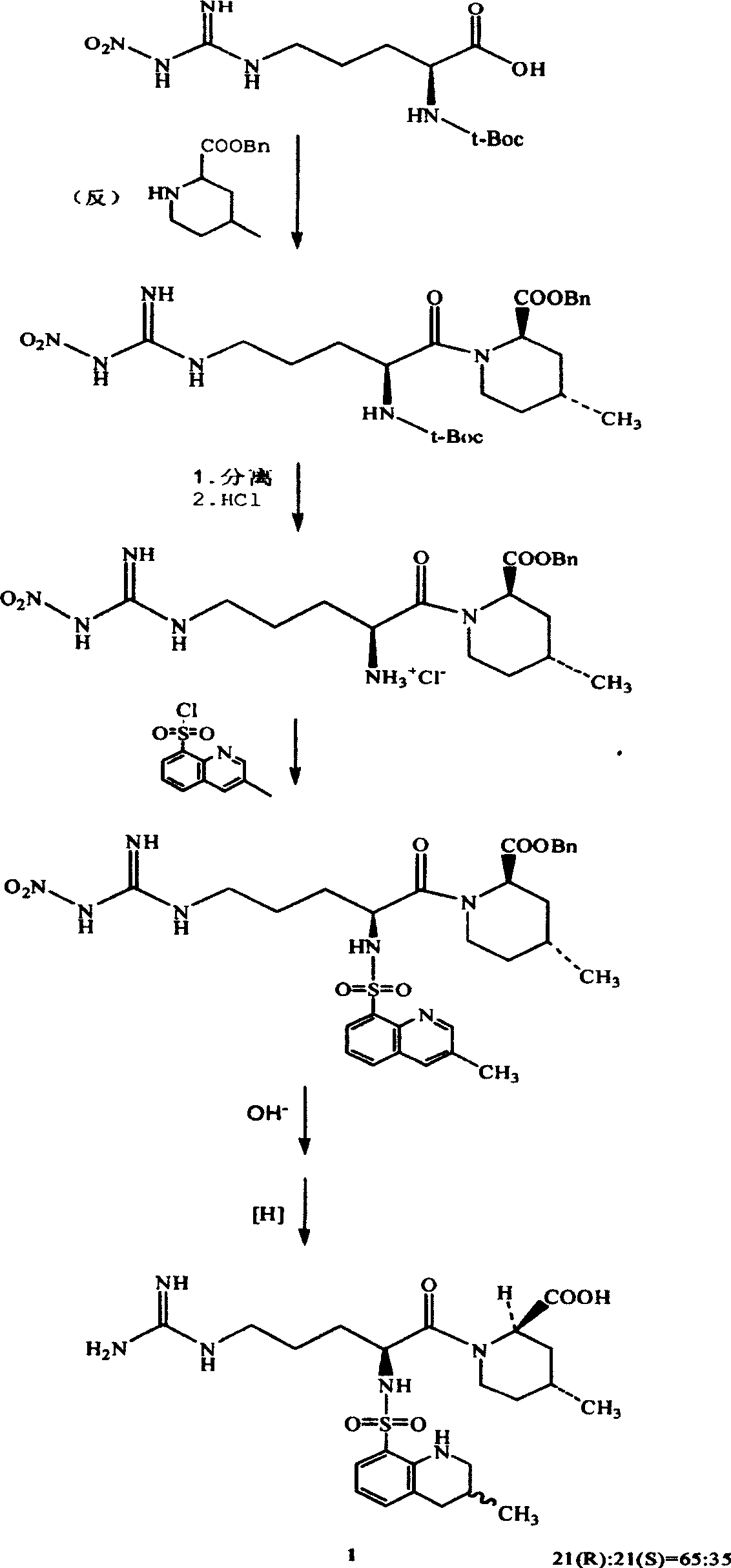
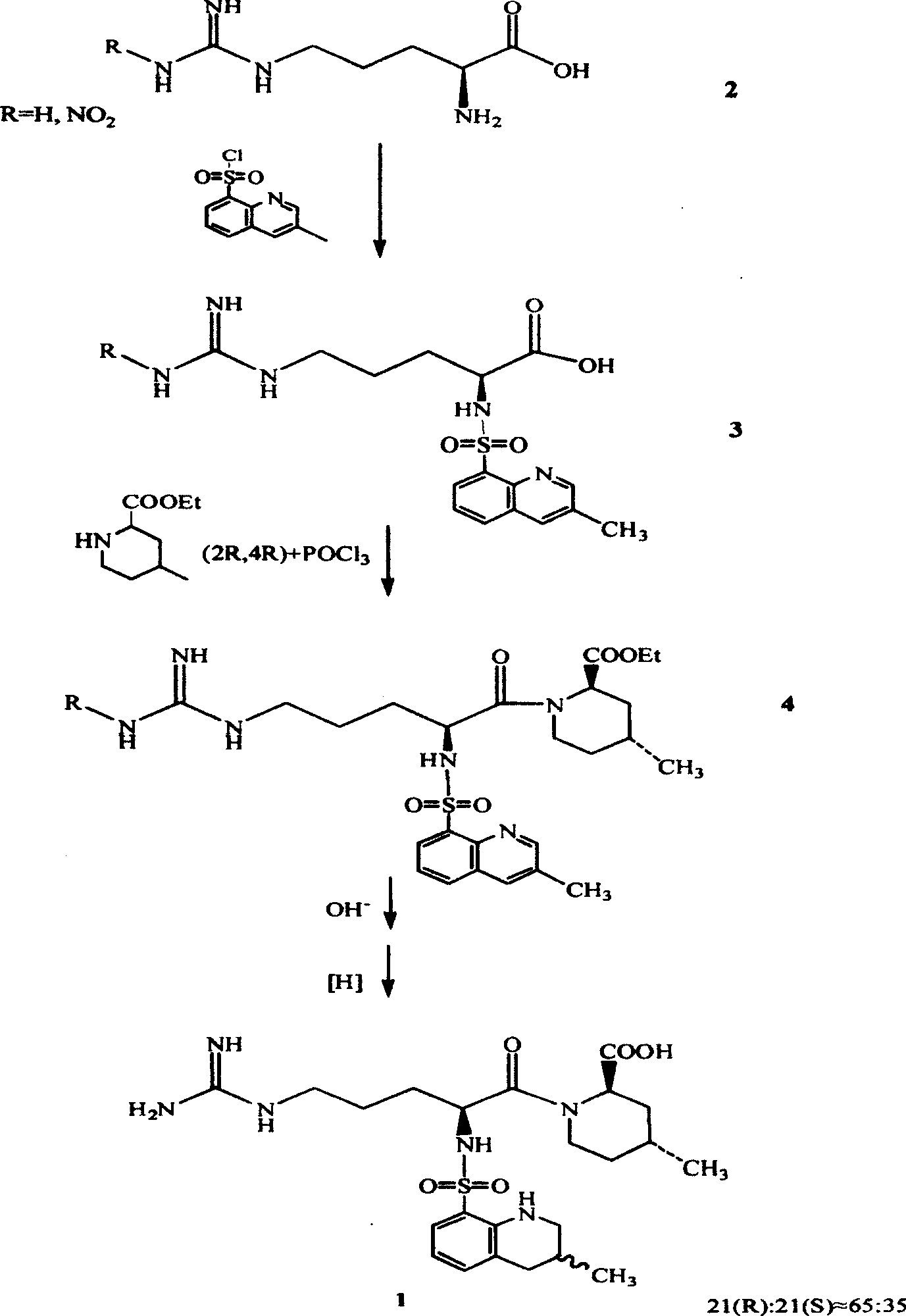
Directional synthesis method for 21(S) argatroban
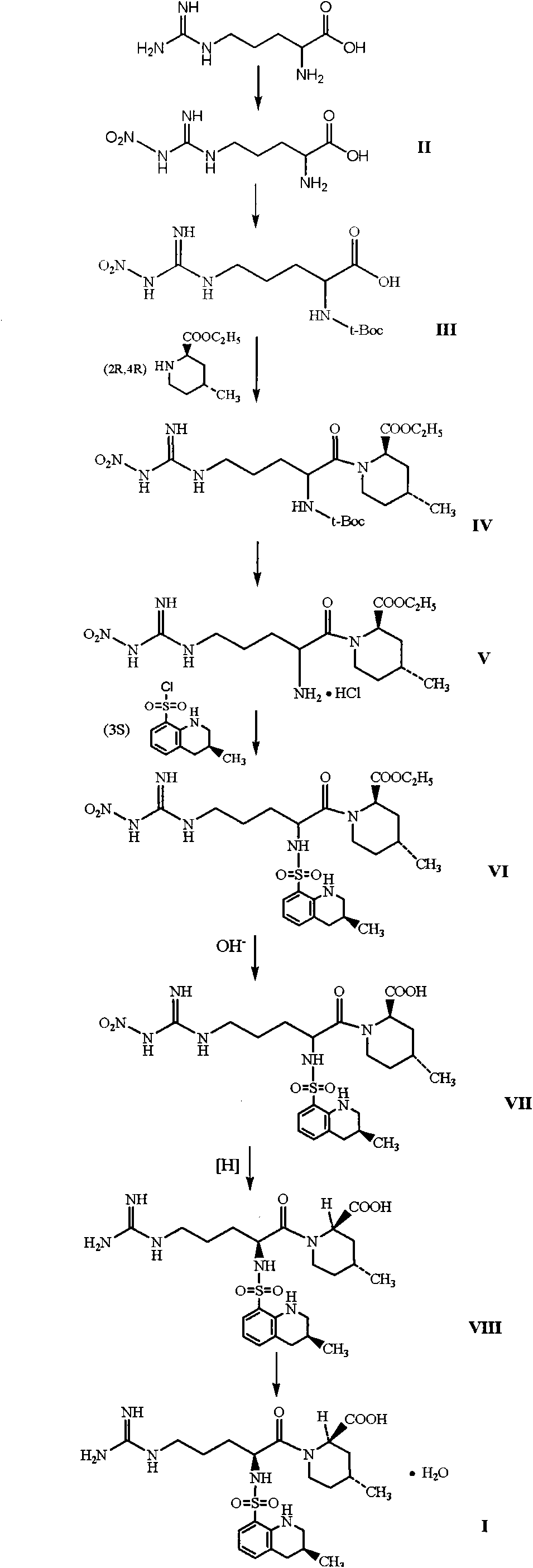
The invention discloses a directional synthesis method for 21(S) argatroban. The directional synthesis method comprises the following steps of: reacting (2R,4R)-1-[NG-nitro-L-arginyl]-4-methyl-2-piperidine ethyl formate hydrochloride serving as a raw material and (3S)-1,2,3,4-tetrahydro-3-methyl-8-quinoline sulfonyl chloride to obtain an intermediate (2R,4R)-1-[NG-nitro-N2-[(3S)-1,2,3,4-tetrahydro-3-methyl-8-quinoline sulfonyl]-L-arginyl]-4-methyl-2-piperidine formic ether, then hydrolyzing and acidifying the intermediate in aqueous solution of sodium hydroxide to obtain an intermediate (2R,4R)-1-[NG-nitro-N2-[(3S)-1,2,3,4-tetrahydro-3-methyl-8-quinoline sulfonyl]-L-arginyl]-4-methyl-2-piperidine formic acid, and finally hydrogenating the intermediate to obtain single diastereoisomer 21(S) argatroban by catalysis of palladium carbon.
Owner:TIANJIN WEIJIE TECH
Biomarker of depression, method for measuring biomarker of depression, computer program, and recording medium
Provided is a method for using low molecular weight compounds found in the body as a biomarker for diagnosing depression. Specifically, more than one compound selected from a group comprising the following are used: ADP-ribose, ATP, ADP, AMP, serotonin, tryptophan, kynurenine, SDMA (symmetrical dimethylarginine), threonine, glyceric acid, serine, N-acetylaspartic acid, glutamic acid, trigonelline, creatine, 2-methylserine, sphingosine, homovanillic acid, piperidine, sulfoxidized methionine, pipecolic acid, sphinganine, gamma-butyrobetaine, guanidinoacetic acid, isobutyric acid, creatinine, sarcosine, 3-methylbutyric acid, nicotinamide, betaine, ornithine, carnitine, ethanolamine, phosphoethanolamine, taurine, hypotaurine, aspartic acid, methionine, and tyrosine.
