Method for manufacturing cis-5-hydroxy-l-pipecolic acid
A manufacturing method, pipecolic acid hydroxylase technology, applied in the direction of biochemical equipment and methods, enzymes, oxidoreductases, etc., to achieve low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Cloning of α-ketoglutarate-dependent pipecolate hydroxylase gene
[0114] The putative L-proline cis-4-hydroxylase XdPH (GenBank accession number CDG16639, sequence number 4) encoding Xenorhabdus doucetiae FRM16 strain source was artificially synthesized by DNA2.0 company for expression in Escherichia coli The codon-optimized gene sequence (xdph_Ecodon, SEQ ID NO: 1) was inserted into pJExpress411 (DNA2.0) to create plasmid pJ411XdPH.
[0115] Similarly, cloning of a representative enzyme gene known to exhibit the 5-hydroxylation activity of pipecolic acid was also carried out. The L-picolic acid cis-5-hydroxylase SruPH (GenBank accession number EFV12517, sequence number 6) encoding Segniliparus rugosus NBRC101839 strain and the source of Sinorhizobium meliloti 1021 strain were artificially synthesized by DNA2.0 company. The codon-optimized gene sequences sruph_Ecodon (SEQ ID NO. 3) and smph_Ecodon of the L-proline cis-4-hydroxylase SmPH (GenBank accession number CAC...
Embodiment 2
[0117] Acquisition of α-ketoglutarate-dependent pipecolate hydroxylase gene expressing bacteria and confirmation of expression level
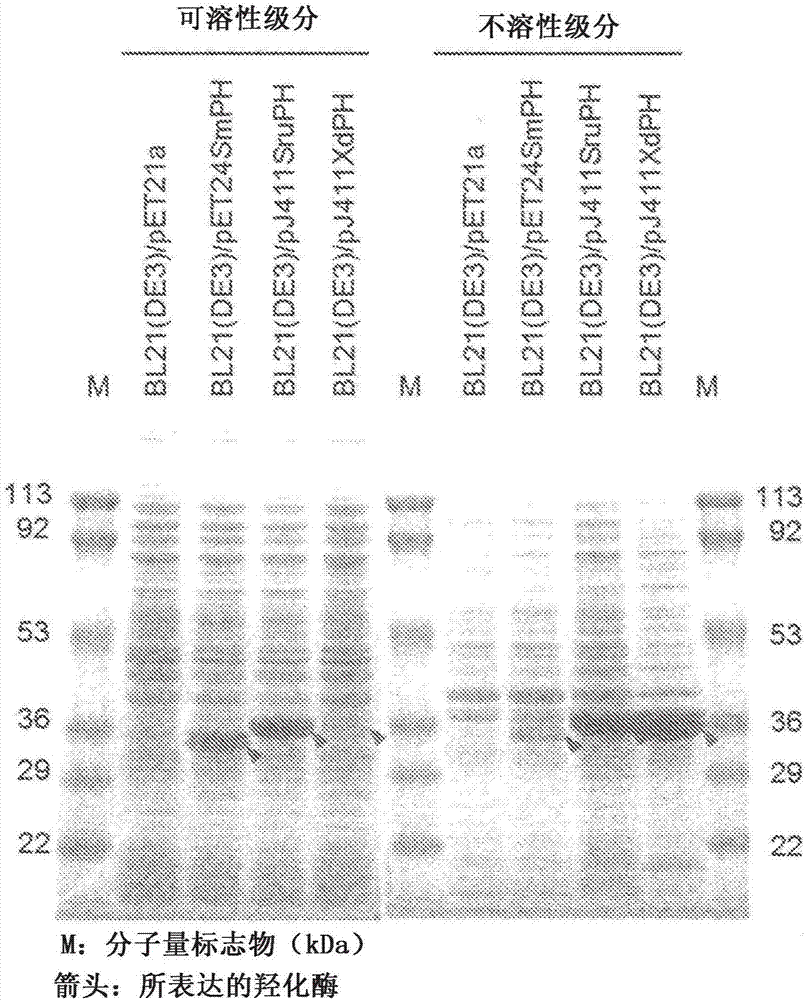
[0118] Next, Escherichia coli (Escherichia coli) BL21(DE3) (manufactured by Invitrogen) was transformed by a conventional method using each of the obtained plasmids to obtain recombinant Escherichia coli BL21(DE3) / pJ411XdPH, BL21(DE3) / pJ411SruPH, BL21(DE3 ) / pET24SmPH. In order to obtain bacterial cells expressing the introduced gene, each recombinant Escherichia coli was cultured at 28°C for 4 to 6 hours in liquid LB medium containing kanamycin and a lac promoter-inducing substance, and then incubated at 15°C. After further culturing for about 40 hours, bacterial cells were collected.
[0119] The obtained recombinant Escherichia coli was measured by turbidity OD 630 Suspended in 50mmol / L MES (2-morpholineethanesulfonic acid) buffer at pH 7 in a mode of about 10. 0.5 mL of this suspension was sonicated on ice, and then centrifuged at 12,00...
