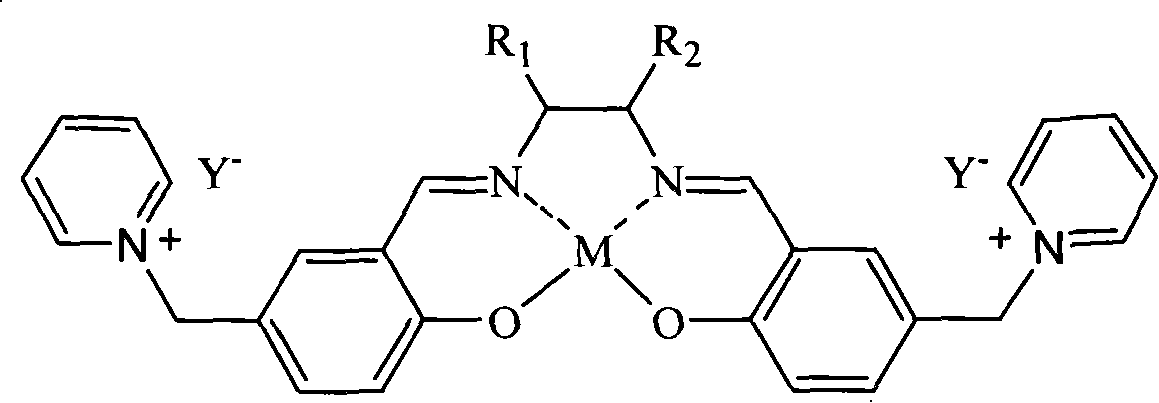
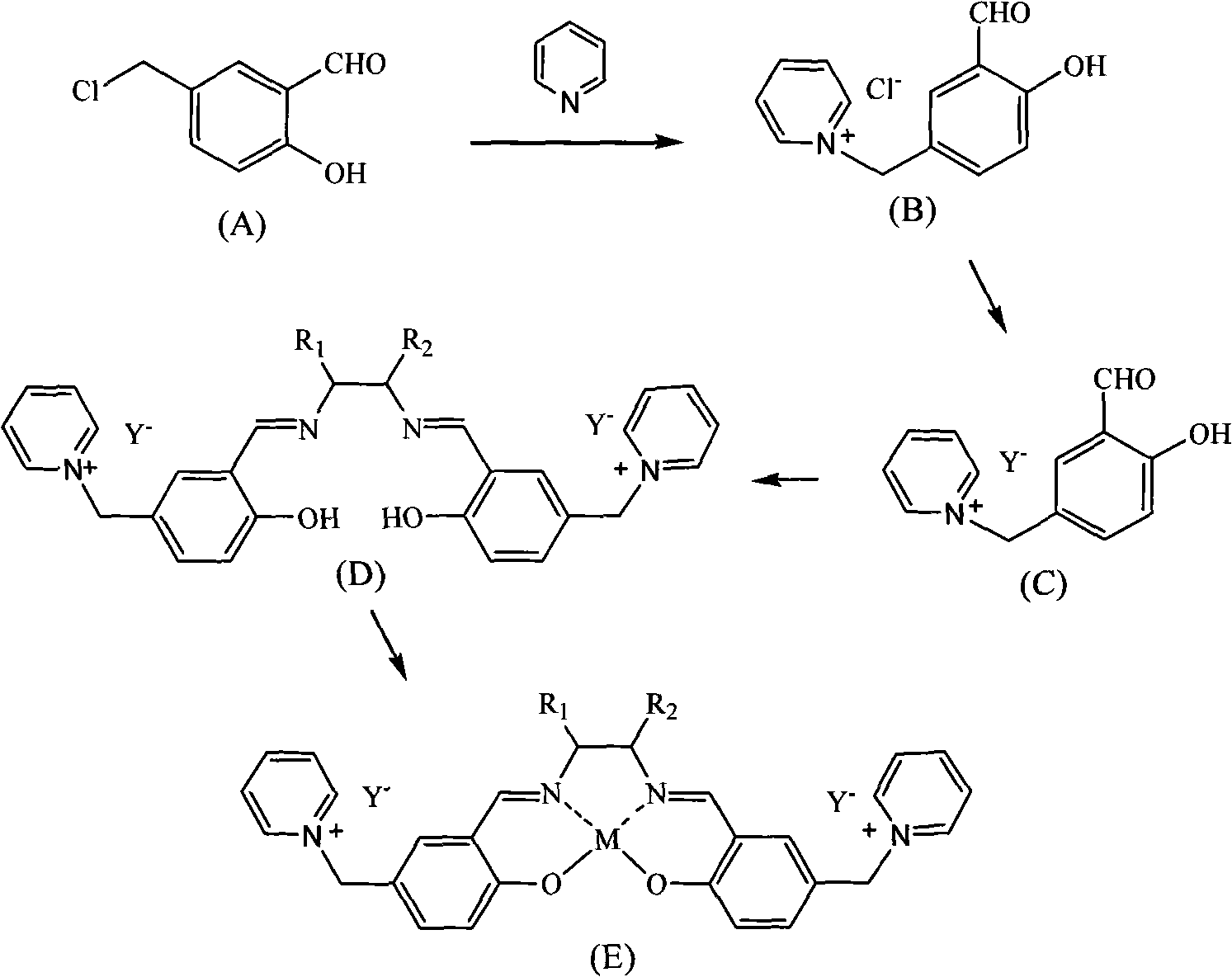
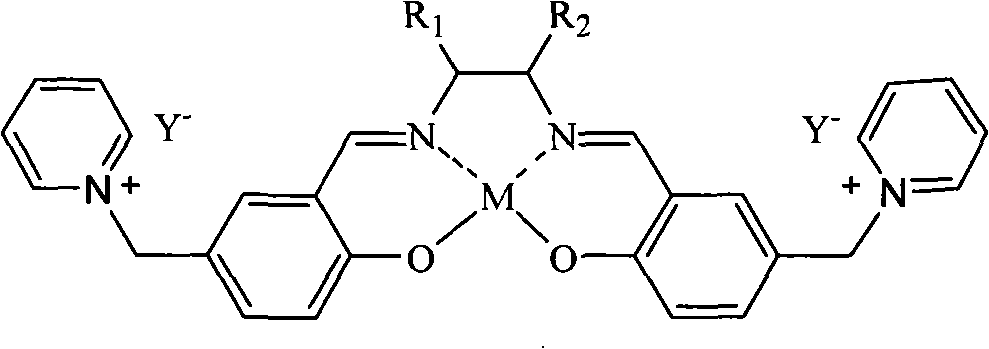
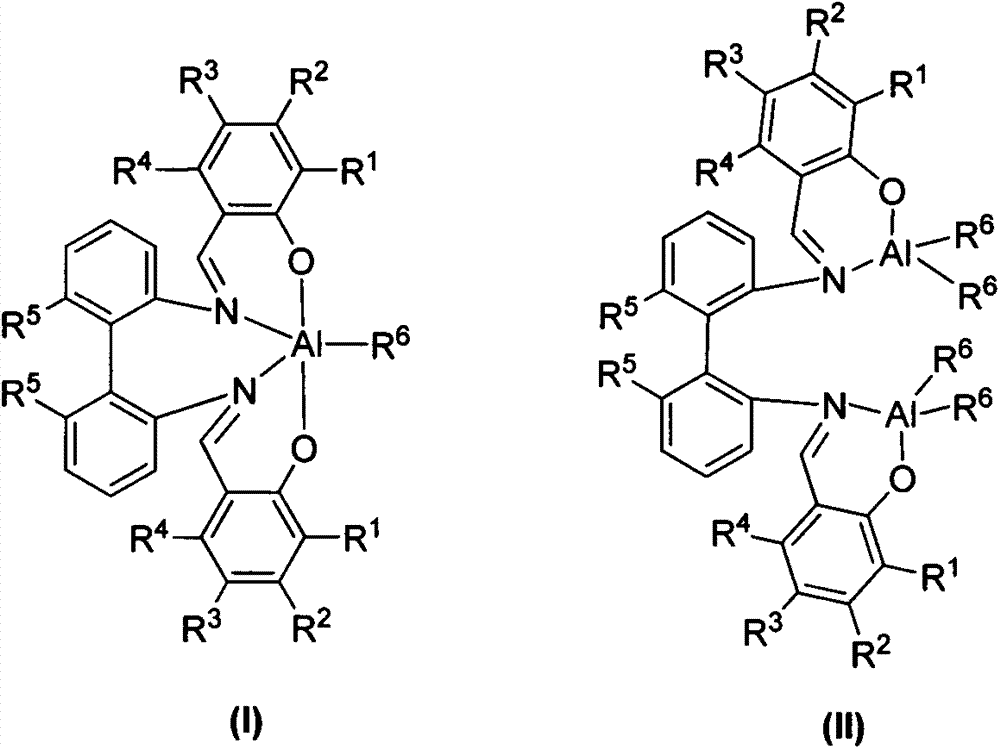
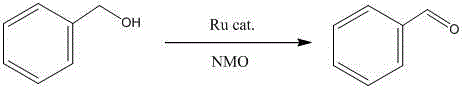Patents
Literature
42 results about "Salen ligand" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
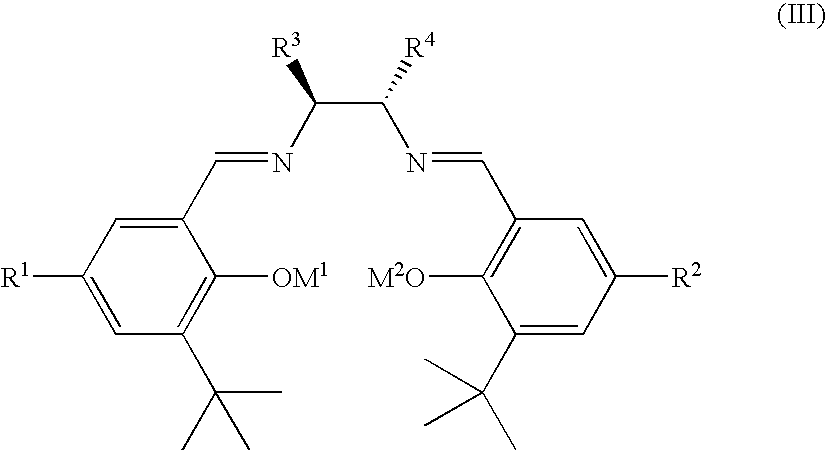
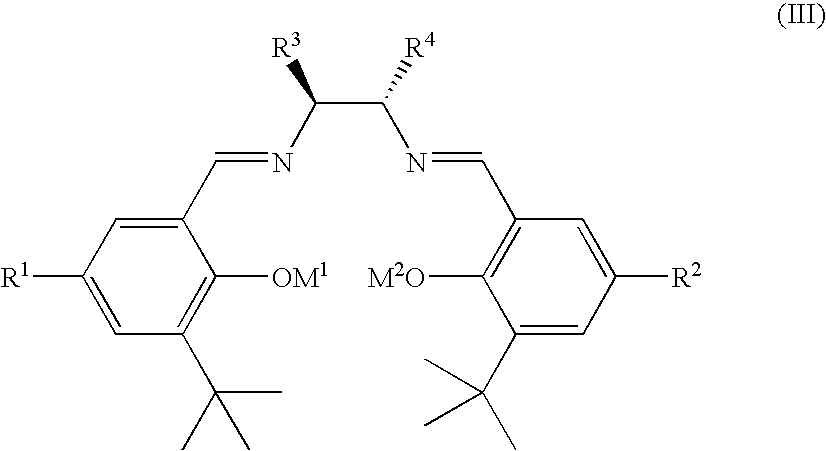
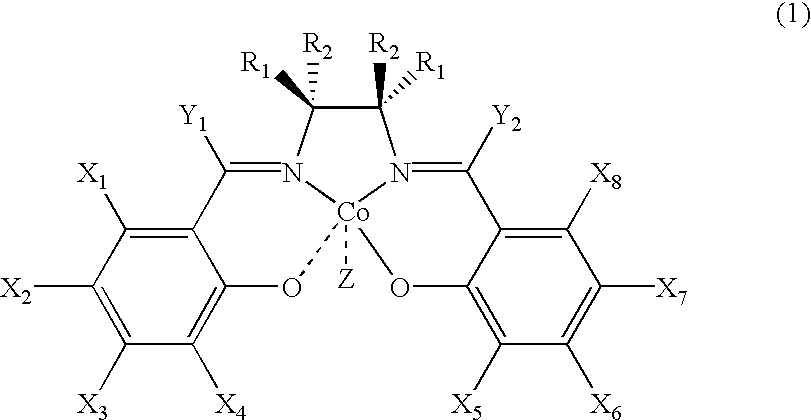
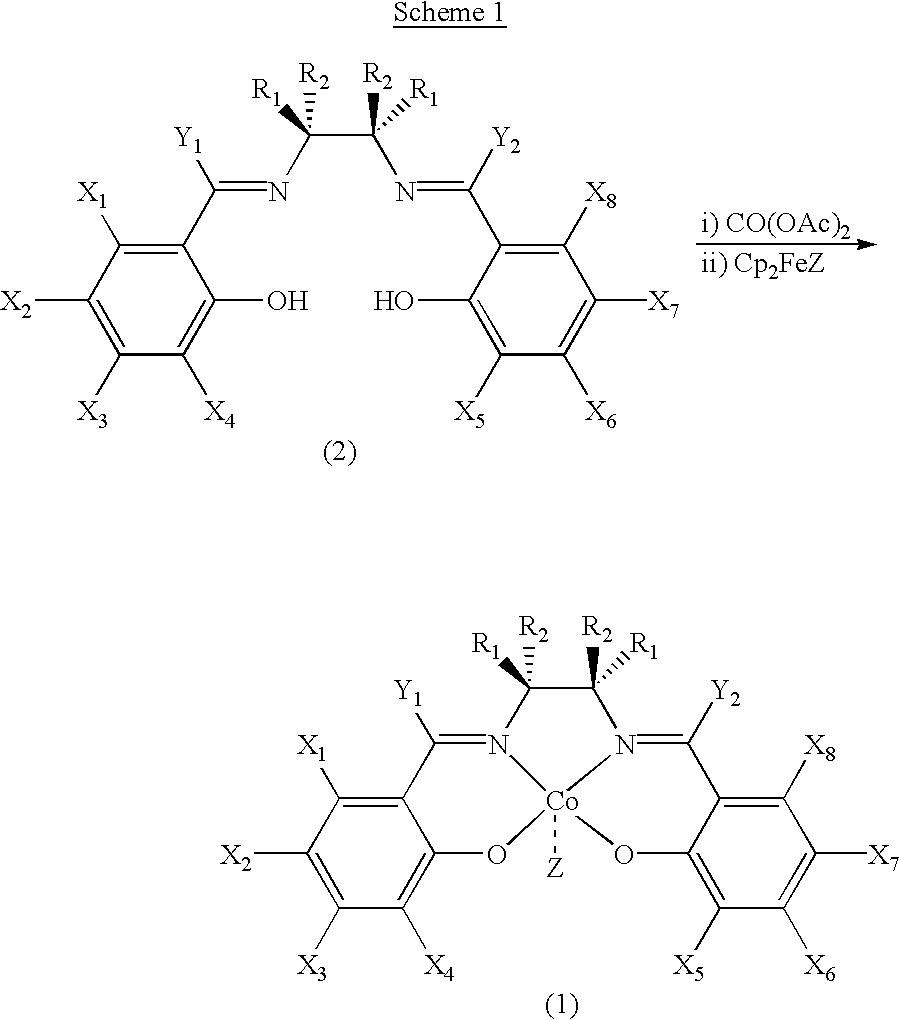