Owner:HUMAN METABOLOME TECH
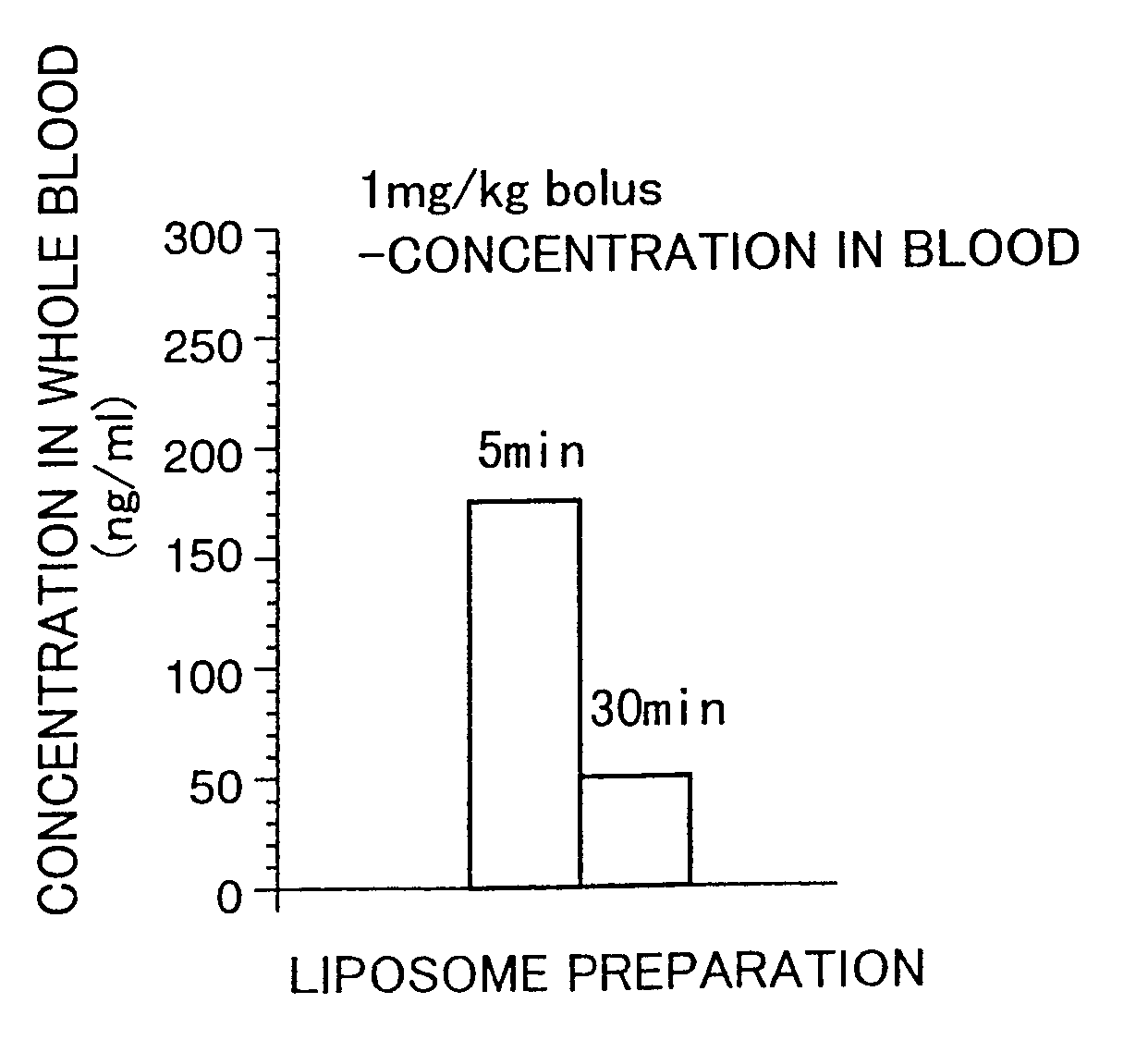
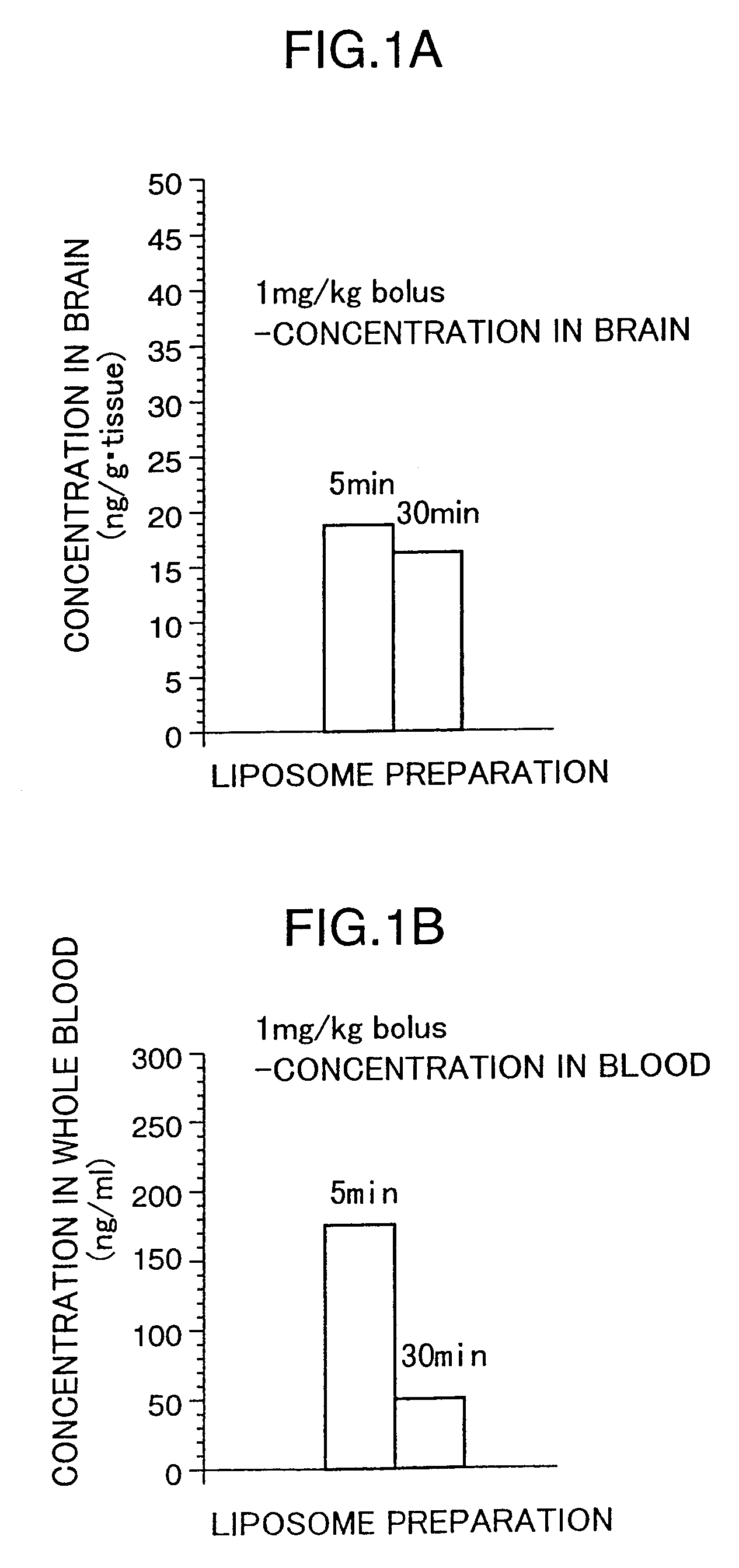
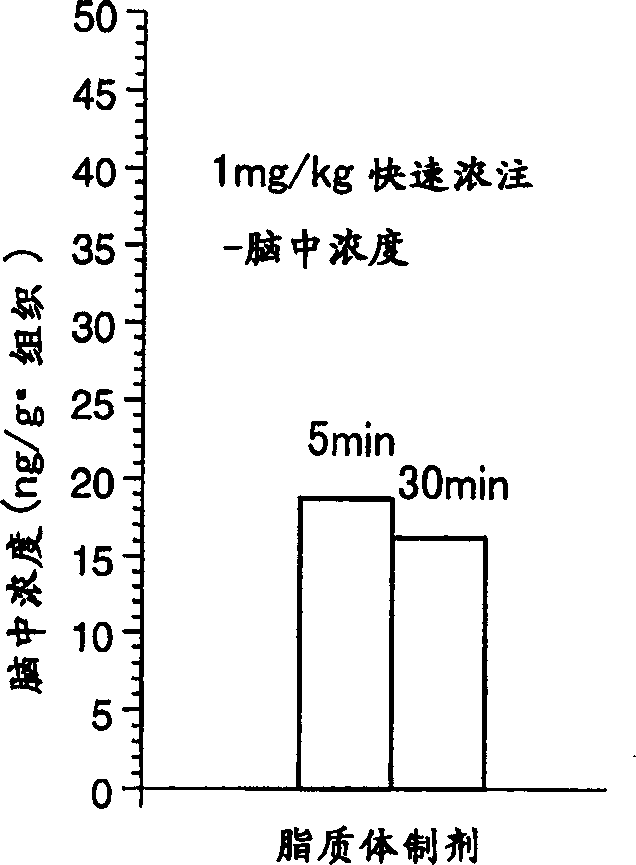
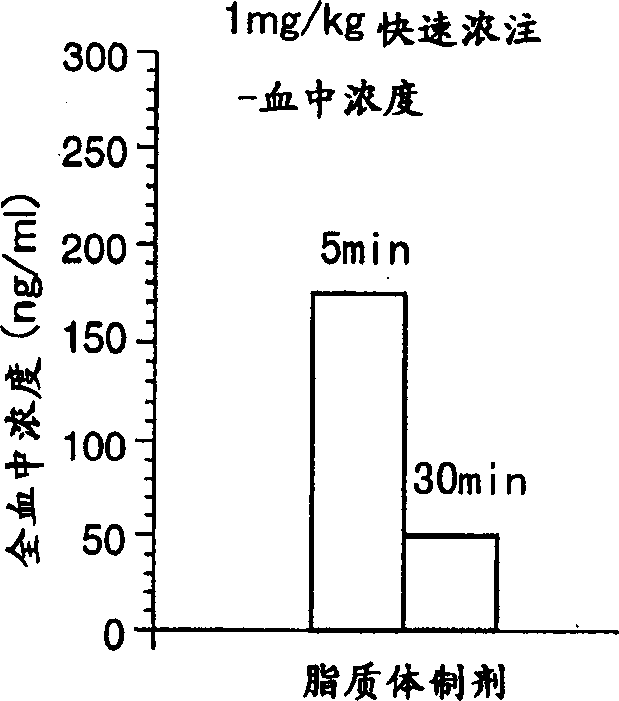
Liposome preparations
The present invention provides a pipecolic acid derivative-containing liposome preparation having has an excellent rapid action capable of coping with an emergent situation such as cerebral infarction.A liposome preparation characterized by comprising, as an active ingredient, a pipecolic acid derivative of the ingredient described in the present specification or a pharmaceutically acceptable salt thereof entrapped into liposomes, wherein lecithin is mainly used as the liposome-forming lipid, said liposome preparation containing no cholesterol as a stabilizer.
Owner:ASTELLAS PHARMA INC
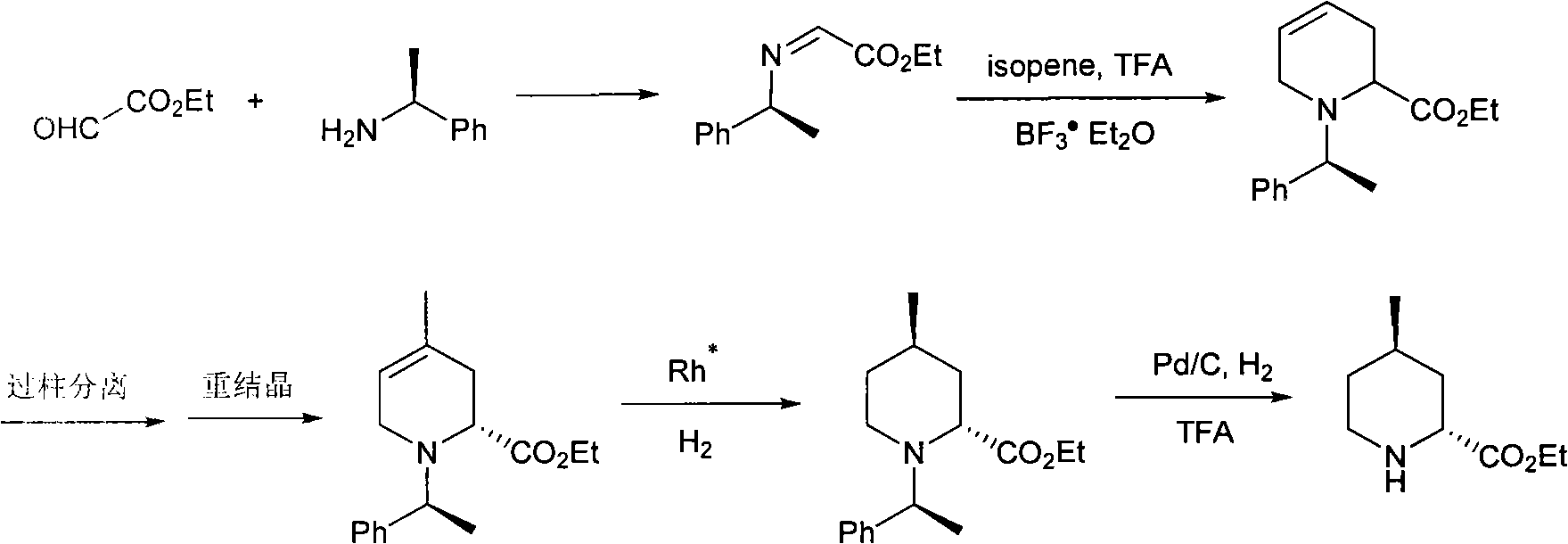
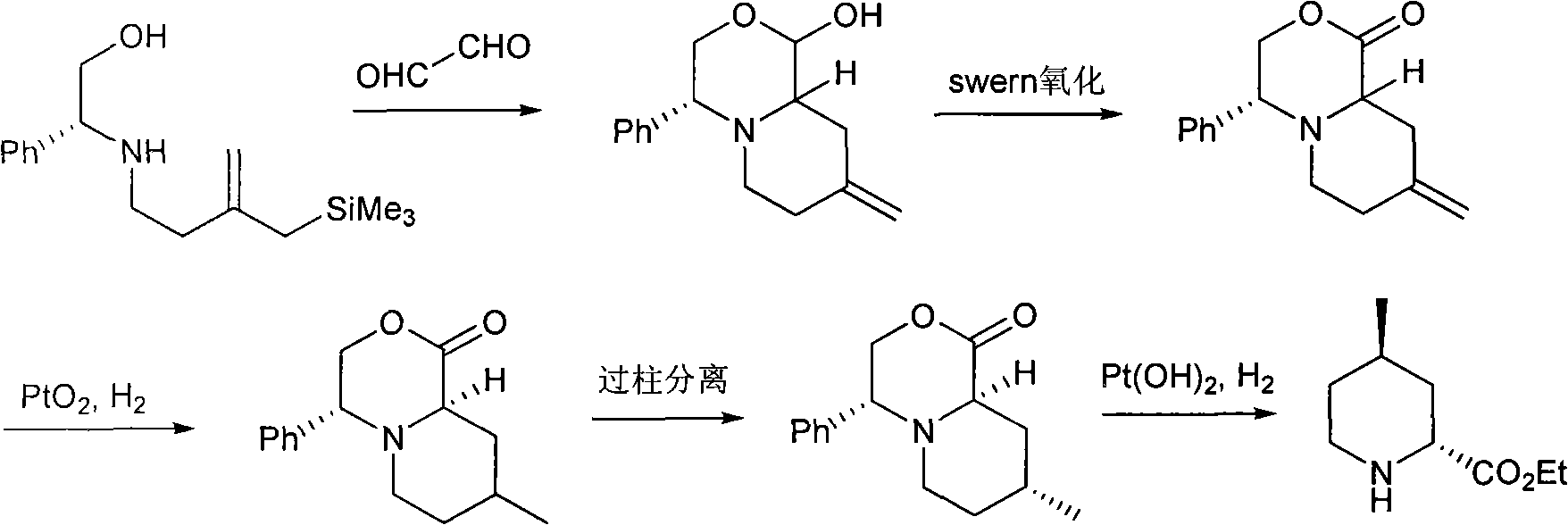
Method for preparing (2R,4R)-4-methyl-2-pipecolic acid
InactiveCN102807523AEasy to manufactureAvoid splittingOrganic chemistryBulk chemical productionL-AspartateFormate
The invention discloses a method for preparing (2R,4R)-4-methyl-2-pipecolic acid (I). The method comprises the following steps that: L-aspartic acid is subjected to selective esterification to obtain L-aspartic acid-4-alkyl ester hydrochlorate (II); the resulting (II) and acrylate are subjected to a reaction and an amino protection reaction to generate N-(2-carboalkoxy(aryloxy))ethyl-N-protective group-L-aspartic acid-4-alkyl ester (III); the resulting (III) is subjected to intramolecular ring closing and decarboxylation under an alkaline environment to generate (R)-N-protective group-4-oxopiperidine-2-formic acid (IV); the carboxyl of the resulting (IV) is subjected to esterification to generate (R)-N-protective group-4-oxopiperidine-2-formate (V); the resulting (V) is subjected to selective carbonyl reduction under an effect of a reducing agent to generate cis (2R,4S)-N-protective group-4-hydroxypiperidine-2-formate (VI); the hydroxyl of the resulting (VI) is activated to generate cis (2R,4S)-N-protective group-4-oxysulfonyl piperidine-2-formate (VII); the resulting (VII) and a nucleophilic methyl metal reagent SN2 are subjected to a substituted reaction to generate trans (2R,4R)-N-protective group-4-methyl piperidine-2-formte (VIII); and amino in the compound (VIII) and the protective group on the acid are removed to generate a target compound (2R,4R)-4-methyl-2-pipecolic acid (I). The method of the present invention has advantages of low cost, environmental friendliness, high selectivity, high yield, and the like, and is suitable for industrial production.
