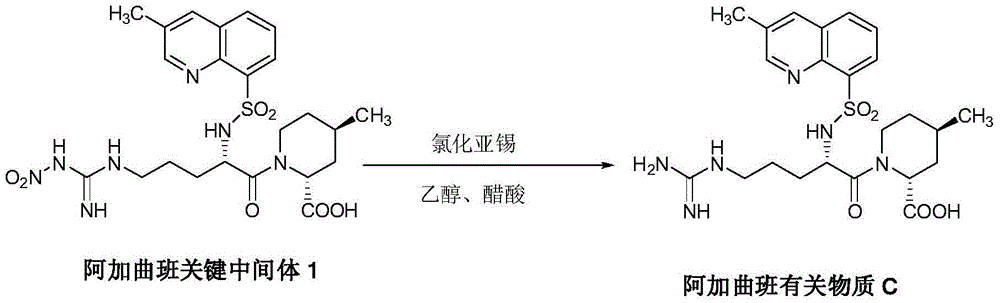
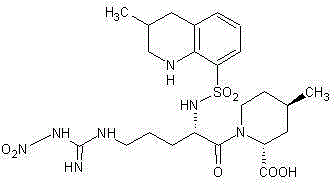
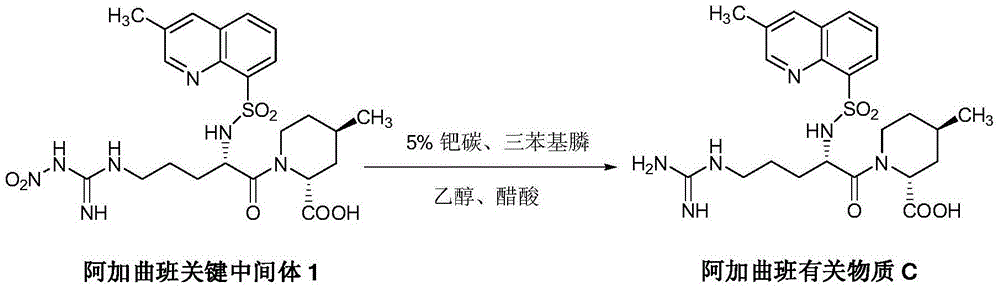
Synthesis and separation identification method for aragatroban related substances
A technology of argatroban and related substances, which is applied in the field of synthesis, separation and identification of related substances of argatroban, can solve the problems that have not been seen in the research reports, and achieve reasonable reaction design, simple and easy operation, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 20ml of fuming nitric acid / 10ml of fuming sulfuric acid into a 100ml three-necked bottle, cool down to below -10°C, slowly add 8.8g of argatroban, after the addition is complete, control the temperature at -10°C to -5°C and stir for 1.5 hours to achieve high efficiency. The liquid phase monitors that the reaction of the raw materials is complete, and the reaction is stopped. Control the low temperature, add 300g of crushed ice to the reaction system, then adjust the pH to 6-7 with concentrated ammonia water, a off-white to light yellow solid precipitates, filter with suction, wash the filter cake with water, and vacuum dry at 40°C for 6h to obtain argatro Class related substance A5.1g, purity 95.6%, yield 53.2%. Product identification: MS (m / z), theoretical value: 553, measured value: 554 [M+H + ].
Embodiment 2
[0034] Add 10ml of fuming nitric acid and 20m of acetic acid into a 100ml three-necked flask, cool down to below -10°C, and slowly add 4.4g of argatroban. After the addition, the temperature was controlled at -10°C to 0°C and the reaction was stirred for 2.5 hours. The high-performance liquid phase was used to monitor the complete reaction of the raw materials, and the reaction was stopped. Control the low temperature, add 200g of crushed ice to the reaction system, adjust the pH to 6-7 with ammonia water, and a off-white to light yellow solid precipitates, filter with suction, wash the filter cake with water, and vacuum-dry at 40°C for 6 hours to obtain related substances of argatroban A1.6g, purity 89.2%, yield 33.4%. Product identification: MS (m / z), theoretical value: 553, measured value: 554 [M+H + ].
Embodiment 3
[0036] Add 3g of argatroban and 90ml of a saturated aqueous solution of barium hydroxide or calcium hydroxide into a 250ml three-necked bottle, stir and heat to reflux, and keep reflux for 12h, and the content of related substance B of argatroban is no longer increased by high performance liquid phase monitoring. Stop responding. Cool to room temperature, add 100ml of ethyl acetate, adjust the pH to neutral with dilute sulfuric acid, and filter with suction to remove insoluble substances. The filtrate was layered, the aqueous phase was extracted with 60ml of ethyl acetate, the organic layers were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered with suction, and the filtrate was concentrated to obtain 0.4g of oily argatroban related substance B with a purity of 93.6%. Yield 14.5%. Product identification: MS (m / z), theoretical value: 466, measured value: 467 [M+H + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com