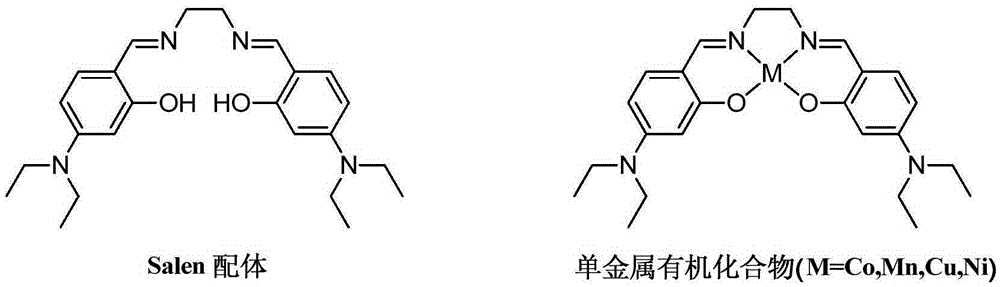
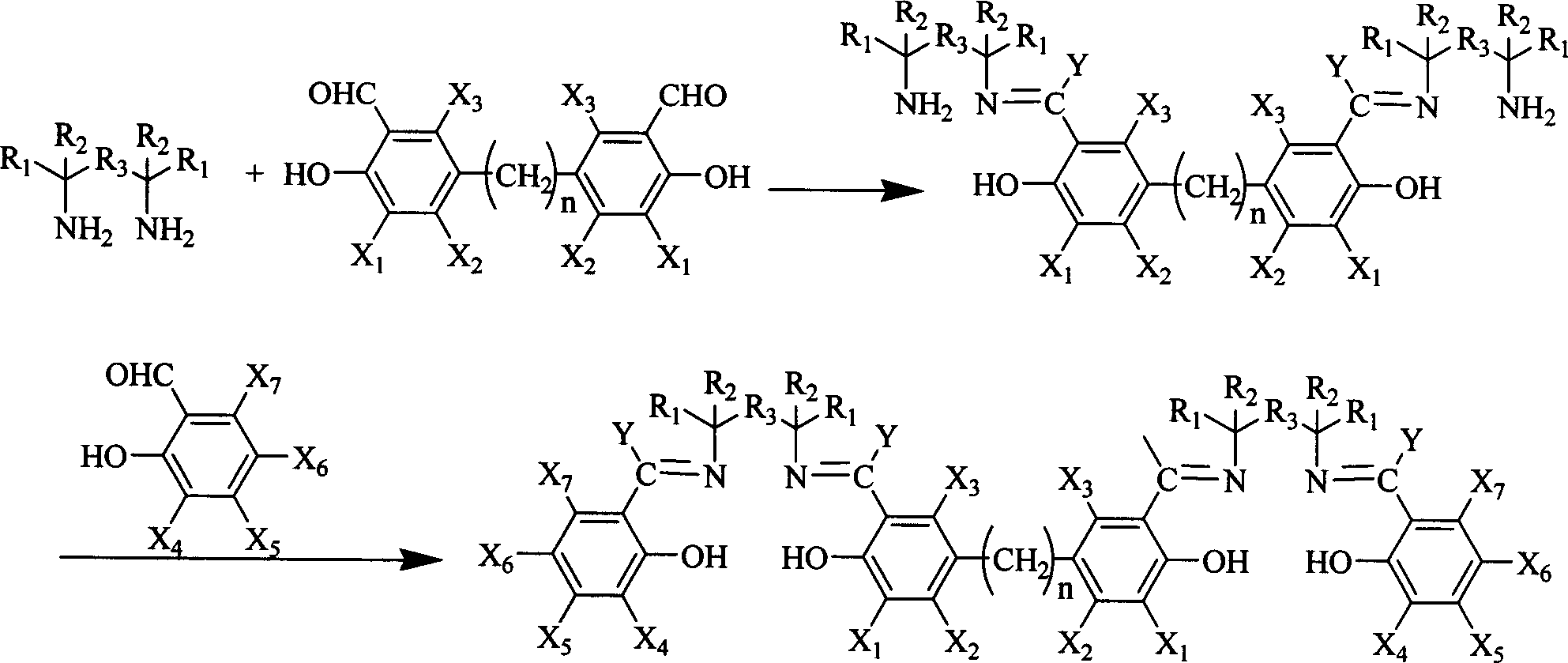
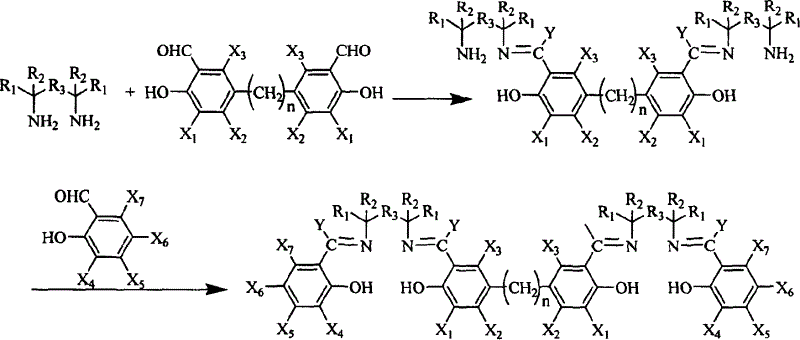
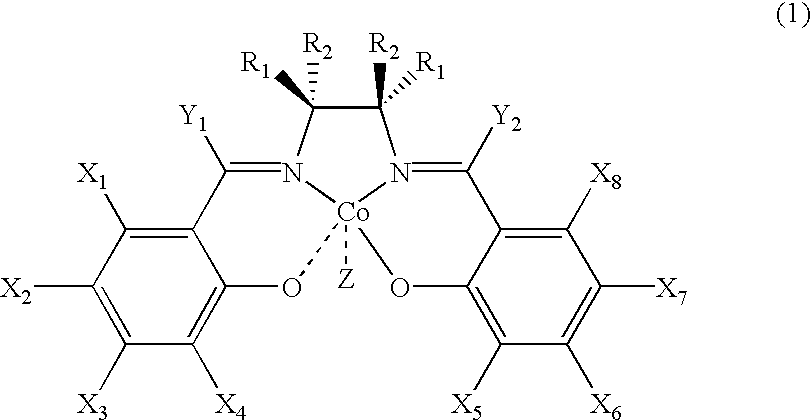
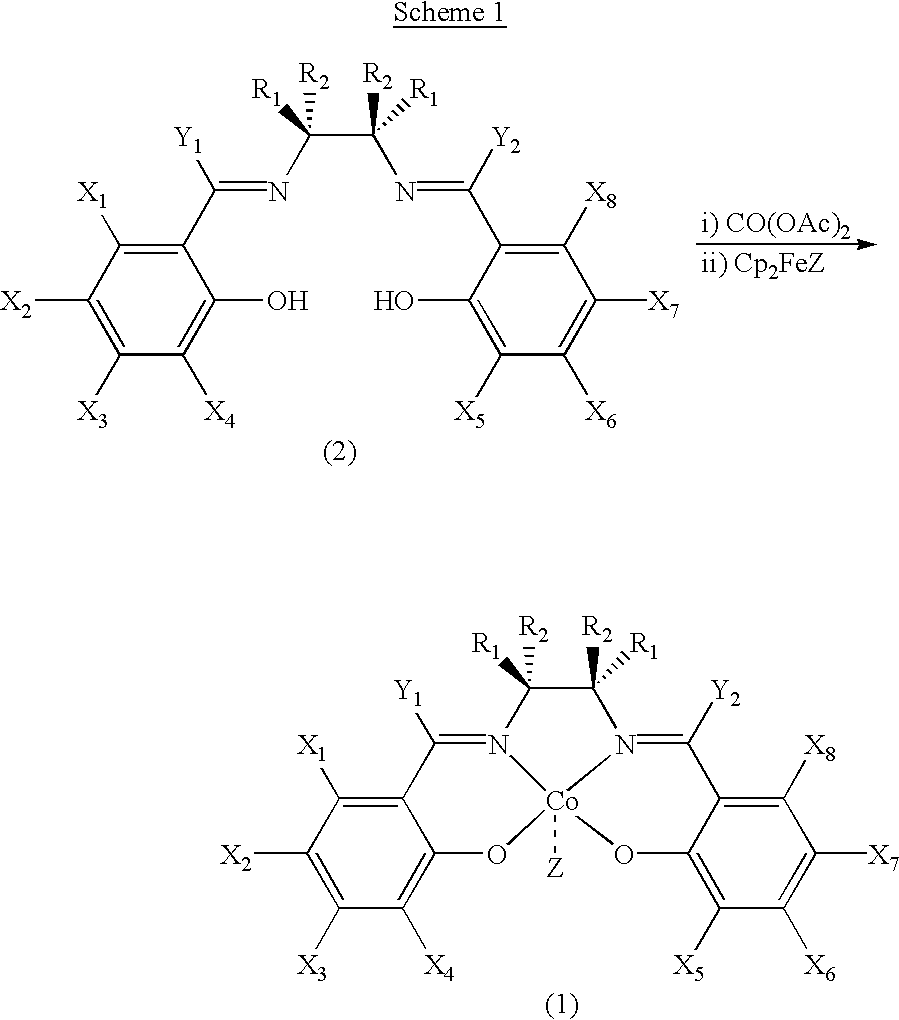
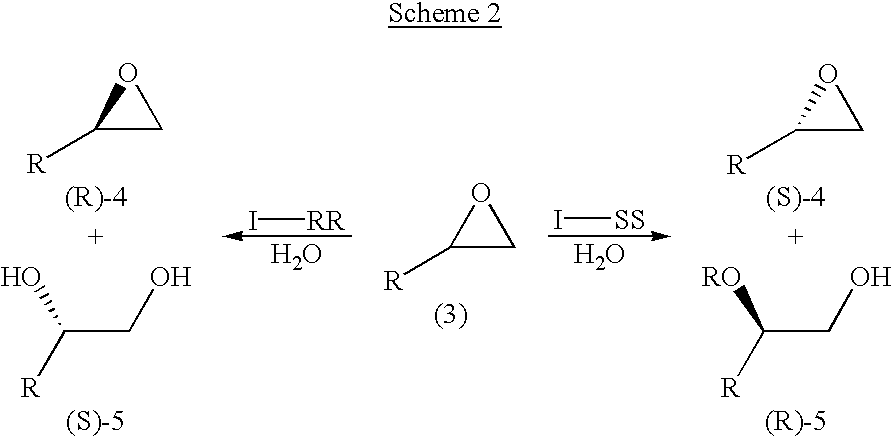
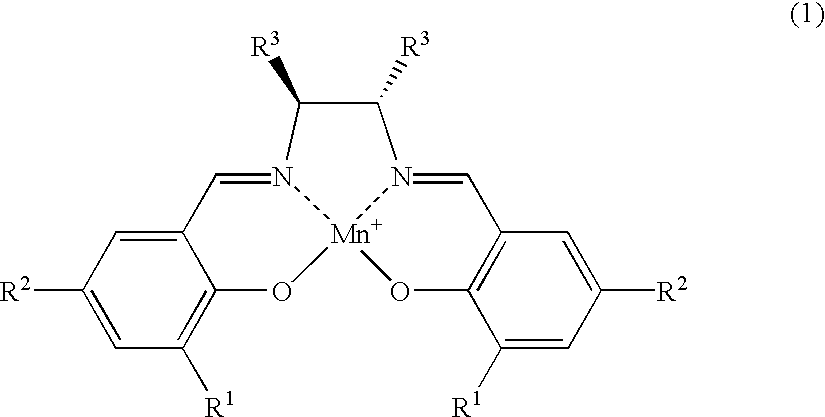
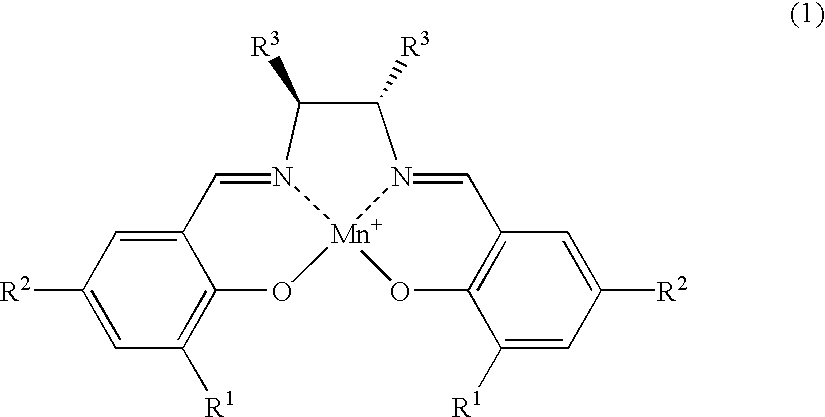
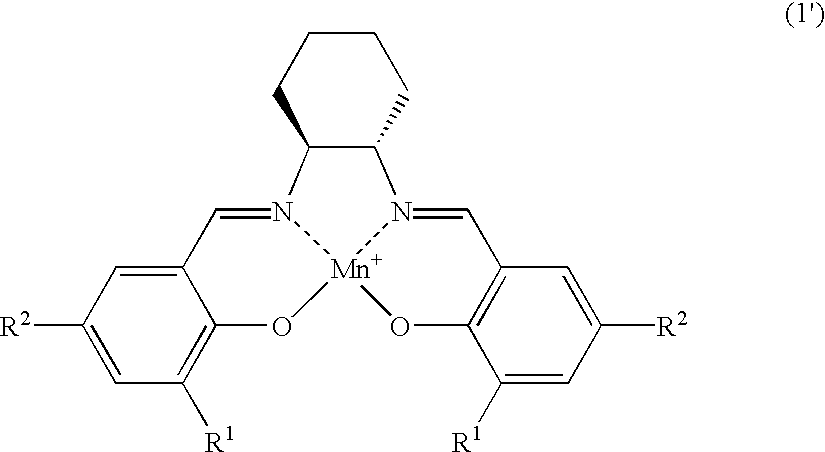
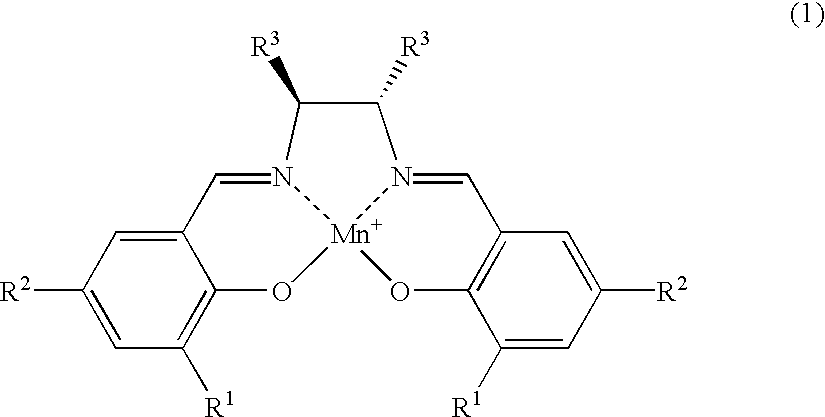
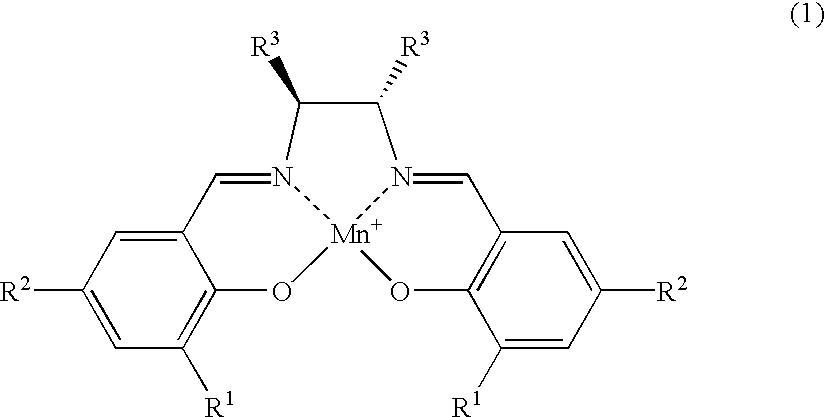