Owner:SHANGHAI AOBO PHARMTECH INC LTD
Preparation method of 2-piperidinecarboxylic acid, 3-piperidinecarboxylic acid and 4-piperidinecarboxylic acid
InactiveCN102174011AThe reaction route is simpleLess side effectsOrganic chemistryHydrogenPipecolic acid
The invention discloses a preparation method of 2-piperidinecarboxylic acid, 3-piperidinecarboxylic acid and 4-piperidinecarboxylic acid. The method is as follows: 2-pyridinecarboxylic acid, 3-pyridinecarboxylic acid and 4-pyridinecarboxylic acid are used as the raw material respectively, hydrogen is used to reduce the raw material to the corresponding piperidinecarboxylic acid in the presence of palladium-carbon catalyst. The method has the advantages of simple route, less side reactions, low hydrogenation pressure and hydrogenation temperature, short hydrogenation time and simple treatment of solid wastes, and is easy to realize industrialization.
Owner:CHANGZHOU DAOU CHEM IND
Method for synthesizing pipecolic acid through one-step enzymatic catalysis based on microchannel reactor
InactiveCN106591387AIncrease the rate of catalytic reactionsShorten the timeFermentationReaction ratePipecolic acid
The invention discloses a method for synthesizing pipecolic acid through one-step enzymatic catalysis based on a microchannel reactor. According to the method, lysine cyclodeaminase, a buffer solution and lysine are mixed, and the mixture is injected into a microchannel chip through a micro feeding pump for reaction, and thus pipecolic acid is prepared. With the adoption of the method provided by the invention, the catalytic reaction rate is obviously improved, and the time needed for catalytic generation of the product of the same total quantity is greatly shortened.
Owner:NANJING UNIV OF TECH
Liposome preparations
InactiveCN1342075ASufficient concentrationOrganic active ingredientsSaccharide with heterocyclic radicalsLipid formationCholesterol
Pipecolic acid derivative-containing liposome preparations which are excellent in the immediate action and thus usable in an urgent situation such as brain infarction. These preparations are characterized by containing pipecolic acid derivatives or pharmaceutically acceptable salts thereof, which comprise the components as described in the description, as the active ingredient and lecithin as the major component of lipids forming liposomes, without resort to cholesterol as a stabilizer.
Owner:FUJISAWA PHARMA CO LTD
Preparation method for aromatic carboxylic acid compounds
ActiveCN102372531AEfficient synthesisImprove tolerancePreparation from nitrilesFunctional group formation/introductionOrganolithium compoundsGrignard reagent
The invention discloses a preparation method for aromatic carboxylic acid compounds. According to the method, corresponding aromatic carboxylic acid compounds are obtained by reacting aryl halides with malononitrile under the conditions of alkali and a copper catalyst. A ligand cocatalyst is also used in the method and is one selected from the group consisting of 2-pipecolic acid, 2-picolinic acid, 2-pralidoxime, glyoxime, 1,10-orthophenanthrolene, N,N-dimethylethylenediamine and L-proline. Compared to the method of oxidation of substituted aromatic hydrocarbons, the method of directly reacting Grignard reagents and organic lithium reagents with carbon dioxide and the method of reacting the compounds of aryl zinc and aryl boron with carbon dioxide under catalysis of transition metals in the prior art, the method provided in the invention has following obvious advantages: the method is based on aryl halides which are easily available; a cheap copper salt is used as a catalyst; little environmental pollution and high yield are realized; high tolerance of a plurality of function groups on aromatic rings is obtained; separation and purification are convenient; etc.
Owner:TSINGHUA UNIV
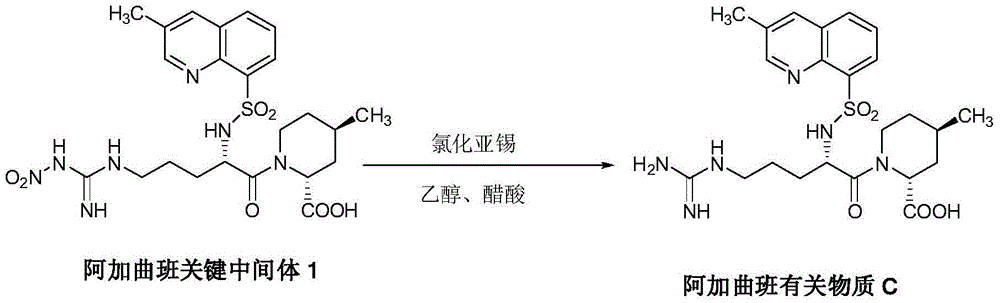
Synthesis and separation identification method for aragatroban related substances
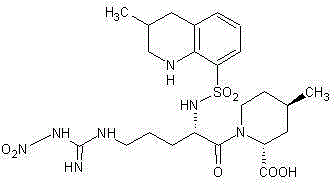
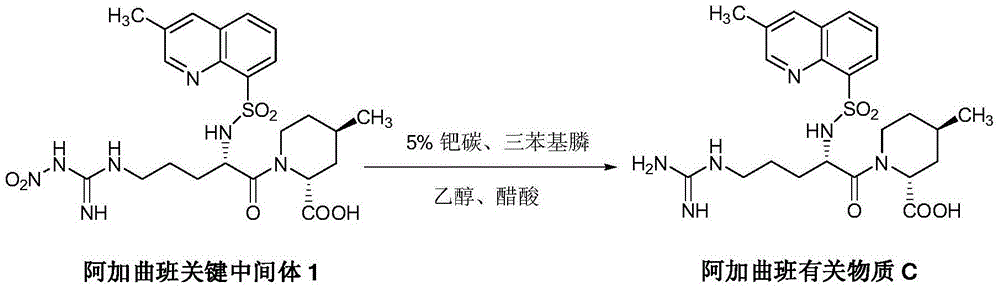
