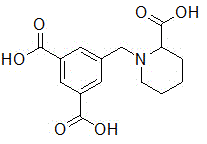
5-((2-carboxypiperidin-1-yl)methyl)isophthalic acid as well as synthetic method and application thereof
A technology of dimethyl bromomethylisophthalate and isophthalic acid, applied in the field of 5-methyl)isophthalic acid and its synthesis, can solve the problem of not retrieving the isophthalic acid compound literature report, not yet Find the problems such as 5-((2-carboxypiperidin-1-yl)methyl)isophthalic acid, and achieve the effect of few reaction steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
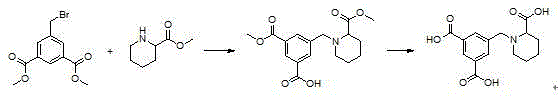
[0032] Step 1: Synthesis of dimethyl 5-((2-methoxycarbonylpiperidin-1-yl)methyl)isophthalate:
[0033] In a 25 mL single-necked flask, weigh methyl 2-piperidinecarboxylate (0.286 g, 2 mmol), dimethyl 5-bromomethylisophthalate (0.572 g, 2 mmol), potassium carbonate (0.414 g, 3 mmol), dissolved in 10 mL of acetonitrile, stirred at room temperature for 16 hours, TLC monitored the reaction was complete, and stopped stirring. Concentrate the reaction solution with a rotary evaporator, extract with ethyl acetate, wash the reaction solution with water to remove excess potassium carbonate, and wash with anhydrous NaSO 4 The organic layer was dried, filtered, and suspended to dryness to obtain dimethyl 5-((2-methoxycarbonylpiperidin-1-yl)methyl)isophthalate as a pale yellow liquid with a yield of 91%.
Embodiment 2
[0035] Step 2: Synthesis of 5-((2-carboxypiperidin-1-yl)methyl)isophthalic acid:
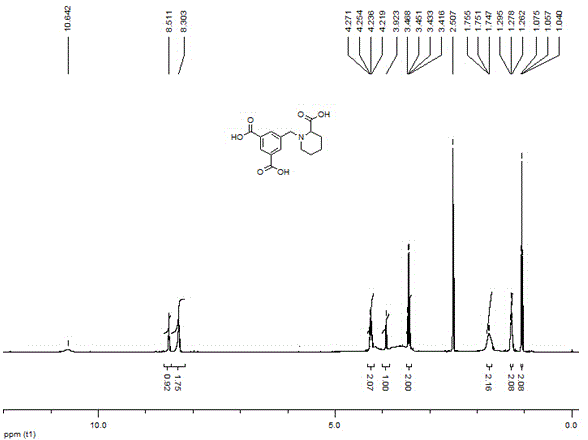
[0036] In a 25 mL single-necked flask equipped with a reflux condenser, the dimethyl 5-((2-methoxycarbonylpiperidin-1-yl)methyl)isophthalate (0.503 g, 1.5 mmol), be dissolved in 2mL water, add potassium hydroxide (0.337 g, 6 mmol), reflux reaction after 6 hours, TLC monitoring reaction is complete, reaction solution is cooled to room temperature, washes with ethyl acetate to remove a small amount of unreacted raw material, Keep the aqueous phase. Then slowly add 1M hydrochloric acid dropwise to adjust the pH of the reaction solution to 3-4. At this time, a large amount of solids precipitated. The precipitated solids were filtered and dried in vacuo to obtain 5-((2-carboxypiperidin-1-yl)methanol Base) isophthalic acid, melting point: 212-214 ° C, yield is 75%. The NMR spectrum of 5-((2-carboxypiperidin-1-yl)methyl)isophthalic acid compound-H figure 1 .
Embodiment 3
[0038] Step 1: Synthesis of dimethyl 5-((2-methoxycarbonylpiperidin-1-yl)methyl)isophthalate:
[0039] In a 25 mL single-necked flask, weigh methyl 2-piperidinecarboxylate (0.286 g, 2 mmol), dimethyl 5-bromomethylisophthalate (0.315 g, 2.2 mmol), potassium carbonate (0.276 g, 2mmol), dissolved in 10mL of acetonitrile, stirred at room temperature for 18 hours, TLC monitoring of complete reaction, stop stirring. Concentrate the reaction solution with a rotary evaporator, extract with ethyl acetate, wash the reaction solution with water to remove excess potassium carbonate, dry the organic layer with anhydrous NaSO, filter, and hang to dryness to obtain 5-((2-methoxycarbonylpiperidine- 1-yl) methyl) dimethyl isophthalate, as light yellow oily liquid, the yield is 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com