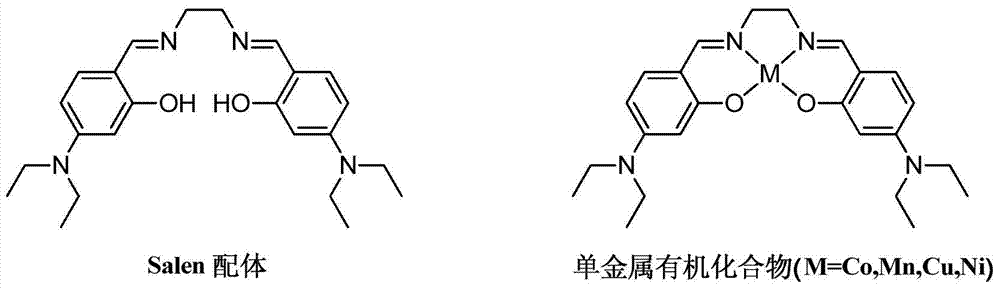
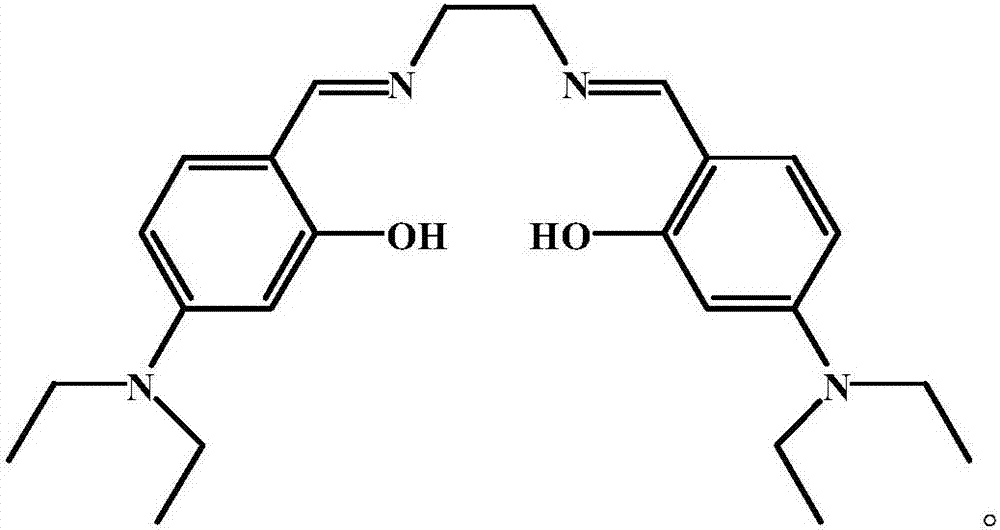
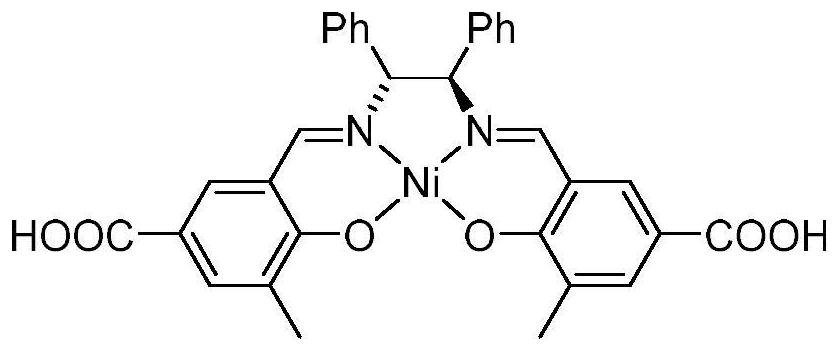
Salen refers to a tetradentate C₂-Symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical Salen ligand, primarily bis-Schiff bases. Salen ligands are notable for coordinating a wide range of different metals, which they can often stabilise in various oxidation states. These metal salen complexes primarily find use as catalysts.