The invention discloses a synthesis and separation identification method for aragatroban two related substances. The method comprises taking aragatroban as a raw material, nitrating by fuming nitric acid, and separating to obtain (2R,4R)-1-[NG-nitro-N2-((R,S)-3-methyl-1,2,3,4-tetrahydro-8- quinolylsulfonyl)-L-arginyl]-4-methyl-2-pipecolinic acid (wherein G in NG and 2 in N2 are both superscripts) (aragatroban related substance A); and taking aragatroban as a raw material, hydrolyzing through an aqueous solution of sodium hydroxide, barium hydroxide or other highly-basic reagent, and separating to obtain (2R,4R)-1-[N-((R,S)-3-methyl-1,2,3,4-tetrahydro-8-quinolylsulfonyl)-L-ornithyl]-4-methyl-2-pipecolinic acid (aragatroban related substance B). The provided preparation method for the aragatroban related substances is simple and practicable, and low in cost, avoids a complex tedious operation process for total synthesis of the aragatroban related substances, and is a suitable preparation method for the aragatroban related substances. The aragatroban related substance A and the aragatroban related substance B are both shown in the specification.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
Biomarker compositions for diagnosing cervicitis and cervical cancer
InactiveCN106124763AComponent separationMaterial analysis by electric/magnetic meansCysteine thiolateCervicitis
The invention discloses biomarker compositions, and provides biomarker compositions for diagnosing cervicitis and a cervical cancer. The biomarker compositions are characterized by at least comprising one or a combination of uridine-5'-monophosphate disodium, histamine, 3'-O-methyl-guanosine and phenyllactic acid. The biomarker composition for diagnosing the cervicitis is characterized by comprising one or a combination of uridine-5'-monophosphate disodium, L-cysteine, isocitric acid, uridine diphosphate glucose, adenosine monophosphate, inosine 5'-monophosphate, L-pipecolic acid, N-acetyl putrescine and saccharose. The biomarker composition for diagnosing the cervical cancer is characterized by comprising one or a combination of histamine, phenyllactic acid, citraconic acid, L-2-aminoadipic acid, 3'-O-methyl-guanosine, beta-D-glucosamine, glycerin and cholesterol sulfate.
Owner:上海阿趣生物科技有限公司
Preparation method of argatroban intermediate
The invention relates to a preparation method of argatroban intermediate (2R,4R)-ethyl-1-((S)-2-(tert-butoxy amido)-5-(3-nitroguanidine)valery)-4-methylpiperidine-2-ethyl carboxylate. The method comprises the following steps: enabling NG-nitro-N2-t-Boc-L-arginine and (2R,4R)-4-methyl-2-ethyl nipecotate to perform condensation reaction in the presence of a condensing agent selected from 1-ethyl-3-(3-dimethylamine propyl)carbodiimide hydrochloride, N,N-diisopropyl carbodiimide and N,N'-carbonyl diimidazole and an aprotic solvent to generate the argatroban intermediate. The raw material for the method is wide in source, cheap and easily-available; the method is mild in reaction condition, short in step, simple in operation, easy to purify the product, low in production cost and environment-friendly, not only suitable for laboratory synthesis, but also suitable for large-scale industrial production.
Owner:SHANGHAI SYNCORES TECH INC
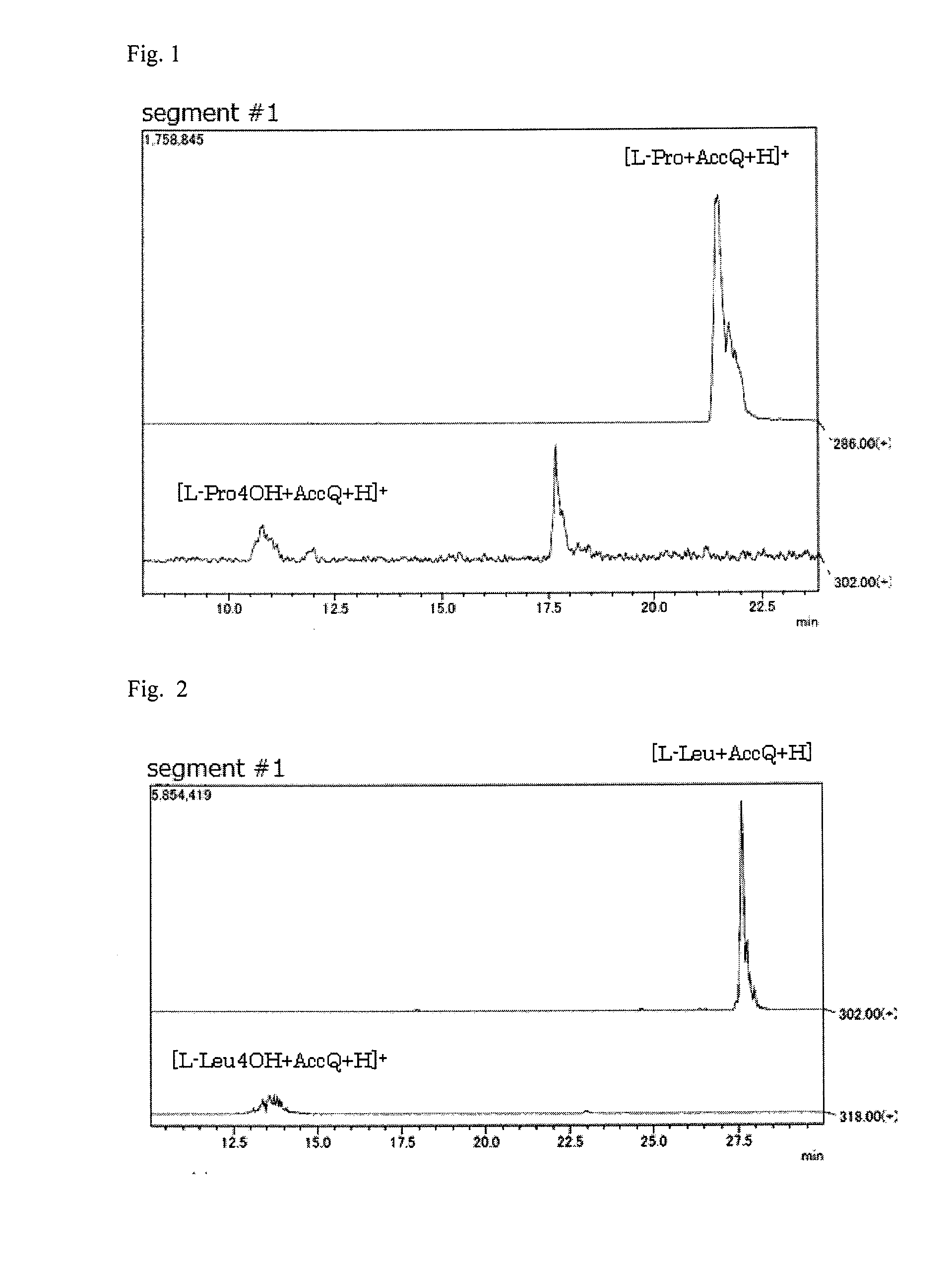
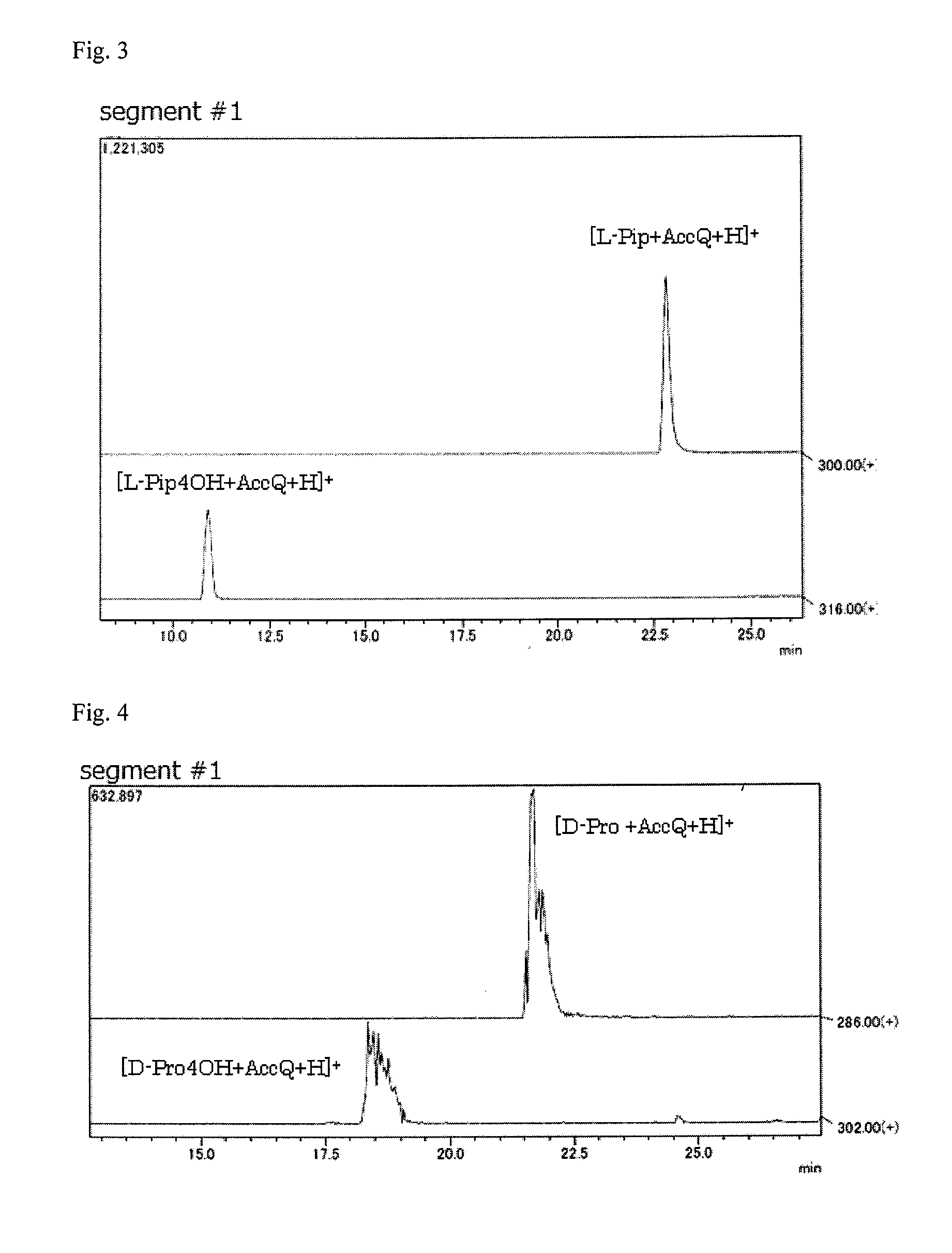
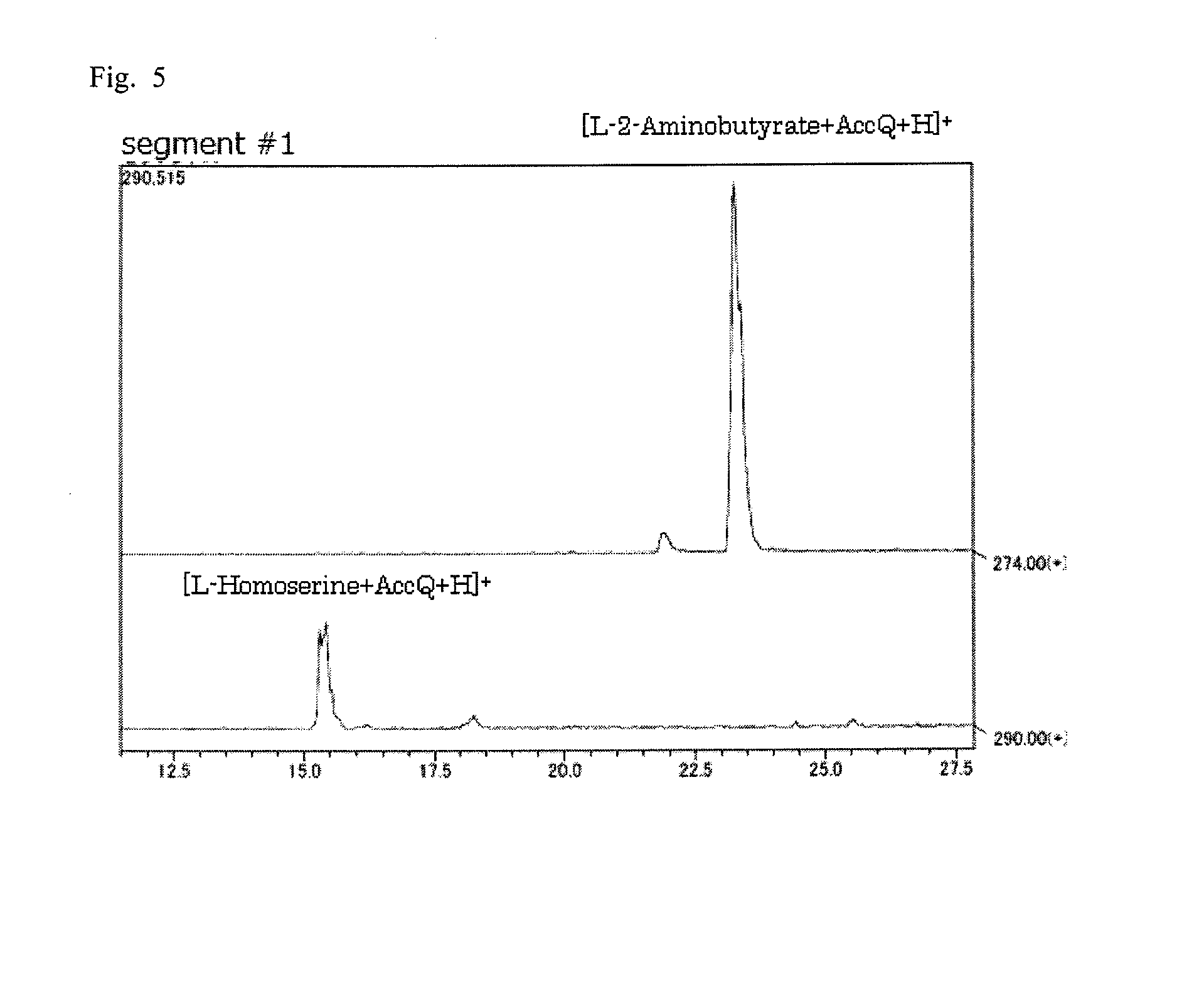
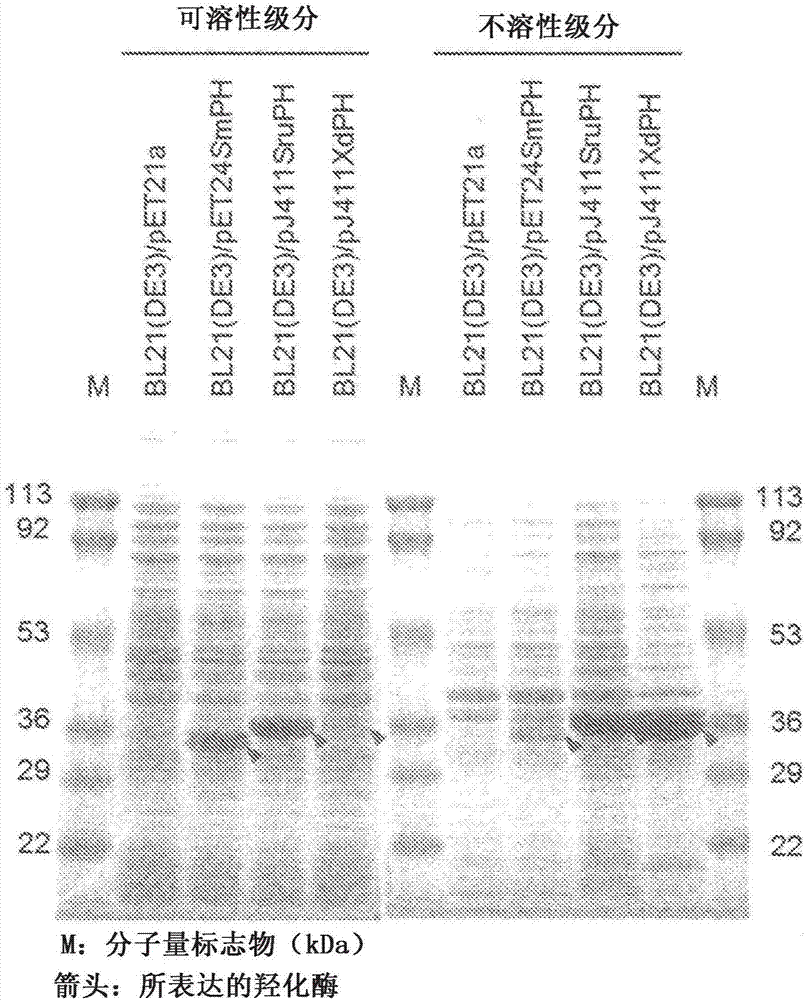
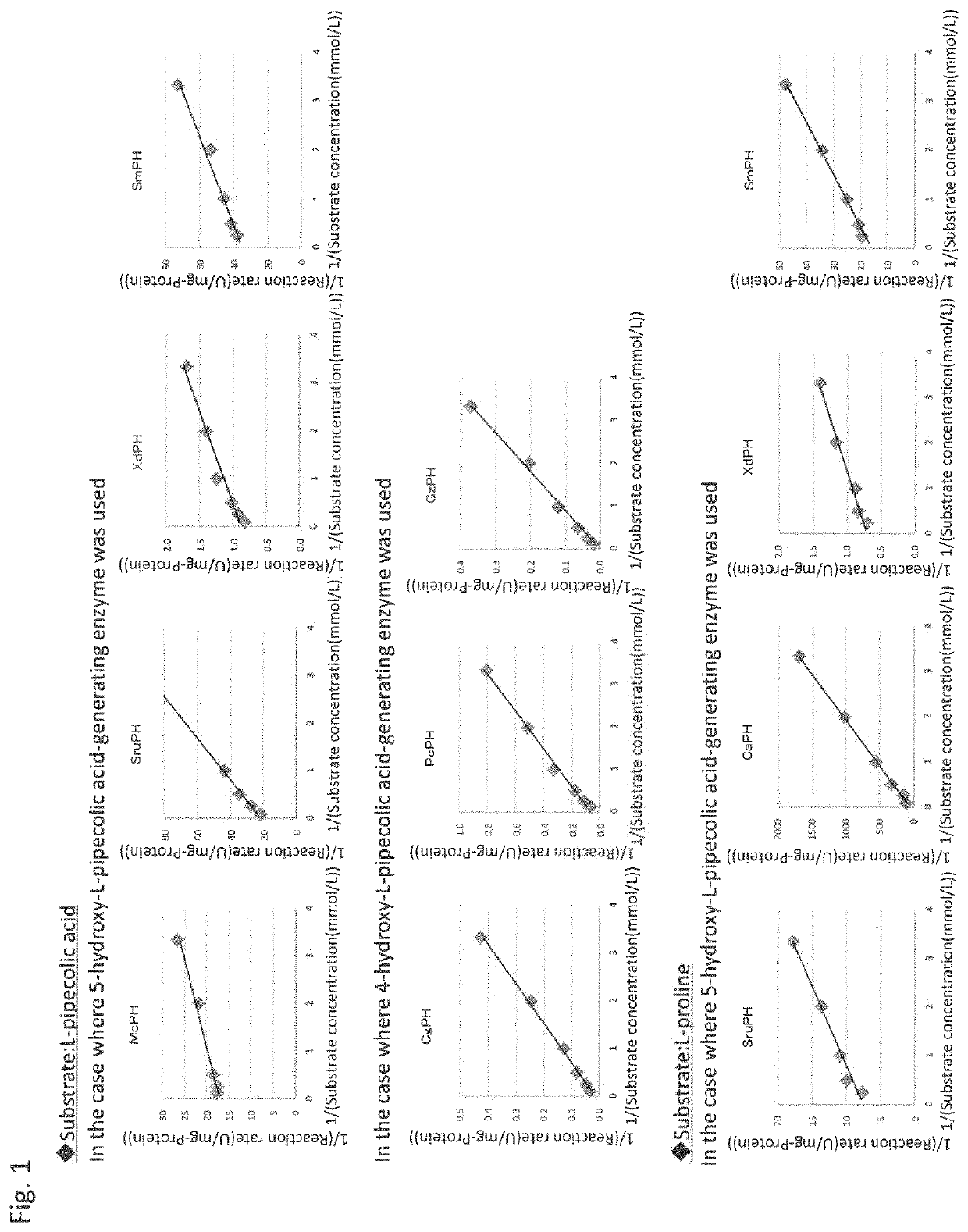
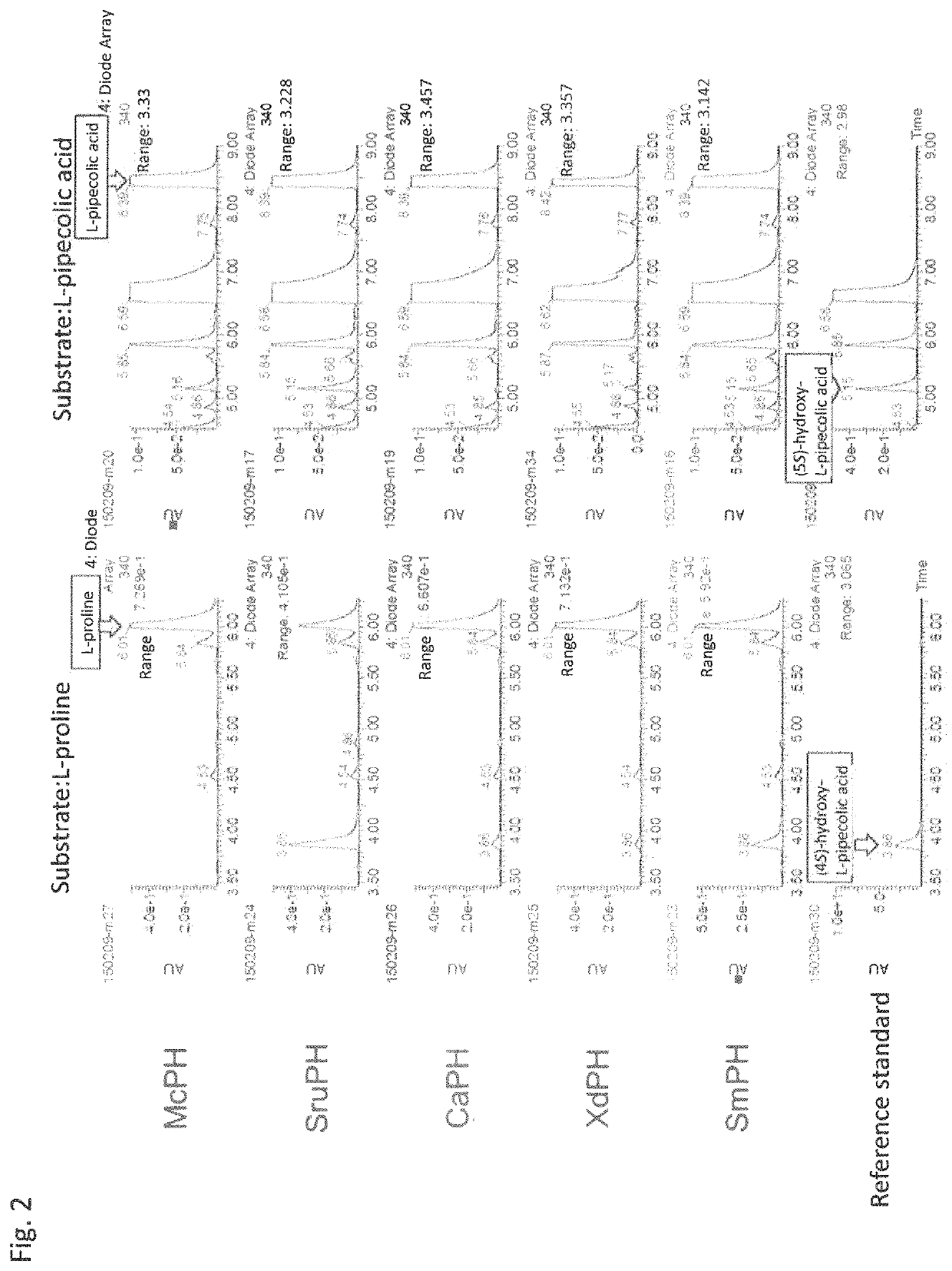
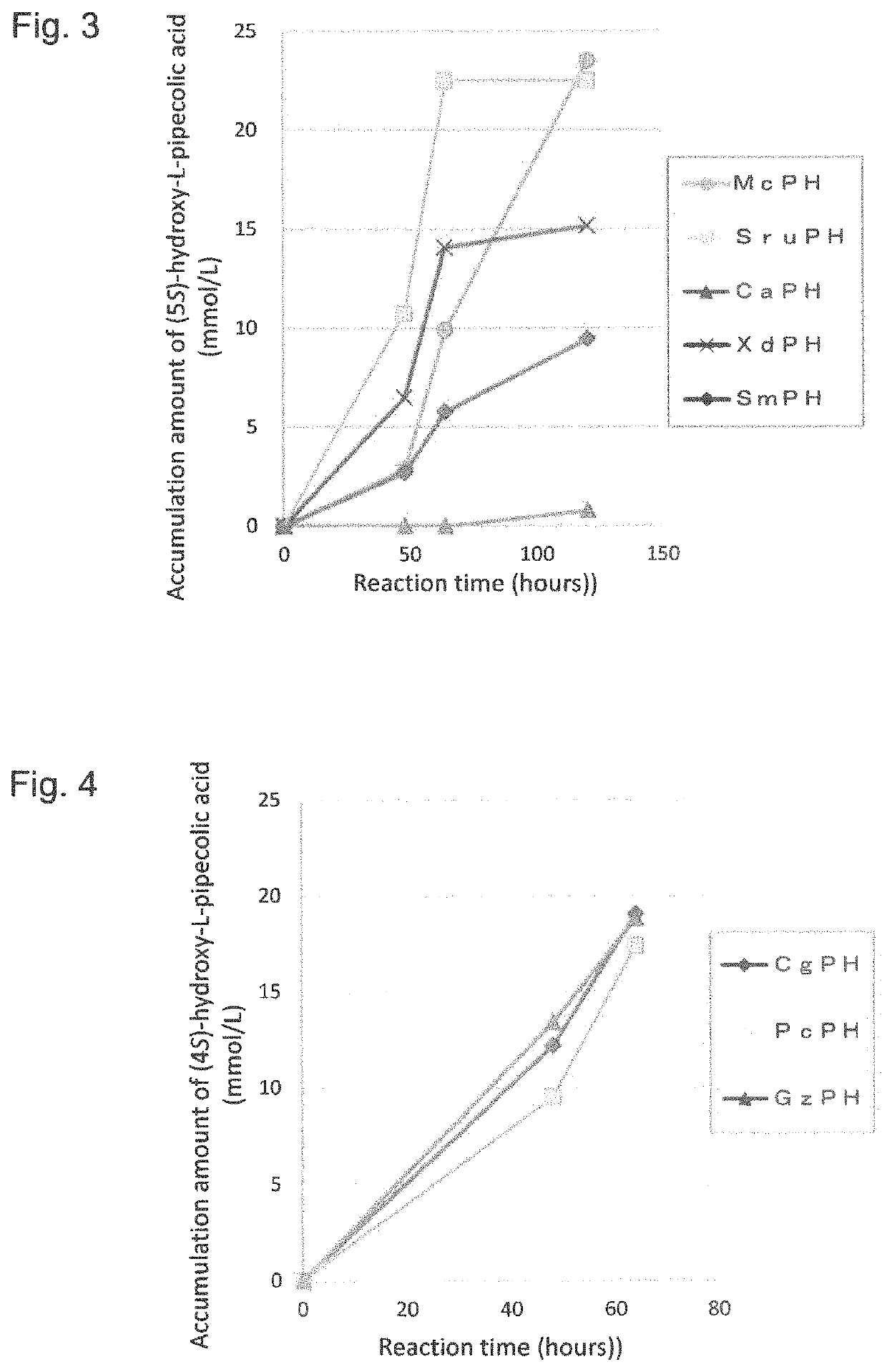
Pipecolinic acid 4-hydroxylase and method for producing 4-hydroxy amino acid using same
ActiveUS20160348081A1Efficient productionHigh optical purityBacteriaOxidoreductasesMugineic acidPipecolic acid
The present invention provides a pipecolic acid 4-hydroxylase protein exemplified by the following (A), (B), and (C), having activity to react with L-pipecolic acid in the presence of 2-oxoglutaric acid and iron(II) ions to produce trans-4-hydroxy-L-pipecolic acid, and a method for producing 4-hydroxy amino acid, which method comprises reacting the pipecolic acid 4-hydroxylase protein, cells containing the protein, a treated product of the cells, and / or a culture liquid obtained by culturing the cells, with α-amino acid to produce 4-hydroxy amino acid:(A) a polypeptide comprising the amino acid sequence represented by SEQ ID NO:2, 4, 6, 8, 10, 12, 16, or 18;(B) a polypeptide comprising the amino acid sequence represented by SEQ ID NO:2, 4, 6, 8, 10, 12, 16, or 18 except that one or several amino acids are deleted, substituted, and / or added, and having pipecolic acid 4-hydroxylase activity; and(C) a polypeptide having an amino acid sequence that is not less than 80% identical to the amino acid sequence represented by SEQ ID NO:2, 4, 6, 8, 10, 12, 16, or 18, and having pipecolic acid 4-hydroxylase activity.