Embodiment 3
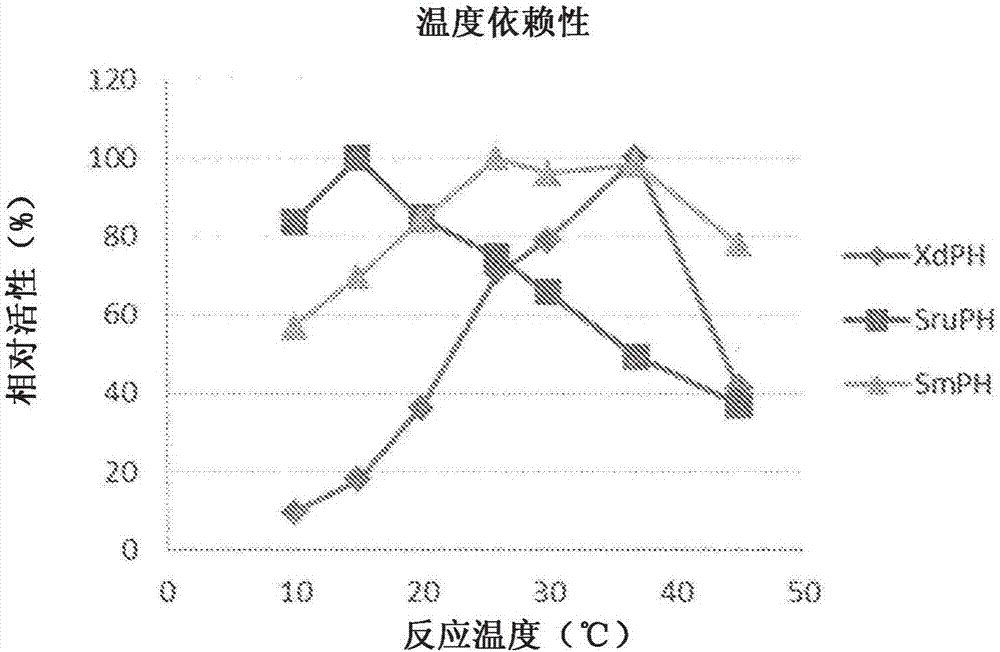
[0122] Activity confirmation of α-ketoglutarate-dependent pipecolate hydroxylase
[0123] 5mmol / L L-picolic acid, 10mmol / L α-ketoglutarate, 1mmol / L L-ascorbic acid, 0.5mmol / L iron sulfate, and the protein concentration of about 2mg / mL were added with Example 2 in a plastic tube. 0.2 mL of each crude enzyme solution obtained in was shaken at 30° C. for 1 hour.
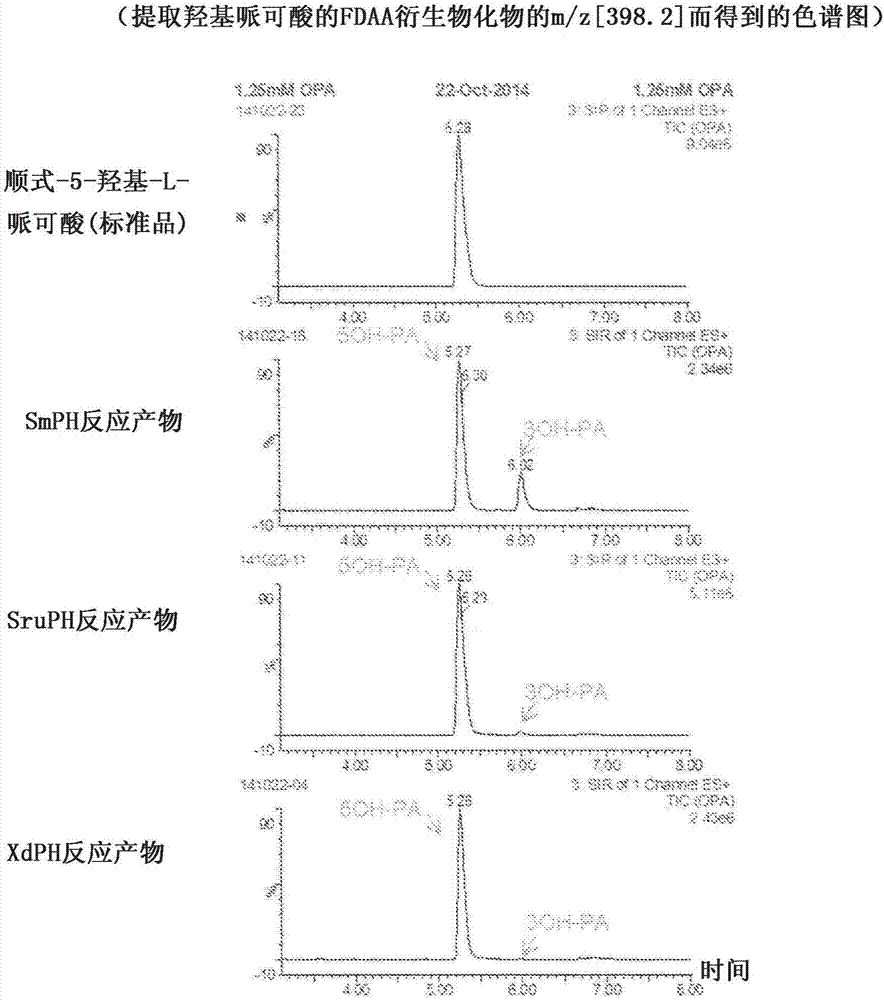
[0124] The reaction product was derivatized with 1-fluoro-2,4-dinitrophenyl-5-L-alaninamide (FDAA), and analyzed by UPLC-MS (manufactured by Waters). The result is as figure 2 As shown, it was confirmed that a compound corresponding to the retention time of the 5-hydroxypicolic acid standard of 5.3 minutes was produced from the solution reacted using each crude enzyme solution. The 5-hydroxypicolic acid production activity (U / g) exhibited by each enzyme liquid was calculated as 4.1 U / g, 8.9 U / g, and 3.1 U / g, respectively, in terms of the unit protein amount (g). The unit (U) here represents the ability to generate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com