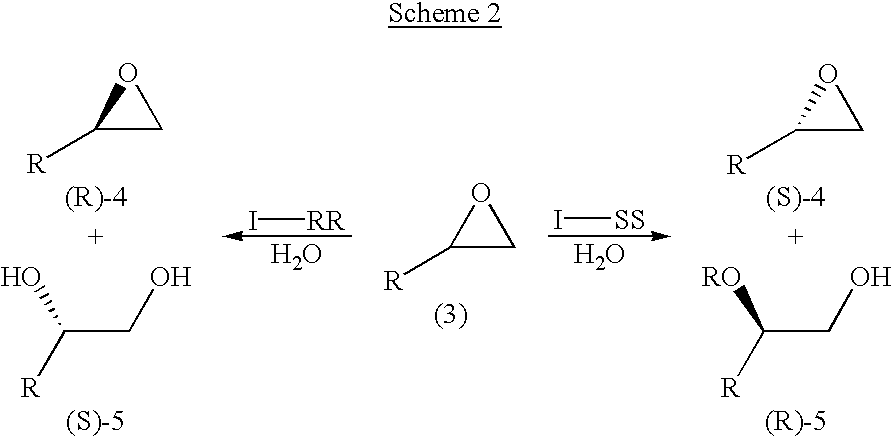
Ruthenium(II) catalysts for use in stereoselective cyclopropanations
ActiveUS7754902B2High stereoselectivityHigh yieldRuthenium organic compoundsCobalt organic compoundsProtonationSalen ligand
Chiral ruthenium catalysts comprising salen and alkenyl ligands are provided for stereoselective cyclopropanation, and methods of cyclopropanation are provided. The chiral ruthenium catalyst is prepared in situ by combining an alkenyl ligand, a deprotonated chiral salen ligand, and a ruthenium (II) metal. A preferred catalyst is prepared in situ by combining 2,3-dihydro-4-venylbenzofuran, deprotonated 1,2-cyclohexanediamino-N,N′-bis(3,5-di-t-butyl-salicylidene) and RuCl2(p-cymene)]2.
Owner:VANDA PHARMA INC
Ruthenium(II) catalysts for use in stereoselective cyclopropanations
ActiveUS20070270593A1High stereoselectivityHigh yieldRuthenium organic compoundsCobalt organic compoundsProtonationSalen ligand
Chiral ruthenium catalysts comprising salen and alkenyl ligands are provided for stereoselective cyclopropanation, and methods of cyclopropanation are provided. The chiral ruthenium catalyst is prepared in situ by combining an alkenyl ligand, a deprotonated chiral salen ligand, and a ruthenium (II) metal. A preferred catalyst is prepared in situ by combining 2,3-dihydro-4-venylbenzofuran, deprotonated 1,2-cyclohexanediamino-N,N′-bis(3,5-di-t-butyl-salicylidene) and RuCl2(p-cymene)]2.
Owner:VANDA PHARMA INC
Process for preparing chiral compounds from recemic epoxides by using chiral salen catalysts
InactiveUS6720434B2Quick responseHigh optical purityOxygen-containing compound preparationOrganic compound preparationSalen ligandContinuous use
The present invention relates to chiral salen catalysts and a process for preparing chiral compounds from racemic epoxides by using them. More particularly, the present invention is to provide chiral salen catalysts and its use for producing chiral compounds such as chiral epoxides and chiral 1,2-diols economically in high yield and high optical purity by performing stereoselective hydrolysis of racemic epoxides, wherein the chiral salen catalyst comprises a cationic cobalt as a center metal of chiral salen ligand and counterions having weak nucleophilic property to resolve disadvantages associated with conventional chiral salen catalysts, and can be used continuously without any activating process of used catalysts because it does not loose a catalytic activity during the reaction process.
Owner:RSTECH CO LTD
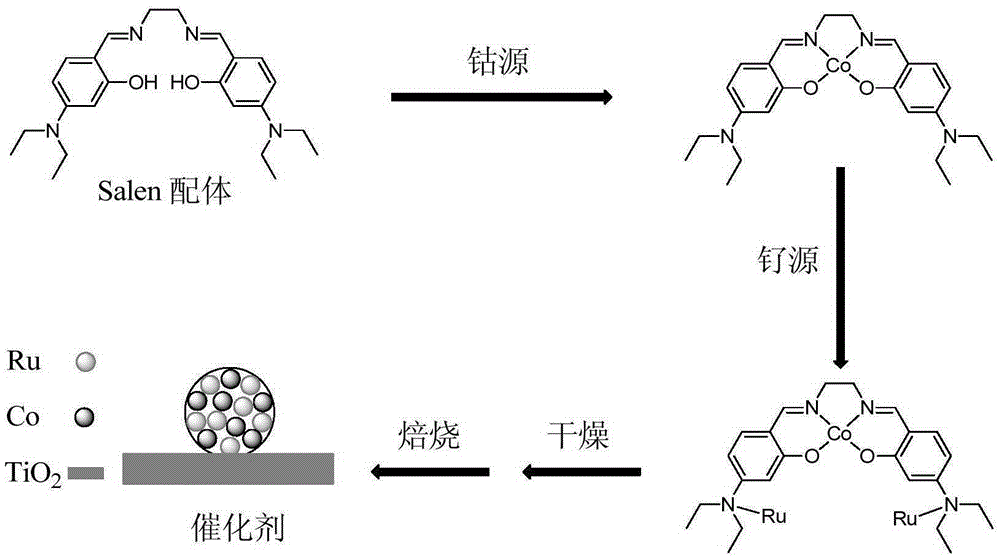
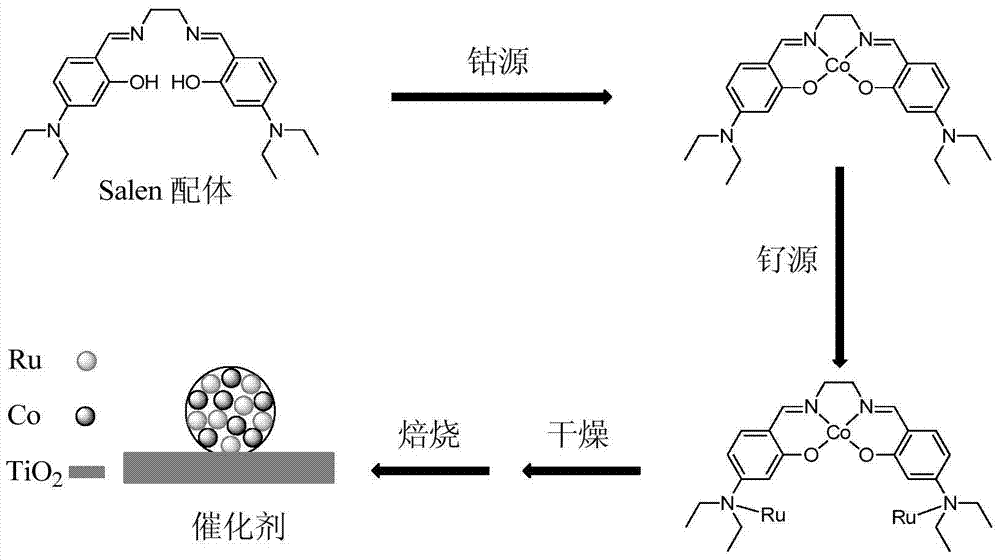
Bimetallic catalyst for catalytic oxidation of VOCs and preparation method and application of bimetallic catalyst
ActiveCN105289651AHigh catalytic activityUniversalOrganic-compounds/hydrides/coordination-complexes catalystsDispersed particle separationSalen ligandActive agent
The invention provides a bimetallic catalyst for catalytic oxidation of VOCs and a preparation method and application of the bimetallic catalyst. According to the catalyst, titanium dioxide is adopted as a carrier, an active agent is an elementary substance of any one of ruthenium, palladium and platinum and / or an oxide of the elementary substance, and a promoter is any one of cobaltosic oxide, manganese oxide, copper oxide and nickel oxide. Nano particles with the active agent and the promoter evenly compounded are synthesized through a nano regulation means and a Salen ligand, a high concerted catalysis function is achieved between the active agent and the promoter, and the catalytic oxidation efficiency of VOCs can be improved. The catalyst is high activity and high in universality, the complete oxidizing temperature on multiple kinds of VOCs ranges from 160 DEG C to 230 DEG C, the overall performance is superior to that of a commercial palladium-platinum catalyst, the selectivity of final reaction products CO2 is high, and good application prospects are achieved.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Catalyst for catalyzing thioether oxidation as well as preparation method and application of catalyst
InactiveCN104588100AEasy to prepareSimple post-processingOrganic chemistryOrganic compound preparationSalen ligandPtru catalyst
The invention discloses a catalyst for catalyzing thioether oxidation as well as a preparation method and application of the catalyst. The preparation method comprises the following steps: synthesizing a bromated Salen ligand from bromosalicylaldehyde and ethanediamine by virtue of Schiff base condensation at first; and then adding a divalent metal salt to coordinate with the Salen ligand, and introducing oxygen for oxidation after complete reaction, thereby obtaining the catalyst for catalyzing thioether oxidation. The catalyst can be used for catalyzing the thioether oxidation of methyl phenyl sulfide, ethyl phenyl sulfide, n-butyl sulfide, dibenzyl sulfide and the like with relatively high activity and relatively high selectivity; and a sulfoxide product is generated when an axial ligand is added, and a sulfone product is generated when the axial ligand is not added.
Owner:FUZHOU UNIV
Mn(III)-Salen catalyst as well as preparation method and application thereof
InactiveCN104030975AEasy to prepareSimple post-processingOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSalen ligandSalicylaldehyde
The invention discloses a Mn(III)-Salen catalyst as well as a preparation method and application thereof. The method comprises the following steps: performing reaction on 5-bromo-3-tert-butyl salicylaldehyde and pyridine-4-boric acid as raw materials to generate pyridine salicylaldehyde derivatives; reacting the pyridine salicylaldehyde derivatives with ethylene diamine to synthesize a Salen ligand with a pyridine functional group; finally coordinating divalent manganese salt with the Salen ligand, oxidizing, evaporating an obtained reaction solution to dryness, washing by water and filtering to obtain the Mn(III)-Salen catalyst. According to the method, a reaction system is simple, a reagent is easily available and low in cost, a reaction product post-treatment process is simple, the product purity is high, and the obtained Mn(III)-Salen catalyst is stable to water and air and capable of catalyzing styrene, 4-tert-butyl styrene, indene, alpha-methyl styrene and the like in a manner of relatively high activity and selectivity to synthesize olefin epoxides.