Owner:API CORP (JP)
Method for manufacturing cis-5-hydroxy-l-pipecolic acid
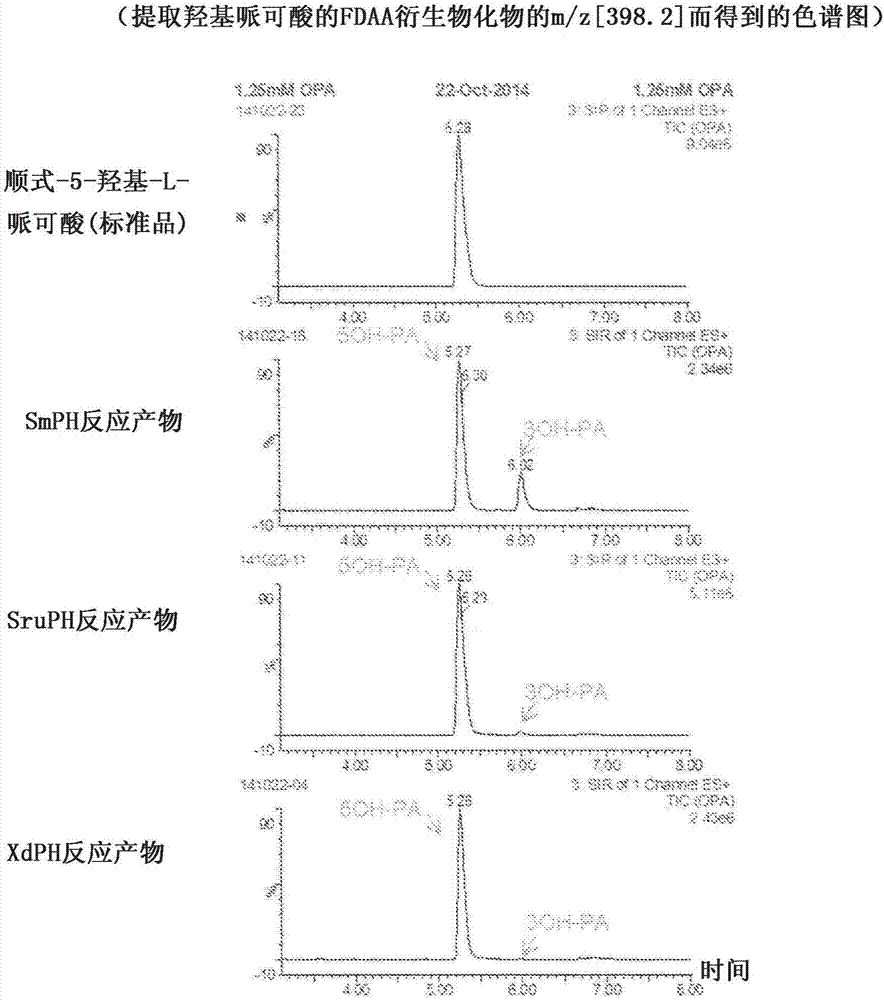
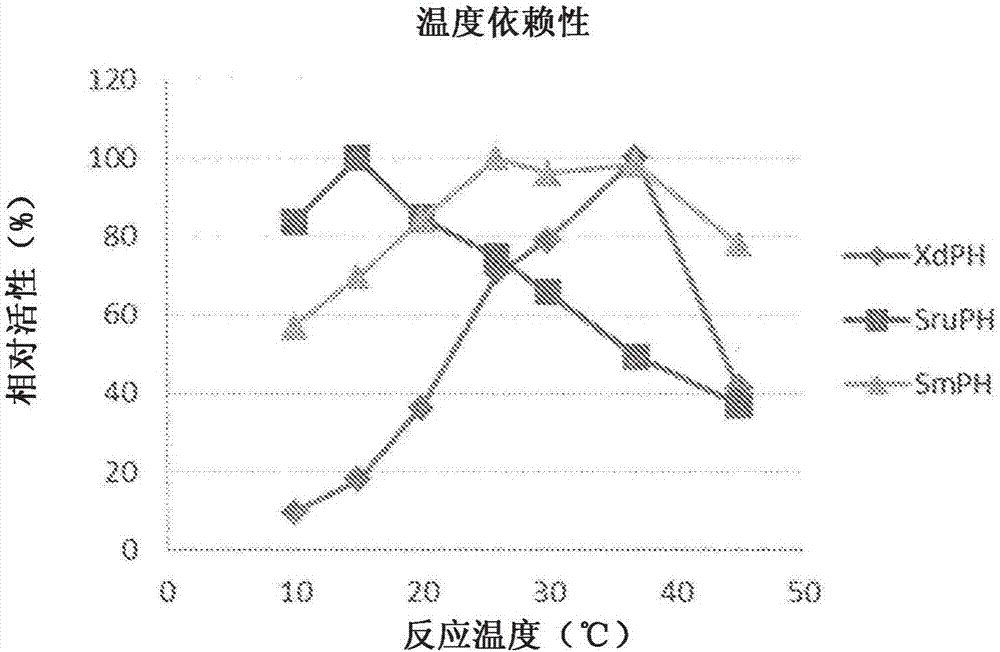
ActiveCN107109446AHigh optical purityHigh purityOxidoreductasesRecombinant DNA-technologyPipecolic acidOxoglutarates
A method for manufacturing cis-5-hydroxy-L-pipecolic acid, characterized in that cis-5-hydroxy-L-pipecolic acid is produced by causing alpha-oxoglutarate-dependent L-pipecolic acid hydroxylase to act on L-pipecolic acid, the alpha -oxoglutarate-dependent L-pipecolic acid hydroxylase including a polypeptide indicated by (A), (B), or (C). (A) A polypeptide having an amino acid sequence represented by SEQ ID NO 4 or 11; (B) a polypeptide having an amino acid sequence in which one or several amino acids have been deleted, substituted, and / or added in an amino acid sequence represented by SEQ ID NO 4 or 11, and further having alpha-oxoglutarate-dependent L-pipecolic acid hydroxylase activity; or (C) a polypeptide having an amino acid sequence having 60% or greater identity with an amino acid sequence represented by SEQ ID NO 4 or 11, and further having alpha-oxoglutarate-dependent L-pipecolic acid hydroxylase activity.
Owner:API CO LTD
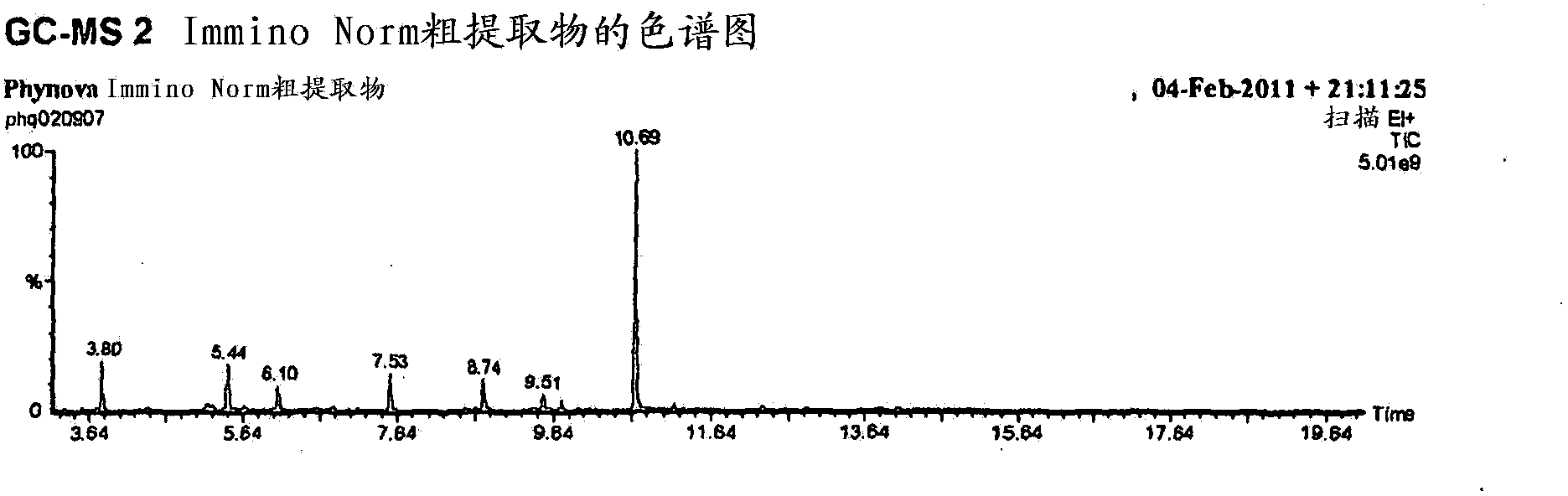
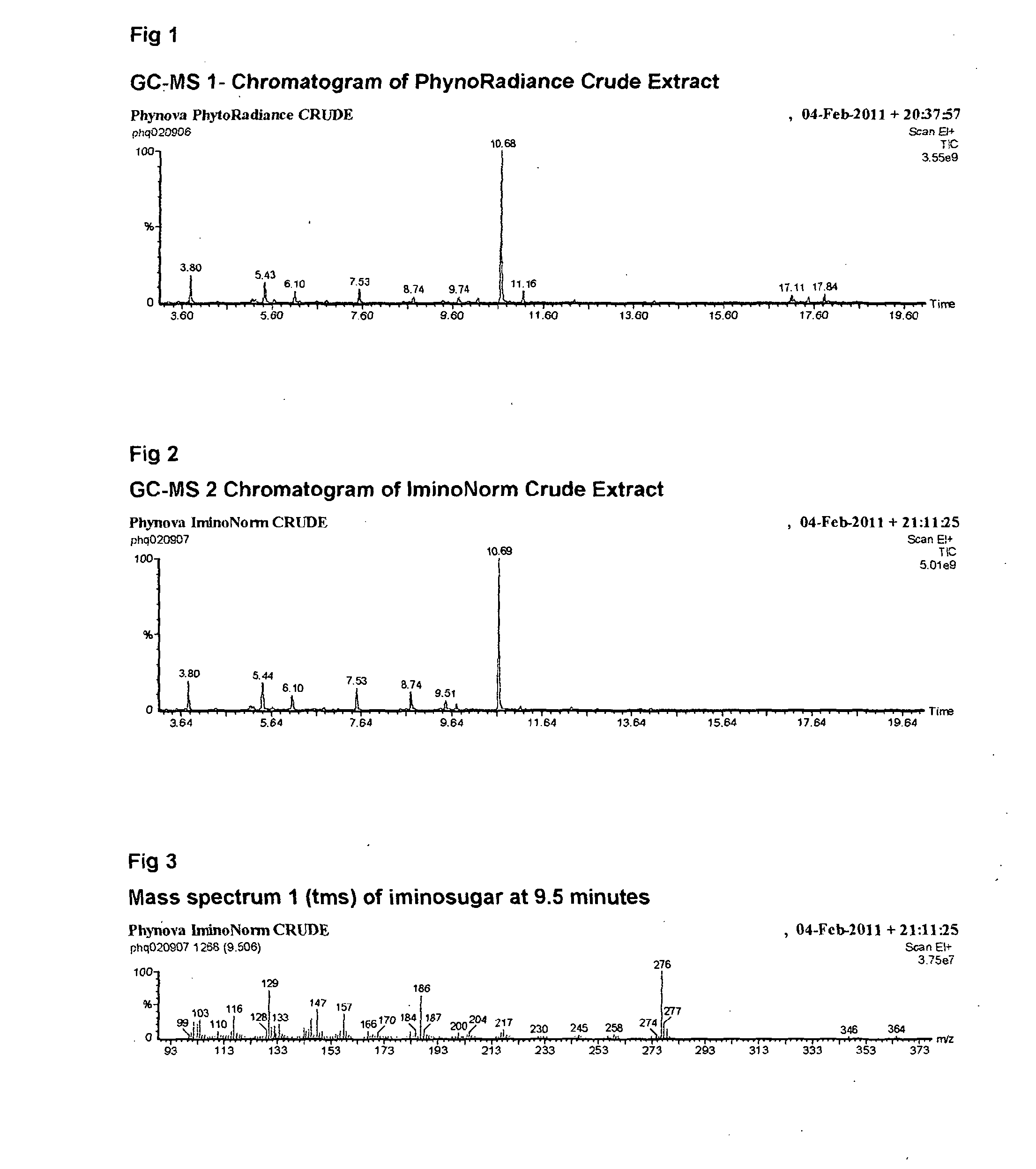
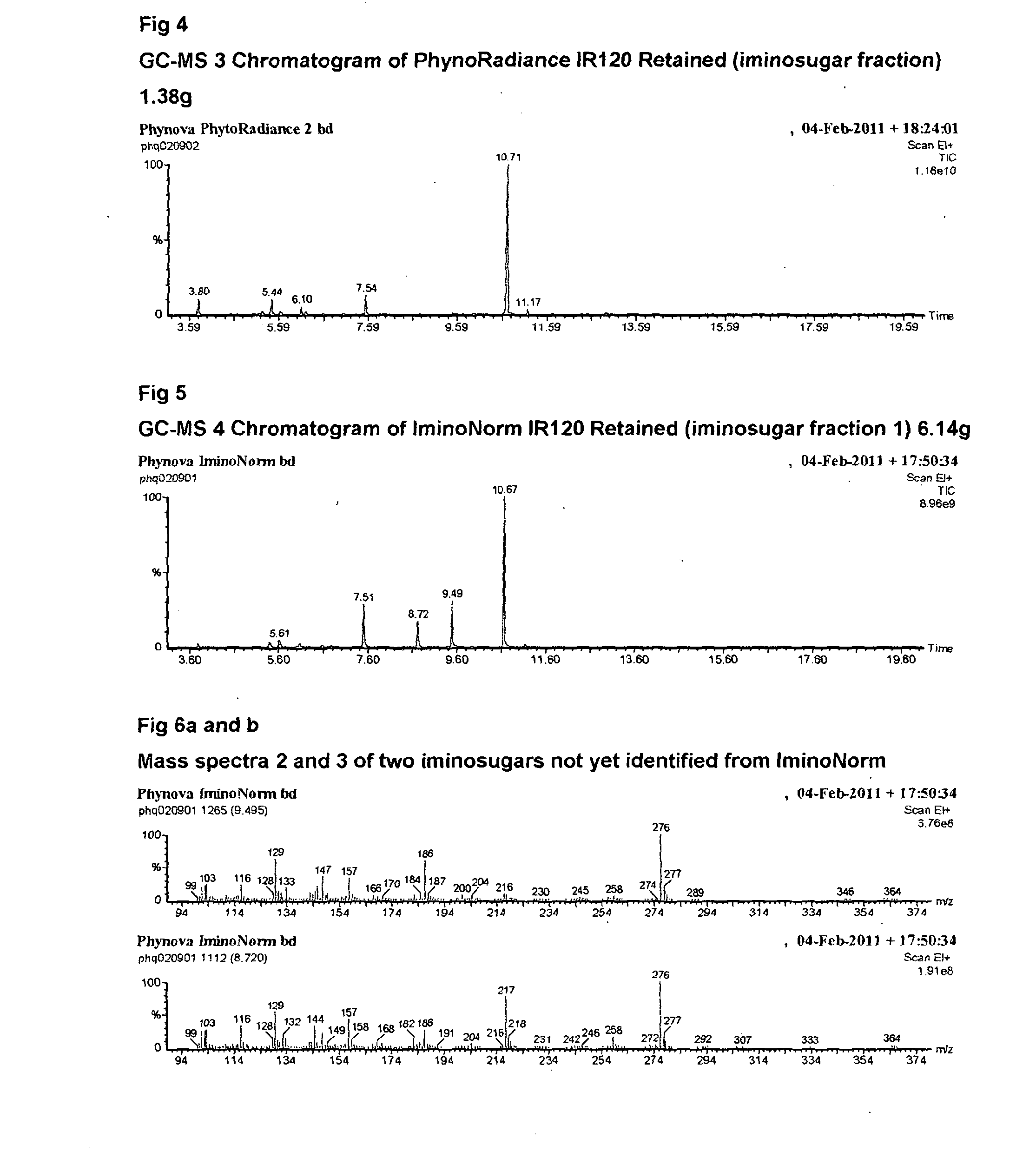
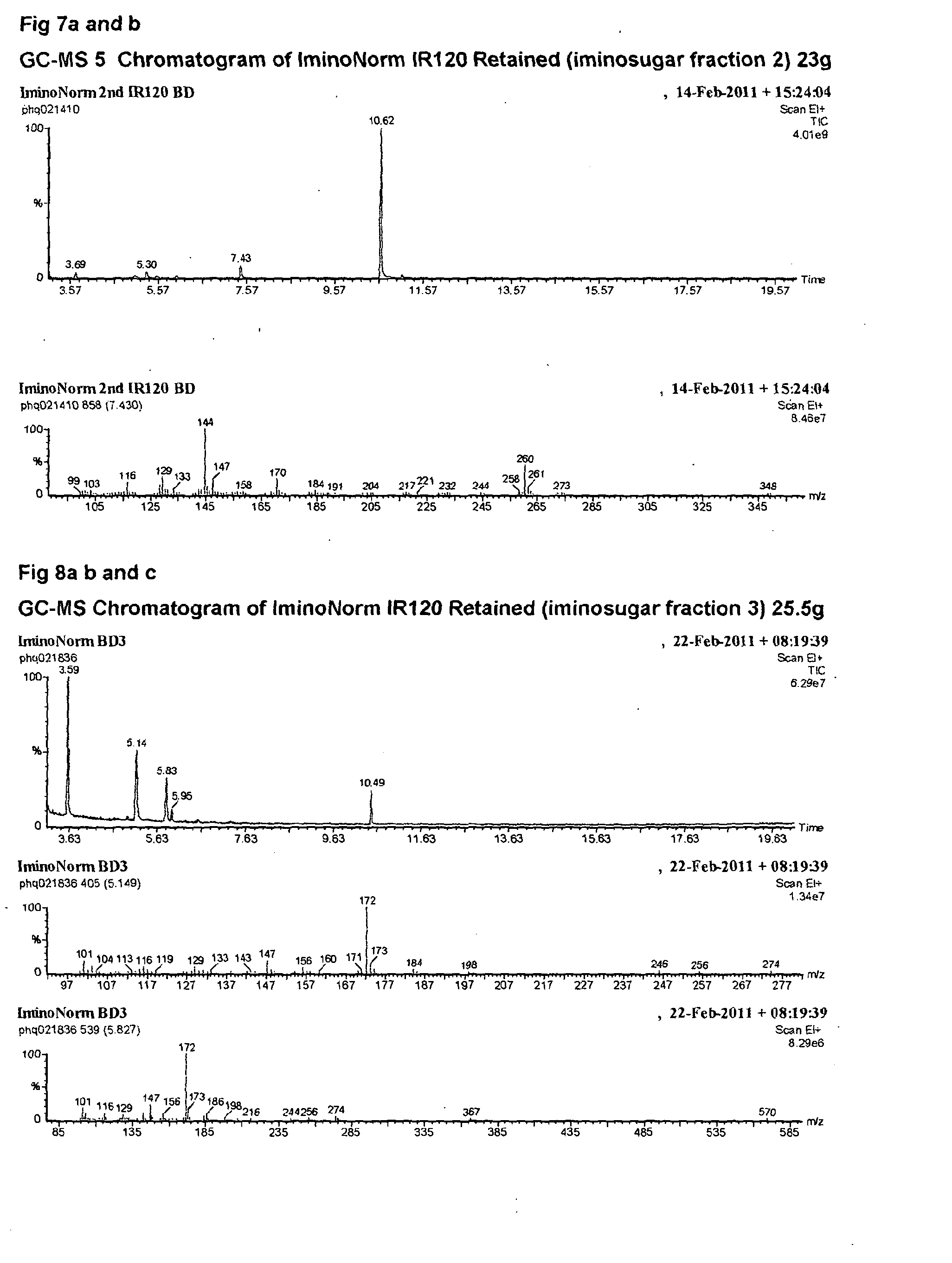
Morus extracts rich in n-acids of imino sugars and or pipecolic acids
ActiveCN104105495AChange hydrophilicity/hydrophobicityImprove three-dimensional structureMetabolism disorderBiological material analysisPipemidic acidDiabetes mellitus
Owner:PHYNOVA LTD
Method for producing hydroxy-l-pipecolic acid
ActiveUS20180273993A1High efficiencyHigh optical purityOxidoreductasesRecombinant DNA-technologyIonL-pipecolic acid
A novel method of producing high-purity hydroxy-L-pipecolic acids in an efficient and inexpensive manner while suppressing the production of hydroxy-L-proline is provided. The method includes allowing an L-pipecolic acid hydroxylase, a microorganism or cell having the ability to produce the enzyme, a processed product of the microorganism or cell, and / or a culture liquid comprising the enzyme and obtained by culturing the microorganism or cell, to act on L-pipecolic acid as a substrate in the presence of 2-oxoglutaric acid and ferrous ion, wherein the L-pipecolic acid hydroxylase has the properties:(1) the enzyme can act on L-pipecolic acid in the presence of 2-oxoglutaric acid and ferrous ion to add a hydroxy group to the carbon atom at positions 3, 4, and / or 5 of L-pipecolic acid; and(2) the enzyme has a catalytic efficiency (kcat / Km) with L-proline that is equal to or less than 7 times the catalytic efficiency (kcat / Km) with L-pipecolic acid.
Owner:API CORP (JP)
Method for synthesizing L-2-piperidinecarboxylic acid through whole-cell catalysis
ActiveCN107287256AOvercome costsOvercome the conditionsMicroorganism based processesFermentationChemical synthesisHigh energy
The invention belongs to the technical field of biocatalysis, and particularly relates to a method for synthesizing L-2-piperidinecarboxylic acid through whole-cell catalysis. According to the method for synthesizing L-2-piperidinecarboxylic acid through the whole-cell catalysis, L-lysine hydrochloride is used as a matrix; and by adding nicotinamide adenine dinucleotide and Arenimonas donghaensis DSM 18148 protein coding gene-containing recombinant host bacteria, or Pseudomonas veronii CIP104663 protein coding gene-containing recombinant host bacteria, or Streptomyces hirsutus ATCC 19091 protein coding gene-containing recombinant host bacteria, the L-2-piperidinecarboxylic acid is prepared through biocatalysis. Therefore, the problems of high cost, harsh condition, low conversion rate, high energy consumption and high pollution of an existing chemical synthesis method are solved. Moreover, a biocatalysis system disclosed by the invention is high in enzyme activity efficiency, short in reaction time and high in ee value of a target product.
Owner:NANJING NUOYUN BIOLOGICAL TECH CO LTD
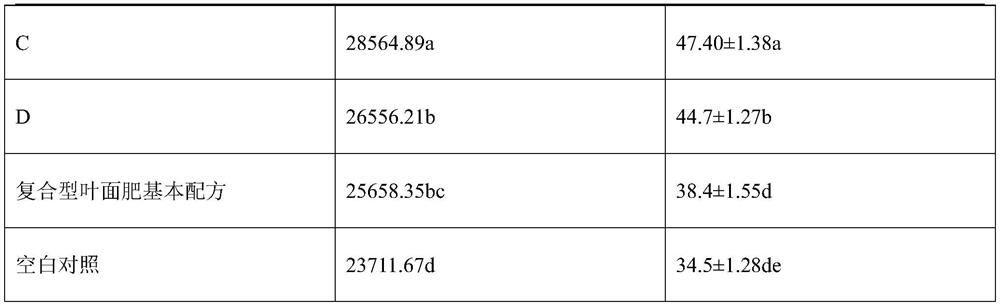
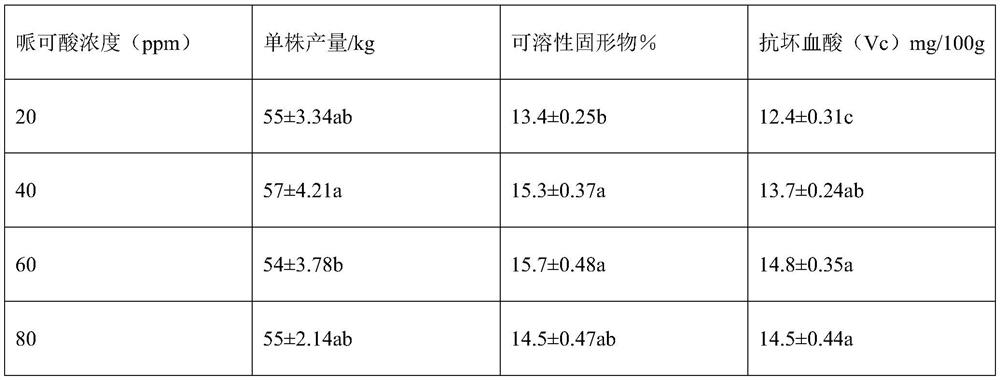
Leaf fertilizer containing organic acid
ActiveCN113735663AImprove inner qualityIncrease productionAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersOrganic acidPipecolic acid
The invention discloses a leaf fertilizer containing organic acid. The pipecolic acid composition contains pipecolic acid or malic acid. The invention discloses the application of pipecolic acid or malic acid in increasing vitamin C content and soluble solid content in fruits and vegetables or increasing vegetable yield. On the basis of the basic formula of the compound foliar fertilizer, by adding pipecolic acid or malic acid, the inherent quality of fruits and vegetables can be obviously improved, for example, the content of soluble solids and vitamin C is increased, the yield is increased, and therefore the compound foliar fertilizer can be used for fruit and vegetable production.
Owner:SOUTH CHINA BOTANICAL GARDEN CHINESE ACADEMY OF SCI +1
Directional synthesis method for 21(S) argatroban
Owner:TIANJIN WEIJIE TECH
Biomarker for depression, method for measuring a biomarker for depression, computer program, and recording medium
Provided is a method for using low molecular weight compounds found in the body as a biomarker for diagnosing depression. Specifically, more than one compound selected from a group comprising the following are used: ADP-ribose, ATP, ADP, AMP, serotonin, tryptophan, kynurenine, SDMA (symmetrical dimethylarginine), threonine, glyceric acid, serine, N-acetylaspartic acid, glutamic acid, trigonelline, creatine, 2-methylserine, sphingosine, homovanillic acid, piperidine, sulfoxidized methionine, pipecolic acid, sphinganine, gamma-butyrobetaine, guanidinoacetic acid, isobutyric acid, creatinine, sarcosine, 3-methylbutyric acid, nicotinamide, betaine, ornithine, carnitine, ethanolamine, phosphoethanolamine, taurine, hypotaurine, aspartic acid, methionine, and tyrosine.