Owner:FUZHOU UNIV
Salen metallic catalyst and preparation method
InactiveCN101327452AEasy to operateStable waterOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSalen ligandSalicylaldehyde
The present invention relates to a Salen metal catalyst and a preparation method thereof. The Salen metal catalyst is characterized in that substituted salicylaldehyde is reacted with organic base to prepare for and obtain chloride; corresponding organic salt is obtained after ion exchange; the organic salt is reacted with diamine to obtain Salen ligand; then the Salen ligand is reacted with metal slat to obtain the Salen metal catalyst. The prepared Salen metal catalyst has the characteristics of easy operation, being stable in water and air and being dissolved in polar solvent, such as water and ethanol, and the like, easily.
Owner:ZHEJIANG UNIV
Preparation method of electrochemical sensor for dissolved oxygen detection
InactiveCN102645470APromote reductionCathodic current enhancementMaterial analysis by electric/magnetic meansPlatinumSalen ligand
The invention relates to a preparation method of an electrochemical sensor for dissolved oxygen detection, comprising the following steps of: firstly, preparing a salen ligand; then dissolving 6.4 mol of the salen ligand into 50 ml of an ethanol solution to form a solution C; dissolving 6.4 mol of nickel acetate into 50 mol of the ethanol solution to form a solution D; gradually dripping the solution C into the solution D to form a mixed solution; drying, precipitating and filtering to obtain a nickel-salen ligand; and finally, carrying out electro-polymerization on the nickel-salen ligand on a platinum electrode with the area of 0.071 cmW so as to obtain the electrochemical sensor. The electrochemical sensor for the dissolved oxygen detection prepared by the invention has high flexibility and stability, and has a better application value.
Owner:WUXI BAILING SENSING TECH
Mononuclear aluminum and binuclear aluminum compounds based on biphenyl skeleton Salen ligand and preparation method and application thereof
InactiveCN103044475AWide variety of sourcesEasy to manufactureGroup 3/13 element organic compoundsSalen ligandLactide
The invention discloses mononuclear aluminum and binuclear aluminum compounds based on biphenyl skeleton Salen ligand, a preparation method of the mononuclear aluminum and binuclear aluminum compounds, and an application of the mononuclear aluminum and binuclear aluminum compounds in ring-opening polymerization of catalytic internal ester. The preparation method of the mononuclear aluminum and binuclear aluminum compounds based on the biphenyl skeleton Salen ligand comprises the following steps of: adding a ligand compound to directly react with aluminum alkyl in an organic medium, filtering, concentrating and recrystallizing to obtain a target compound; and adjusting the mole ratio of the ligand compound to the aluminum alkyl, thus obtaining the mononuclear aluminum or binuclear aluminum compound respectively. The mononuclear aluminum and binuclear aluminum compounds based on the biphenyl skeleton Salen ligand are efficient catalysts for ring-opening polymerization of internal ester, and can be used for polymerization of lactide, epsilon-caprolactone and the like; and the source of raw materials is wide, the synthesis is easy, the product yield is high, the performance is stable, the catalytic activity is high, a polymer with high molecular weight can be obtained, and the requirements of industrial departments can be met. The structural formulae of the compounds are as shown in the specification.
Owner:EAST CHINA UNIV OF SCI & TECH
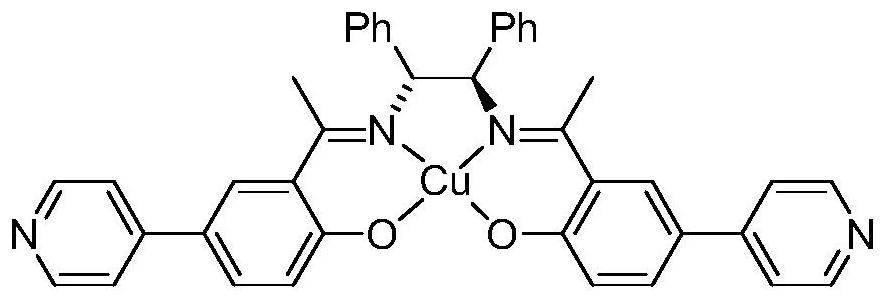
Pyridine type chiral Cu(II)-Salen ligand metal organic framework crystal material as well as preparation method and application thereof
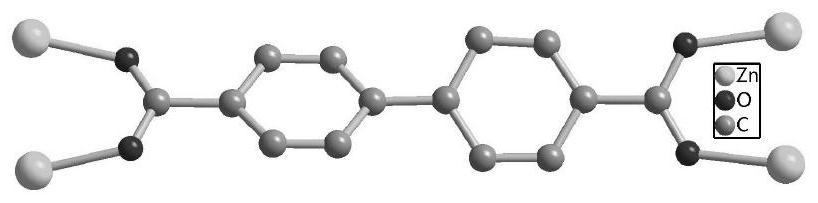
ActiveCN111690145ANovel structureUnique structureProductsOther chemical processesEthylenediamineSalen ligand
The invention relates to a pyridine type chiral Cu(II)-Salen ligand metal organic framework crystal material as well as a preparation method and application thereof. The material has the following chemical formula: {[Zn2(L)(BPDC)2].DMF.5H2O}n, wherein L is (R,R)-N,N'-bis(5-(4-pyridyl) diyl-2-hydroxyacetophenone)-1,2-diphenylethylenediamine copper (II), BPDC is a 4,4'-biphenyl dicarboxylate divalent anion, and n is the degree of polymerization. The metal organic framework crystal material provided by the invention adopts a solvothermal synthesis method, and is simple to operate, low in cost, high in yield and easy for large-scale industrial production. The prepared metal organic framework crystal material has a relatively high specific surface area (BET specific surface area is 752 m<2> / g),and the adsorption capacities of CO2 and N2 under 1atm and 273 K are 3.47 mmol / g and 0.57 mmol / g respectively. TEMPO is used as an additive, selective oxidation of benzyl alcohol is catalyzed in a water phase to generate benzaldehyde, the yield reaches 99%, the catalyst is recycled for five times with almost no activity loss, and the catalyst is a good heterogeneous catalyst.
Owner:ZUNYI MEDICAL UNIVERSITY
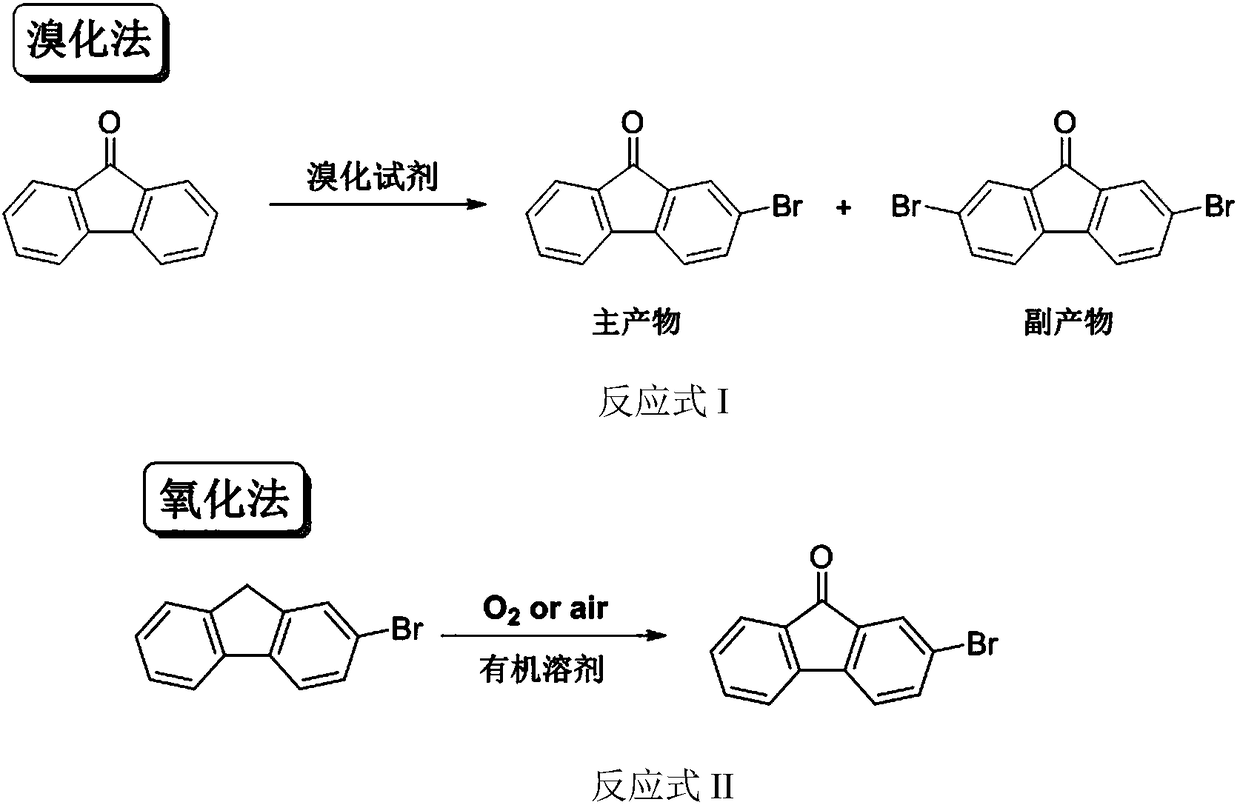
Method for preparing 2-bromofluorenone by catalyzing oxidizing of molecular oxygen in water phase
ActiveCN108276261AHigh catalytic activityReduce wasteOrganic compound preparationCarbonyl compound preparationOrganic solventSalen ligand
The invention provides a method for preparing 2-bromofluorenone by catalyzing oxidizing of molecular oxygen in a water phase. The method is characterized in that a transition metal Salen ligand is used as a catalyst; under the condition of air bubbles, 2-bromofluorene is oxidized to generate the 2-bromofluorenone in the water phase, the reaction temperature is 60 to 100 DEG C, and the reaction time is 8 to 24h. The method has the advantages that the reaction is performed in the water phase, the organic solvent as the reaction medium is not needed, and the environment-friendly effect is betterin comparison with the traditional oxidizing method; the transition metal Salen catalyst has higher catalytic activity in the water phase medium; the water phase medium can be utilized for multiple times, the amount of wastes is fewer, and the industrial application prospect is stronger.