Owner:HUMAN METABOLOME TECH
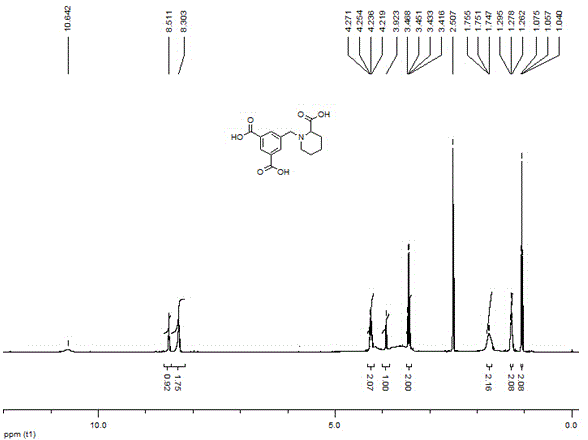
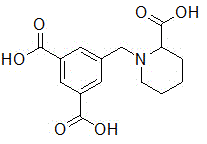
5-((2-carboxypiperidin-1-yl)methyl)isophthalic acid as well as synthetic method and application thereof
The invention discloses 5-((2-carboxypiperidin-1-yl)methyl)isophthalic acid as well as a synthetic method and an application thereof. The synthetic method comprises the following steps of reacting dimethyl 5-(bromomethyl)isophthalate with methyl 2-piperidinecarboxylate to obtain dimethyl 5-((2-methoxycarbonylpiperidin-1-yl) methyl) isophthalate; then carrying out hydrolysis reaction on dimethyl 5-((2-methoxycarbonylpiperidin-1-yl) methyl) isophthalate under a basic condition and then carrying out acidic adjustment on the reaction solution for precipitating a product, filtering the precipitated solid and drying to obtain the target product. The compound can be used as a hydrophilic chain extender to prepare a waterborne polyurethane emulsion.
Owner:天津致嫣达远科技发展有限公司
A preparation method of argatroban intermediates
The invention discloses a preparing method of Argatroban intermediate, which is characterized by the following: adopting NG-nitro-N2-tBoc -L-arginine and (2R,4R)-4-methyl-2-piperidine ethyl formate to condensate; making DCC as dehydrant; reacting under -5-35deg.c for 1-5h and stirring; cooling reacting liquid; filtering to remove solid; stratifying filtrate; removing water layer; washing organic layer through sodium hydroxide solution, citrate solution and saturated salt water; drying; evaporating dichloromethane; obtaining yellow solid product.
Owner:TIANJIN WEIJIE TECH
Synthetic method of (2S,5S or 5R)-N-tert-butoxycarbonyl-5-hydroxy-2-piperidinecarboxylic acid ethyl ester
ActiveCN108239019ALow priceLow toxicityOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupPipecolic acid
The invention discloses a synthetic method of (2S,5S or 5R)-N-tert-butoxycarbonyl-5-hydroxy-2-piperidinecarboxylic acid ethyl ester. Existing methods for synthesizing (2S,5S or 5R)-N-tert-butoxycarbonyl-5-hydroxy-2-piperidinecarboxylic acid ethyl ester have obvious deficiencies and further have difficulty in the construction of cis-piperidine acid hexatomic rings. According to the synthetic method, (2S,5S)-N-tert-butoxycarbonyl-5-hydroxy-2-piperidinecarboxylic acid ethyl ester or / and (2S, 5R)-N-tert-butoxycarbonyl-5-hydroxy-2-piperidinecarboxylic acid ethyl ester is / are prepared from a starting raw material, namely 5-hydroxy-2-pyridine carboxylic acid through nine steps of esterification salinization, reduction, Boc treatment, oxygen, protection treatment, lipase chiral resolution, Boc treatment, deprotection and reductase reduction. The synthetic method has the beneficial effects that reaction conditions are mild and easily controlled, the operation is simple and convenient, adopted reaction reagents are cheap, the production cost is greatly lowered, reaction byproducts are relatively low in toxicity and environmentally friendly, and the method is beneficial to the large-scale production.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Morus extracts rich in n-acids of imino sugars and or pipecolic acids
ActiveUS20140328950A1Good curative effectEliminate side effectsBiocideMetabolism disorderDiseaseImino Sugars
This invention relates to extracts rich in N-acids of imino sugars (as opposed to neutral and basic imino sugars) and / or pipecolic acids obtainable from the plant leaves of mulberry (Morus). The extracts have been shown to have enzymatic activities making extracts rich in these compounds, and the compounds isolated from these extracts, to be good candidates for use in the treatment of diseases, particularly, but not exclusively, metabolic disorders, such as, for example, diabetes.
Owner:PHYNOVA LTD
Pipecolinic acid 4-hydroxylase and method for producing 4-hydroxy amino acid using same
ActiveUS20180282709A1Efficient productionHigh optical purityBacteriaOxidoreductasesPipemidic acidGlutaric acid
The present invention provides a pipecolic acid 4-hydroxylase protein exemplified by the following (A), (B), and (C), having activity to react with L-pipecolic acid in the presence of 2-oxoglutaric acid and iron(II) ions to produce trans-4-hydroxy-L-pipecolic acid, and a method for producing 4-hydroxy amino acid, which method comprises reacting the pipecolic acid 4-hydroxylase protein, cells containing the protein, a treated product of the cells, and / or a culture liquid obtained by culturing the cells, with α-amino acid to produce 4-hydroxy amino acid:(A) a polypeptide comprising the amino acid sequence represented by SEQ ID NO:2, 4, 6, 8, 10, 12, 16, or 18;(B) a polypeptide comprising the amino acid sequence represented by SEQ ID NO:2, 4, 6, 8, 10, 12, 16, or 18 except that one or several amino acids are deleted, substituted, and / or added, and having pipecolic acid 4-hydroxylase activity; and(C) a polypeptide having an amino acid sequence that is not less than 80% identical to the amino acid sequence represented by SEQ ID NO:2, 4, 6, 8, 10, 12, 16, or 18, and having pipecolic acid 4-hydroxylase activity.
Owner:API CORP (JP)
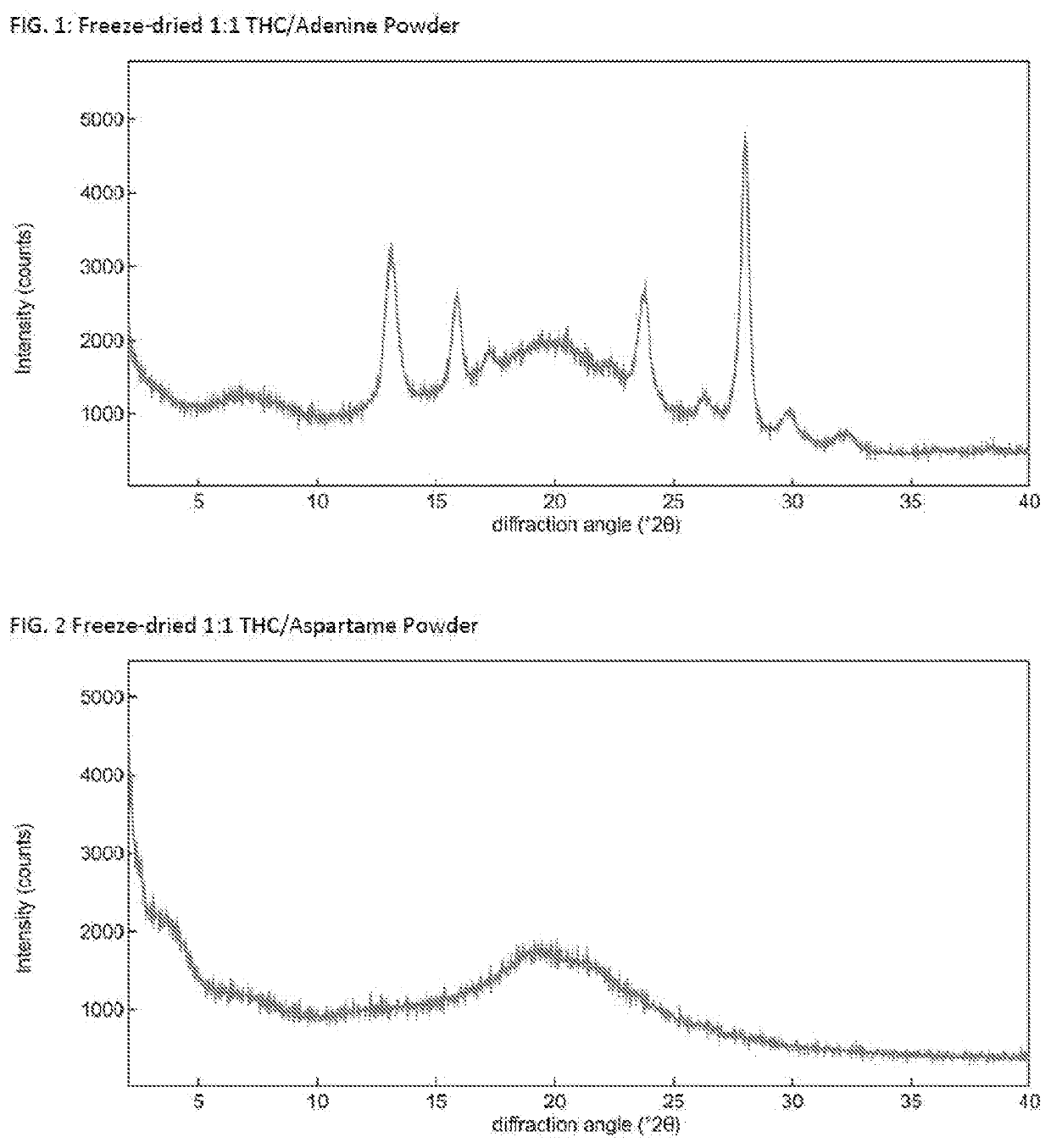
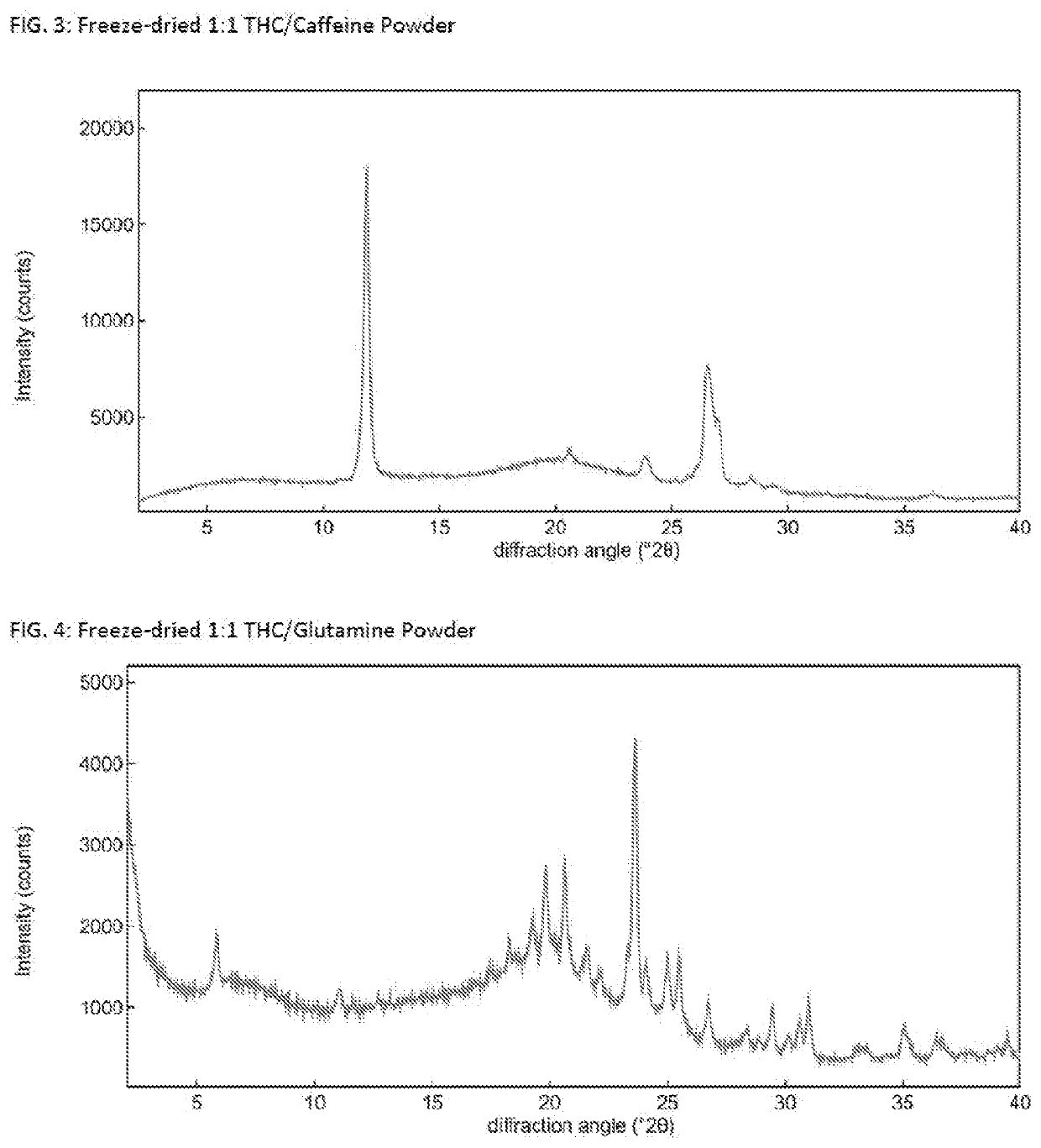
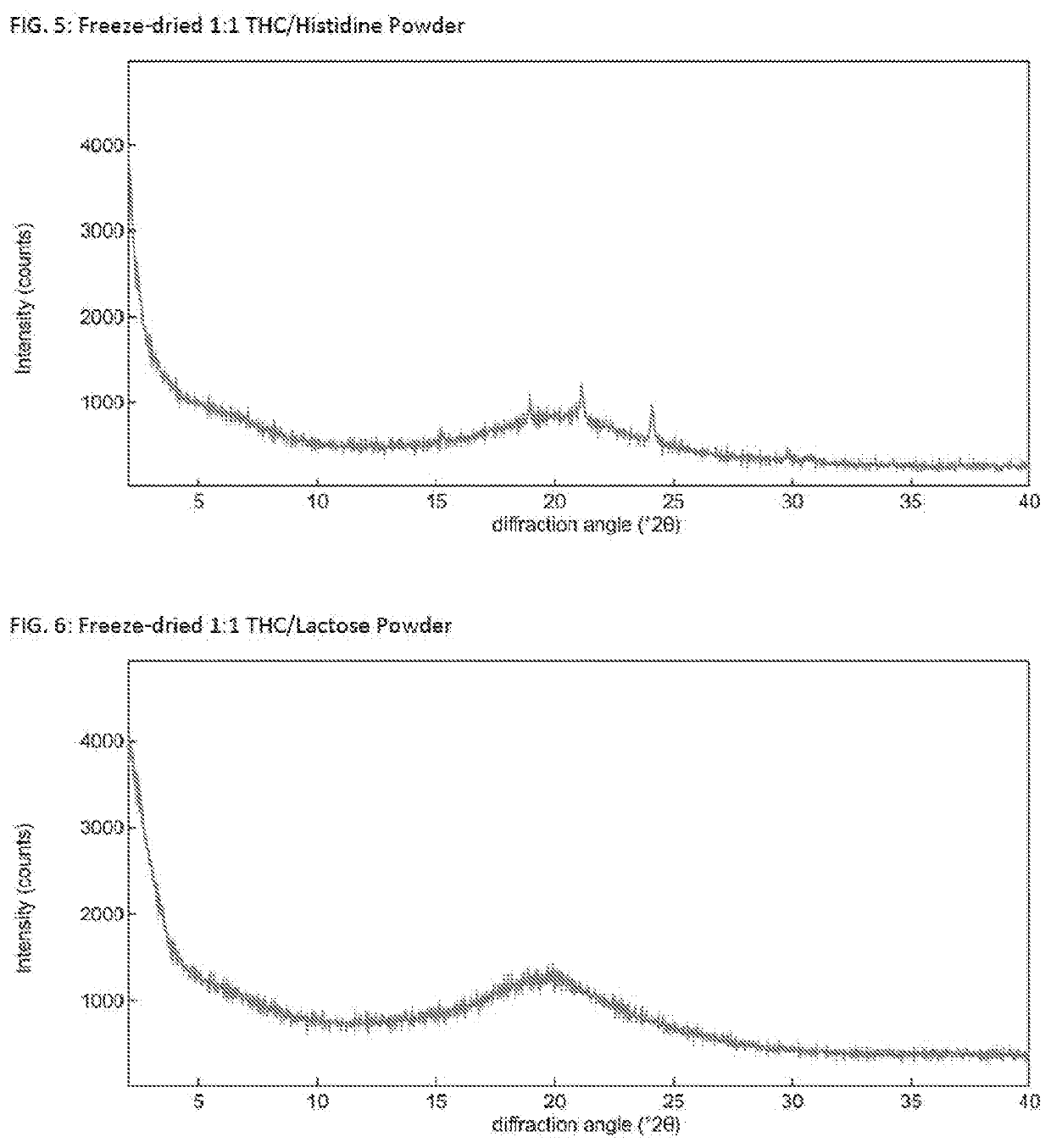
Solid delta9-tetrahydrocannabinol (delta9-thc) compositions
The invention relates to a solid Δ9-THC composition containing Δ9-THC and a powder former and having a molar ratio of Δ9-THC to powder former to form a flowable Δ9-THC powder. The powder former is selected from the group consisting of adenine, aspartame, caffeine, lactose, mannitol, nicotinamide, β-nicotinamide adenine dinucleotide, pipecolic acid, saccharin, aspartic acid, glutamic acid, glutamine, histidine, leucine, methionine, phenylalanine, proline, serine, tryptophan, valine, Epigallocatechin Gallate (EGCG), 2-Hydroxypropyl-beta-cyclodextrin (HPbCD), and Trimethyl-beta-cyclodextrin (TOMBC) and mixtures thereof. The invention also relates to methods of making a solid Δ9-THC composition of the invention. The Δ9-THC may be synthetic Δ9-THC or may be extracted Δ9-THC. The invention provides pharmaceutical or nutraceutical composition containing a solid Δ9-THC composition of the invention and a pharmaceutically- or nutraceutically-acceptable carrier where Δ9-THC is present in a pharmaceutically or nutraceutically effective amount. The invention also provides methods of treating a disease, disorder, or condition by administering to a patient in need thereof a therapeutically effective amount of a solid Δ9-THC composition. A solid Δ9-THC composition of the invention may also be incorporated into food and beverage products.
Owner:CANNA CHEMISTRIES LLC
Method for producing hydroxy-L-pipecolic acid
ActiveUS10954539B2High optical purityImprove efficiencyTransferasesRecombinant DNA-technologyPipemidic acidMicroorganism
Owner:API CORP (JP)
Method for producing pipecolamide derivative
Owner:MICROBIOPHARM JAPAN CO LTD
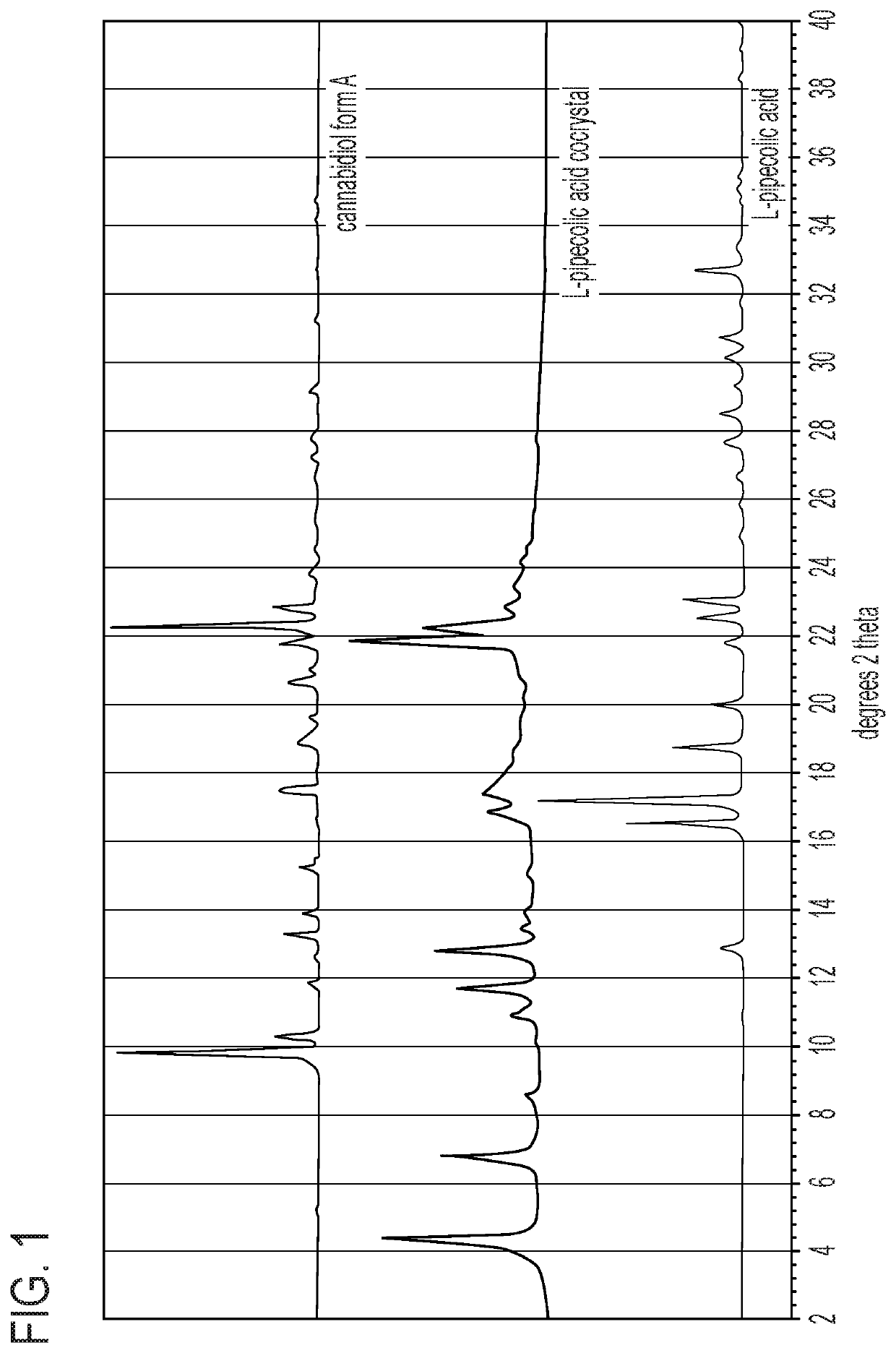
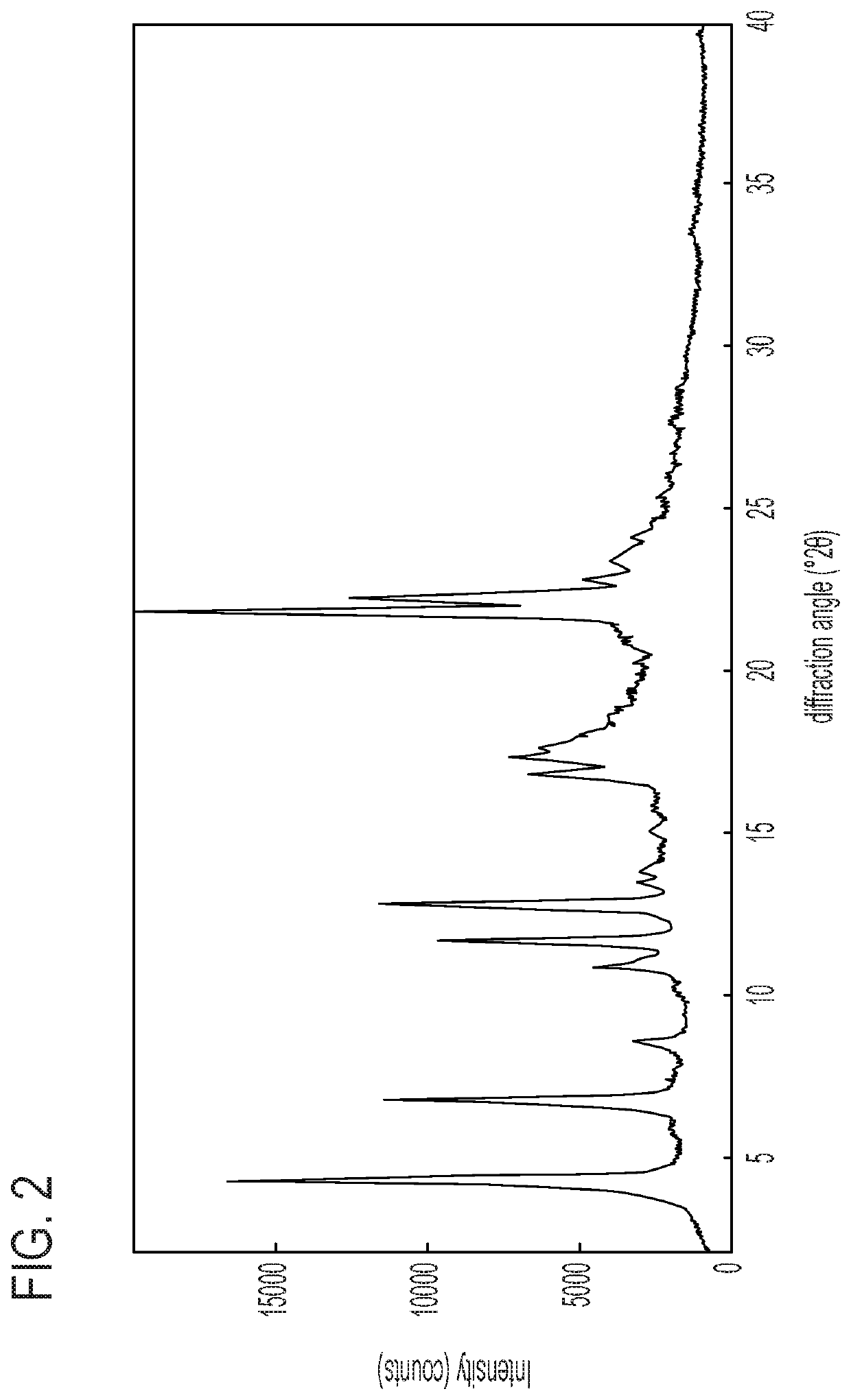
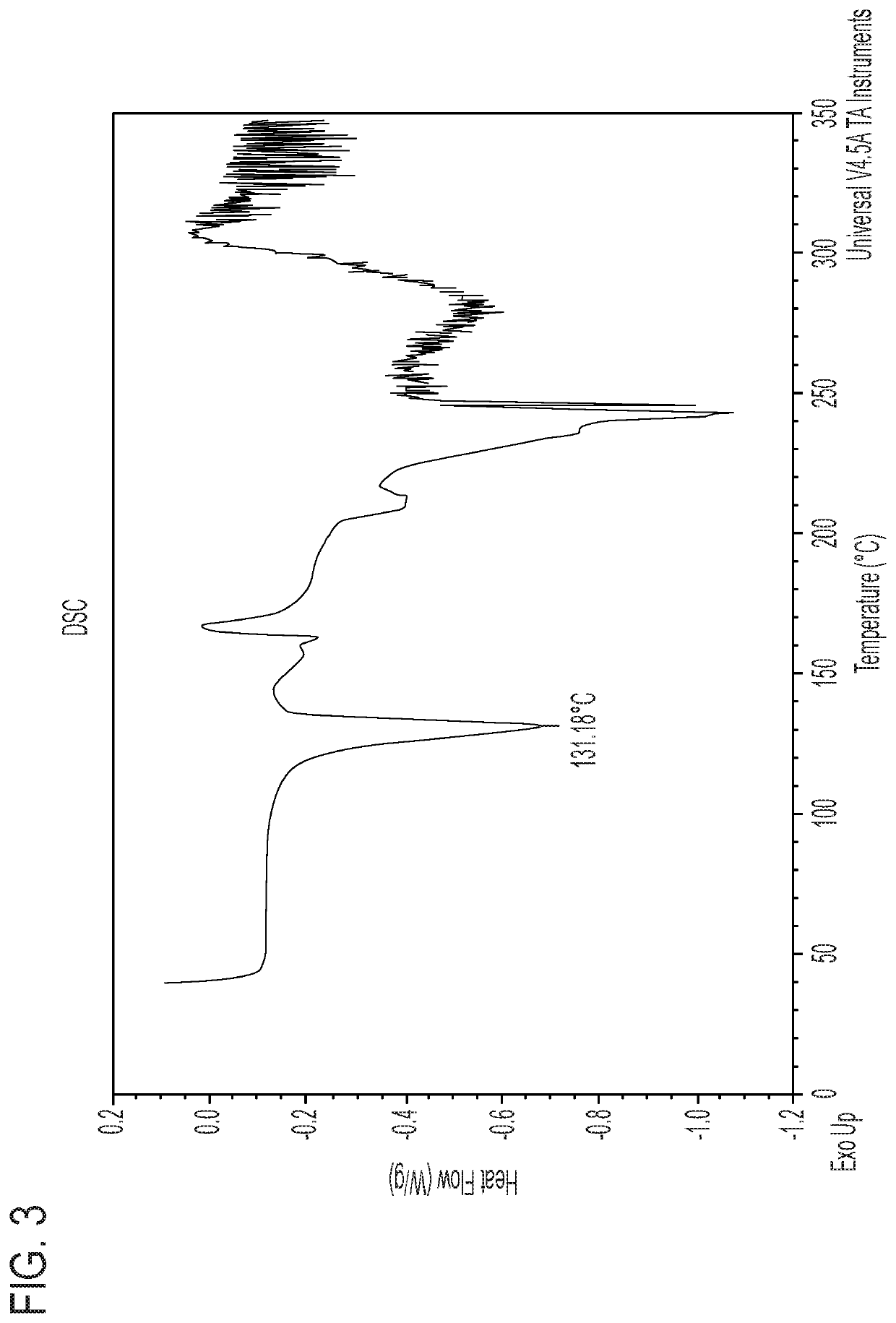
L-pipecolic acid cocrystal of cannabidiol
A cocrystal of cannabidiol is disclosed, specifically a 1:1 cannabidiokL-pipecolic acid cocrystal The beneficial and therapeutic uses of the cocrystal and of compositions containing the cocrystal are also disclosed. The disclosure sets out methods of making and characterizing the cocrystal.
Owner:EBERS TECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com