Owner:XINXIANG RUNYU NEW MATERIAL TECH CO LTD
Ru (II)-Salen metal complexes and preparation methods thereof
InactiveCN104804044APromote oxidation reactionImprovement of oxidation reactionGroup 8/9/10/18 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsEthylenediamineSalen ligand
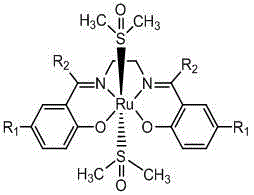
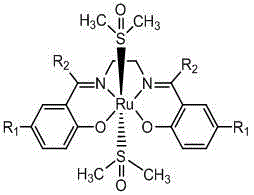
The invention provides Ru (II)-Salen metal complexes and preparation methods thereof, and relates to metal complexes and preparation methods thereof. According to the three Ru (II)-Salen metal complexes, Ru(DMSO)4Cl2 is taken as the metal precursor, and Salen ligands are H2L<1>, H2L<2> and H2L<3> obtained through condensation reactions of ethanediamine with salicylaldehyde, 5-bromosalicylaldehyde and hydroxyacetophenone respectively. The three Ru (II)-Salen metal complexes Ru(DMSO)2(H2L<1>), Ru(DMSO)2(H2L<2>) and Ru(DMSO)2(H2L<3>) are prepared with a step-by-step synthesis technique by changing conditions of each reaction process. The three novel complexes have clear structures as well as stability on water and air; the reaction conditions are mild and the post-processing is simple in catalysis of oxidizing reaction of alcohol, and the three Ru (II)-Salen metal complexes have good catalytic activity and selectivity.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Preparation method of chiral Salen-Co(III) catalyst loaded by utilizing Merrifield resin
InactiveCN105618141AGood swelling propertiesIncrease load capacityOrganic compound preparationOrganic chemistry methodsHydrolysis kineticsPolymer science
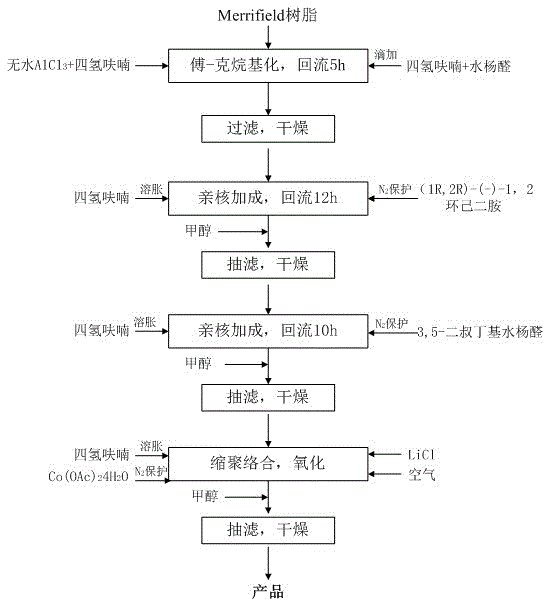
The invention relates to a preparation method of a catalyst, in particular to a preparation method of a chiral Salen-Co(III) catalyst loaded by utilizing Merrifield resin. The preparation method comprises the following steps: utilizing Friedel-Crafts alkylation reaction, and taking the Merrifield resin and a salicylic aldehyde solution as raw materials to obtain Merrifield resin-based salicylic aldehyde; then enabling reaction with (1R, 2R)-(-)1,2-cyclohexanediamine and 3-5-di-tert-butyl salicylaldehyde to prepare a Merrifield resin-loaded chiral Salen ligand; through complexing with Co(OAc)2*4H2O, oxidizing to obtain the Merrifield resin-loaded chiral Salen-Co(III) catalyst; the catalyst is applied to the hydrolytic resolution of chiral epichlorohydrin. The preparation method provided by the invention is environment-friendly, free from pollution and obvious in economic benefits and easy to industrialize and has an important significance in the production development of hydrolytic kinetic resolution of a terminal epoxy compound as well as the technical progress.
Owner:JILIN INST OF CHEM TECH
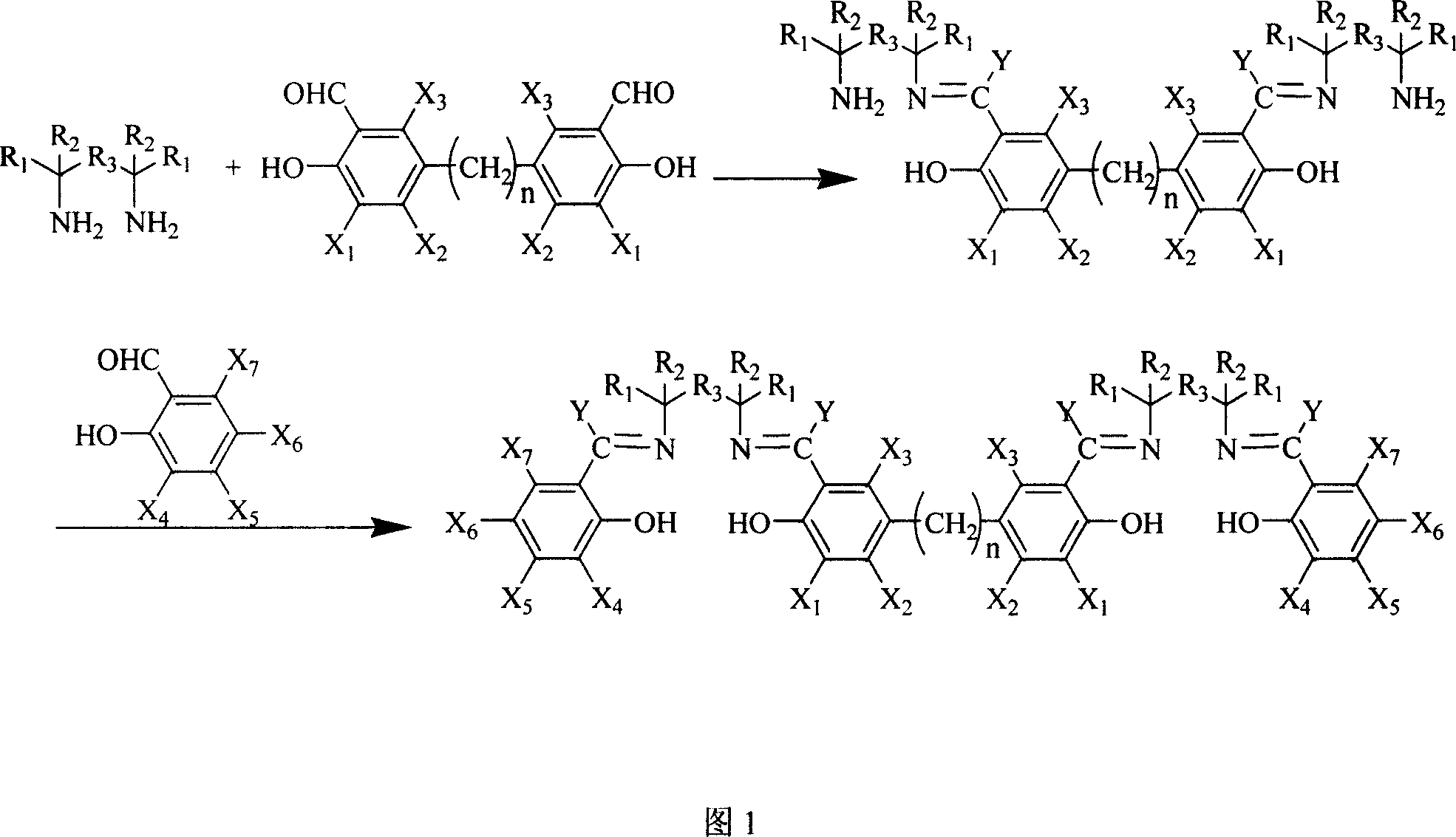
Synthesis method for preparing Salen ligand in chiral or not chiral binuclear
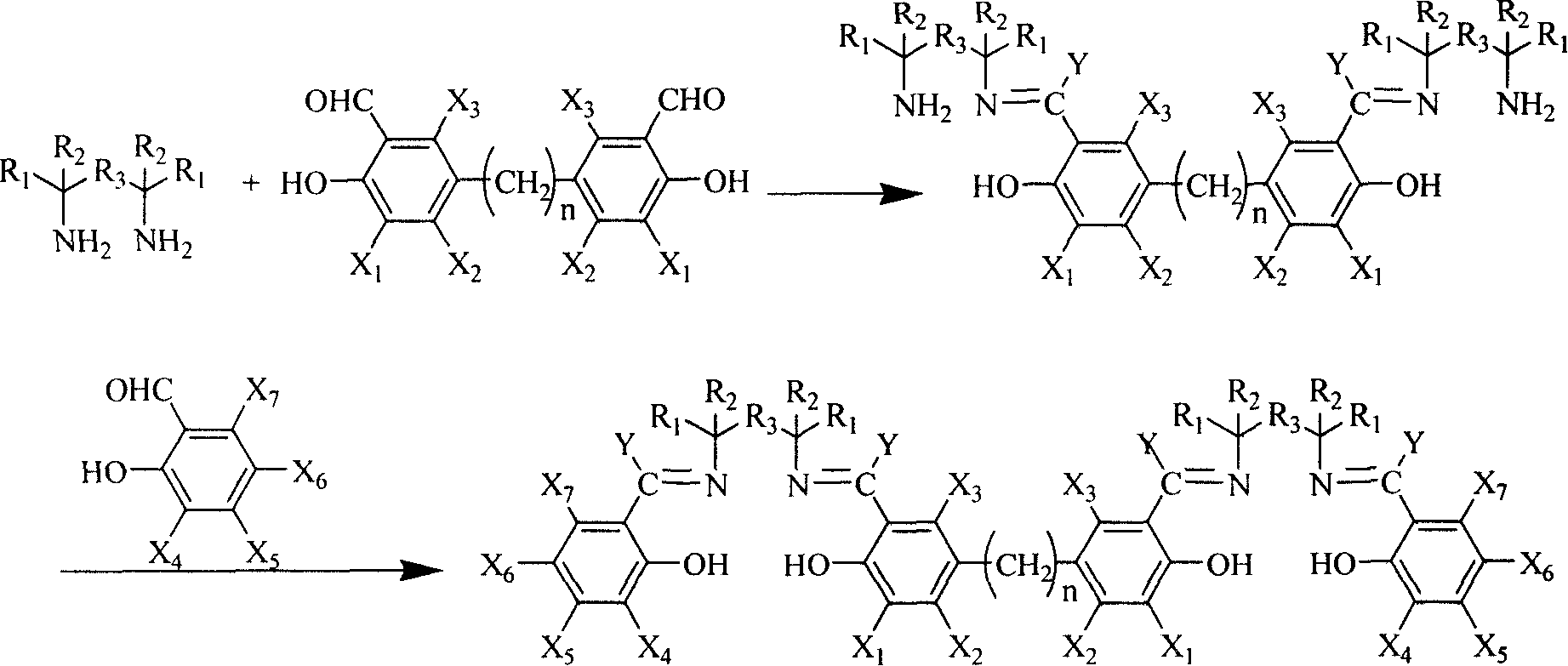
InactiveCN1687020AReduce generationGet efficientlyImino compound preparationSalicylaldehydeSalen ligand
The present invention provides a synthesis method for preparing chiral or achiral binuclear Salen ligand by utilizing chiral or achiral diamine and derivative of monosalicylal and disalicylal. Said method adopts the following steps: firstly, making derivative of disalicylal be reacted with excess diamine, then adding excess derivative of monosalicylal to produce synthesis reaction to prepare the invented product. The described diamine can be chiral or achiral diamine and its ammonium salt.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Novel chiral salen catalysts, and a process for preparing chiral compounds from racemic epoxides for using them
InactiveUS20030032821A1Decrease in optical purityLong reaction timeOxygen-containing compound preparationOrganic compound preparationSalen ligandContinuous use
The present invention relates to chiral salen catalysts and a process for preparing chiral compounds from racemic epoxides by using them. More particularly, the present invention is to provide chiral salen catalysts and its use for producing chiral compounds such as chiral epoxides and chiral 1,2-diols economically in high yield and high optical purity by performing stereoselective hydrolysis of racemic epoxides, wherein the chiral salen catalyst comprises a cationic cobalt as a center metal of chiral salen ligand and counterions having weak nucleophilic property to resolve disadvantages associated with conventional chiral salen catalysts, and can be used continuously without any activating process of used catalysts because it does not loose a catalytic activity during the reaction process.
Owner:RSTECH CO LTD
Metal coordination compounds with zinc (II) and platinum (II) in different doping proportions of based on poly-Salen ligands as well as preparation methods and applications thereof
InactiveCN102199286AThe synthesis method is simpleEasy to operateLuminescent compositionsPlatinumSalen ligand
The invention relates to organic metal coordination compounds as well as synthesis methods and applications thereof, in particular to a series of poly-Salen ligands and metal coordination compounds with zinc (II) and platinum (II) in different doping proportions as well as synthesis methods and applications thereof in the field of organic luminance. The metal coordination compounds with zinc (II)and platinum (II) in different doping proportions based on the poly-Salen ligands are characterized in that the structures of the metal coordination compounds are shown in the below structural formula, wherein the synthesis methods of the poly-Salen ligands and the metal coordination compounds have the advantages of simplicity, convenience, simple technological operation, high yield, low cost andeasiness for molecular design.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Preparation method and application of catalyst
InactiveCN106563509AImprove catalytic performanceEasy to useCatalyst carriersOrganic compound preparationEthylenediamineSalen ligand
The invention discloses a preparation method and application of a catalyst. The preparation method of the catalyst is characterized by comprising the following steps that (1) in a polar aprotic solvent solution, in the presence of alkali, salicylaldehyde and allyl haloalkane are made to perform a nucleophilic substitution reaction, so that a nucleophilic substitution product is obtained; secondly, the product generated in the step (1) is heated to perform a Claisen rearrangement reaction; (3) the product obtained in the step (2) and ethylenediamine are condensed in an organic solvent, so that a Salen ligand is obtained; (4), in an ether solvent, the Salen ligand and divinyl benzene are copolymerized in the presence of a radical initiator and an auxiliary initiator, so that a Salen organic polymer of a mesoporous structure is obtained; and (5) in a mixed solvent of alcohol and water, the Salen organic polymer is used as a carrier, and mesoporous Salen organic polymer Co catalyst is obtained through preparation. The catalyst prepared through the preparation method is applied to catalytic oxidation of benzoin and has good catalytic activity, and meanwhile, even the catalyst is repeatedly used, the catalytic activity of the catalyst cannot be lowered.
Owner:SHAOXING UNIVERSITY
Process for preparing amino compounds by using salen-manganese complexes as the catalyst
InactiveUS6723879B2Organic compound preparationOrganic chemistry methodsSalen-manganese complexSalen ligand
Disclosed is a process for the enantioselective production of an optically active amino compound by the amination of the C-H bond of an organic compound. The amino compound is produced by converting the C-H bond at the allyl position of an alkene or the C-H bond at the benzyl position of an alkylarene to the corresponding C-N bond, using a salen-manganese complex as the catalyst and N-substituted iminoaryliodinane as the amination agent. Both the catalytic activity and the enantioselectivity are very high when there is used a catalyst in which the 3- and 5-positions of the salicylaldehyde moiety of the salen ligand are substituted with an electron-withdrawing group, particularly with a halogen atom.
Owner:JAPAN SCI & TECH CORP
Process for preparing amino compounds by using salen-manganese complexes as the catalyst
InactiveUS20030139627A1Organic compound preparationOrganic chemistry methodsSalen ligandOrganic compound
Disclosed is a process for the enantioselective production of an optically active amino compound by the amination of the C-H bond of an organic compound. The amino compound is produced by converting the C-H bond at the allyl position of an alkene or the C-H bond at the benzyl position of an alkylarene to the corresponding C-N bond, using a salen-manganese complex as the catalyst and N-substituted iminoaryliodinane as the amination agent. Both the catalytic activity and the enantioselectivity are very high when there is used a catalyst in which the 3- and 5-positions of the salicylaldehyde moiety of the salen ligand are substituted with an electron-withdrawing group, particularly with a halogen atom.
Owner:JAPAN SCI & TECH CORP
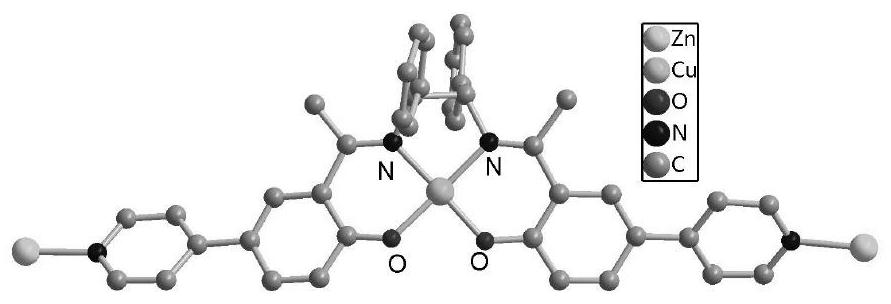
Ni (II)-Salen ligand metal organic framework crystal material and preparation method and application thereof
ActiveCN111732736ANovel structureUnique structureProductsOther chemical processesEthylenediamineEpoxy
The invention discloses a Ni (II)-Salen ligand metal organic framework crystal material and a preparation method and application thereof. The chemical formula of the material is {[Zn4O (L) 6]. DMF.H2O} n, wherein L is a dicarboxylic acid radical divalent anion of (R, R)-N, N '-bis (3-methyl-5-carboxyl salicylidene)-1, 2-diphenylethylenediamine nickel (II), and n is the polymerization degree. The metal organic framework crystal material is synthesized by a solvothermal synthesis method, which is simple to operate, low in cost, high in yield and easy for large-scale industrial production. The prepared metal organic framework crystal material has high thermal stability (400 DEG C), and the BET specific surface area is 228m <2> / g. And the adsorption quantity of CO2 at 273K and 1atm is 18.8 m<3> / g. In the presence of an oxidizing agent, styrene is catalyzed to be selectively oxidized to generate benzaldehyde in a water phase, the yield reaches 99%, the catalyst is recycled for five times, and the activity loss is small. In the presence of tetrabutylammonium bromide, 1 atm and 50 DEG C solvent-free catalysis is performed on epoxy styrene and CO2 to react to generate styrene carbonate, the yield is 91%, and the catalyst is recycled for five times and still keeps activity. The material is a good heterogeneous catalyst.
Owner:ZUNYI MEDICAL UNIVERSITY
Novel coordination circular polarization luminescent crystalline compound and preparation method and application thereof
ActiveCN113292581APotential application value is goodSimple processOrganic chemistry methodsEnergy efficient lightingSalen ligandSalicylaldehyde
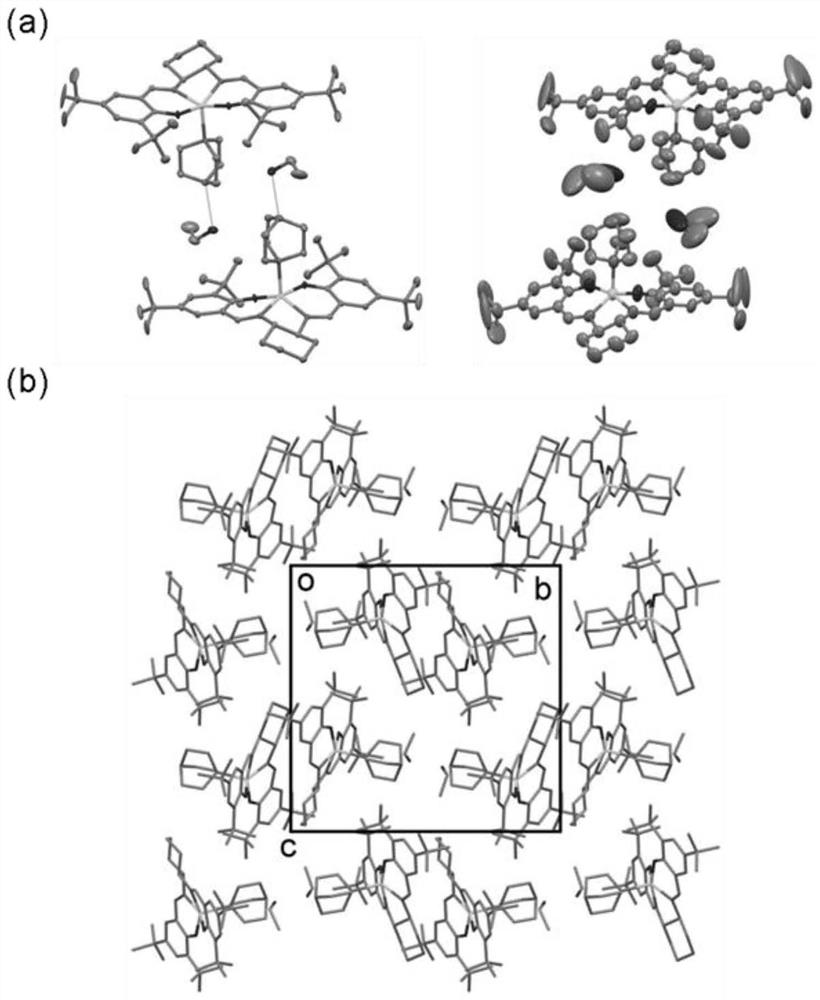
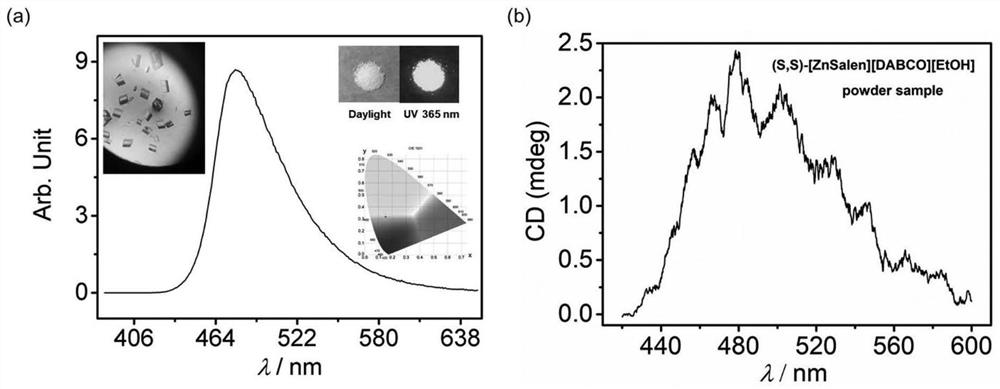
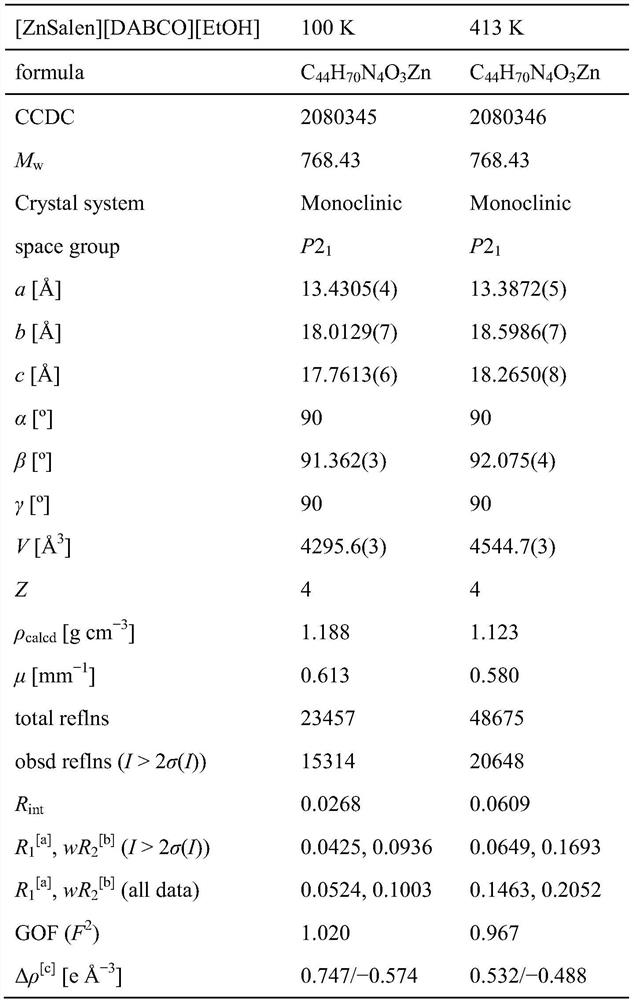
The invention discloses a novel coordinated circular polarization luminescent crystalline compound [ZnSalen] [DABCO] [EtOH], the molecular formula of the compound is C44H70N4O3Zn, Salen is 3, 5-di-tert-butyl salicylaldehyde-S, S-1, 2-cyclohexyl imine, DABCO is triethylene diamine, a central metal zinc atom of the compound is of a five-coordinated pyramid type configuration, N2O2 of a Salen ligand occupies four corners of the bottom surface, and another N atom of DABCO occupies the vertex. The complex presents a T-shaped conformation, and the other N atom of DABCO and an ethanol guest molecule form hydrogen-bond interaction. The complex has polarity, adopts a P21 space group, emits cyan light under 365nm ultraviolet irradiation, and can emit light in a light-induced circular polarization manner. The invention also discloses a preparation method of the metal organic compound crystal. The prepared [ZnSalen] [DABCO] [EtOH] metal fluorescent crystalline state complex has good potential application in the aspects of optical materials (such as nonlinear optical materials, circular polarization luminescence, fluorescence sensing and the like).
Owner:SOUTHEAST UNIV
Synthesis method for preparing Salen ligand in chiral or not chiral binuclear
InactiveCN1314661CReduce generationGet efficientlyImino compound preparationSalicylaldehydeSalen ligand
The present invention provides a synthesis method for preparing chiral or achiral binuclear Salen ligand by utilizing chiral or achiral diamine and derivative of monosalicylal and disalicylal. Said method adopts the following steps: firstly, making derivative of disalicylal be reacted with excess diamine, then adding excess derivative of monosalicylal to produce synthesis reaction to prepare the invented product. The described diamine can be chiral or achiral diamine and its ammonium salt.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Preparation method of Cis-ruthenium-salen N-heterocyclic carbene
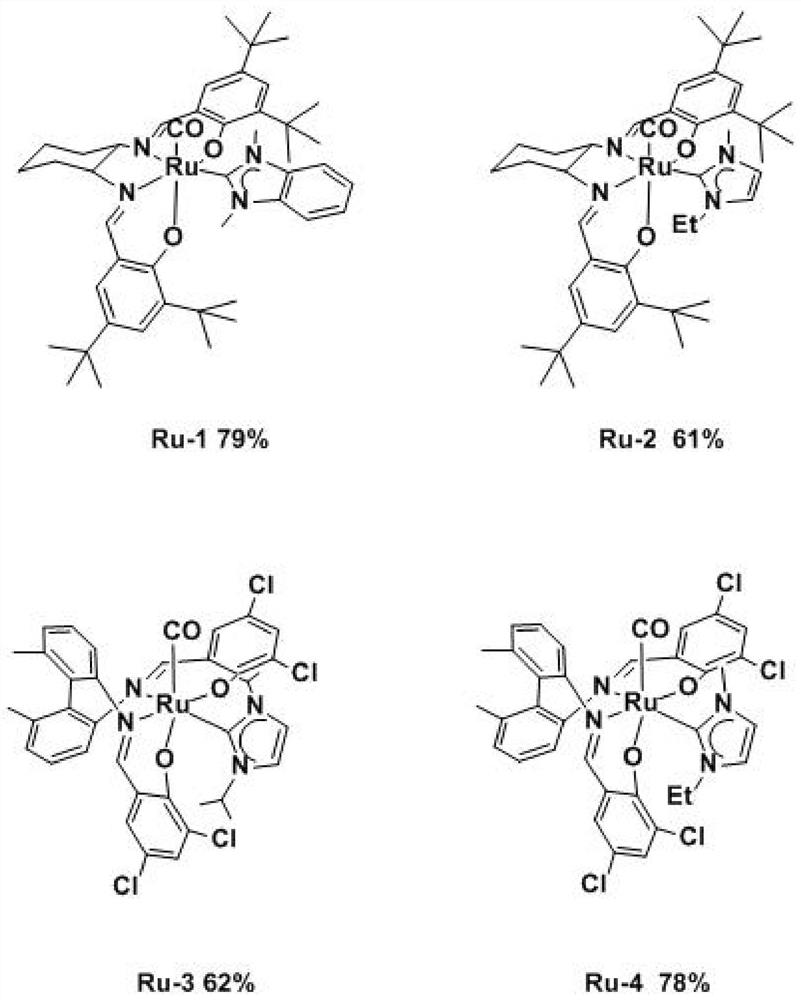
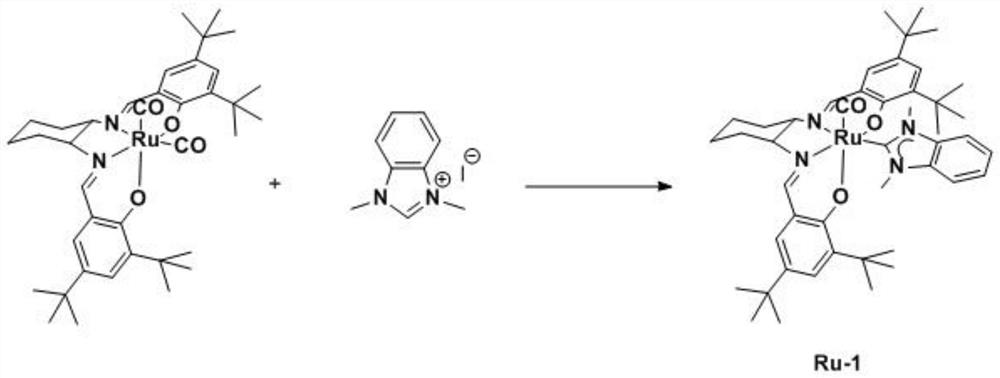
ActiveCN112300217ANo light requiredHigh reaction yieldRuthenium organic compoundsChlorobenzeneSalen ligand
The invention discloses a preparation method of Cis-ruthenium-salen N-heterocyclic carbene. The preparation method comprises the following steps: adding 1 mmol of dodecacarbonyl triruthenium, 1 mmol of salen ligand and 10 ml of 1, 2, 4-trichlorobenzene into a reaction flask under the protection of nitrogen, heating to 185 DEG C, reacting for 6 hours, cooling to room temperature, and separating andpurifying by a silica gel chromatographic column to obtain a cis-ruthenium-salen dicarbonyl complex; the method comprises the following steps: adding 1 mmol of a cis-ruthenium-salen dicarbonyl complex, 4 mmol of silver oxide, 2 mmol of an N-heterocyclic carbene precursor and 100 ml of dehydrated tetrahydrofuran into a reaction flask under the protection of nitrogen, reacting at 50 DEG C for 8 h after the reactants are added, cooling to room temperature, and separating and purifying by a silica gel chromatographic column to obtain the cis-ruthenium-salen N-heterocyclic carbene complex. The preparation method is performed under mild conditions without light, and a series of novel cis-ruthenium-salen N-heterocyclic carbenes are obtained at an excellent reaction yield of 61%-79%.
Owner:JIANGSU OPEN UNIV
A kind of bimetallic catalyst for catalytic oxidation VOCs and its preparation method and application
ActiveCN105289651BHigh catalytic activityUniversalOrganic-compounds/hydrides/coordination-complexes catalystsDispersed particle separationSalen ligandPtru catalyst
The invention provides a bimetallic catalyst for catalyzing and oxidizing VOCs, a preparation method and application thereof. The catalyst uses titanium dioxide as a carrier, the active agent is a simple substance and / or an oxide of any element in ruthenium, palladium or platinum, and the cocatalyst is any one of tricobalt tetroxide, manganese oxide, copper oxide or nickel oxide. Through nano-control means, Salen ligands are used to synthesize nanoparticles uniformly composited with active agents and co-catalysts. There is a strong synergistic catalytic effect between the two, which can improve the catalytic oxidation efficiency of VOCs. The catalyst of the present invention has high activity and strong universality. The complete oxidation temperature for various VOCs is between 160 and 230°C. It has a good application prospect.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
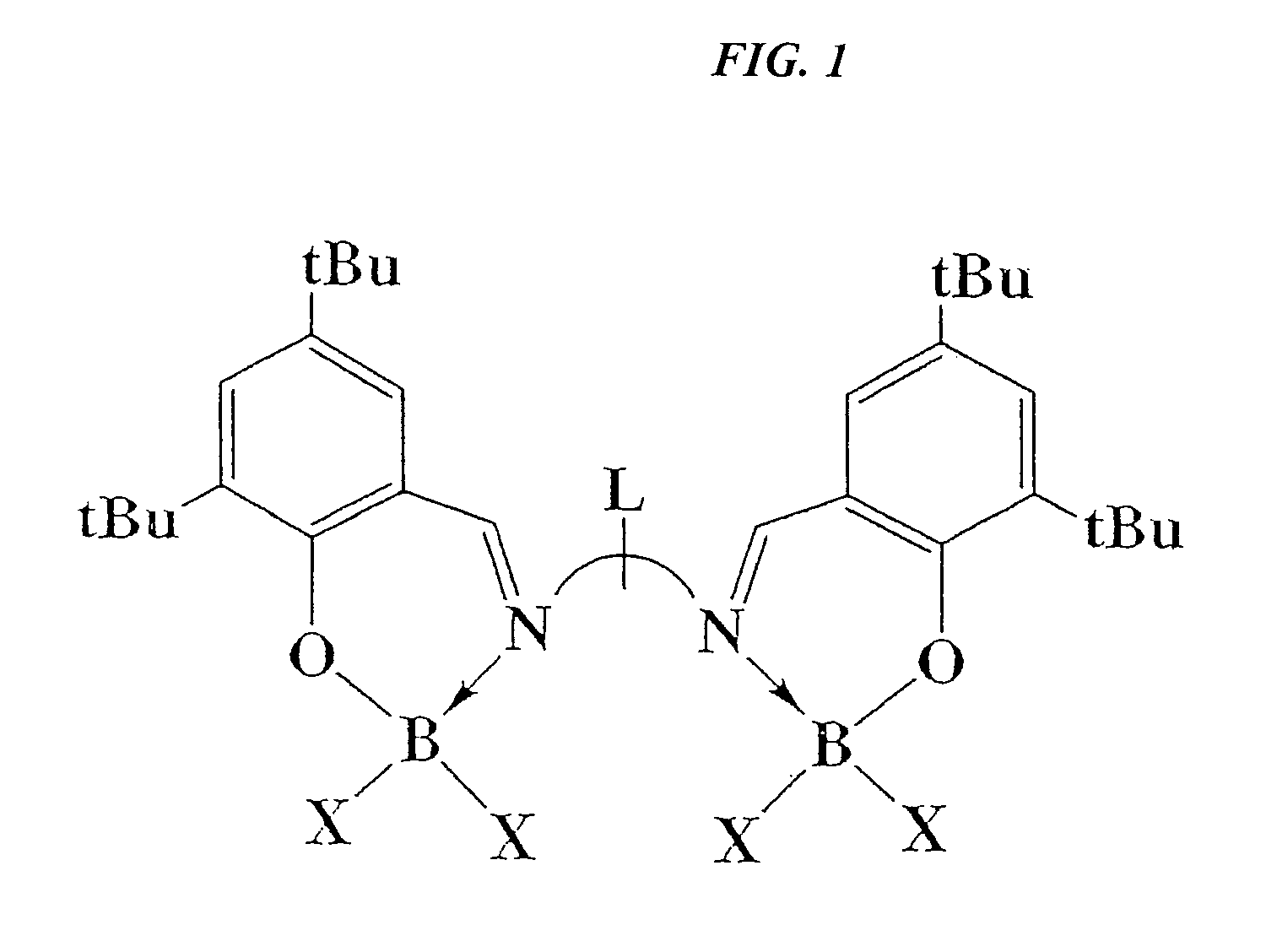
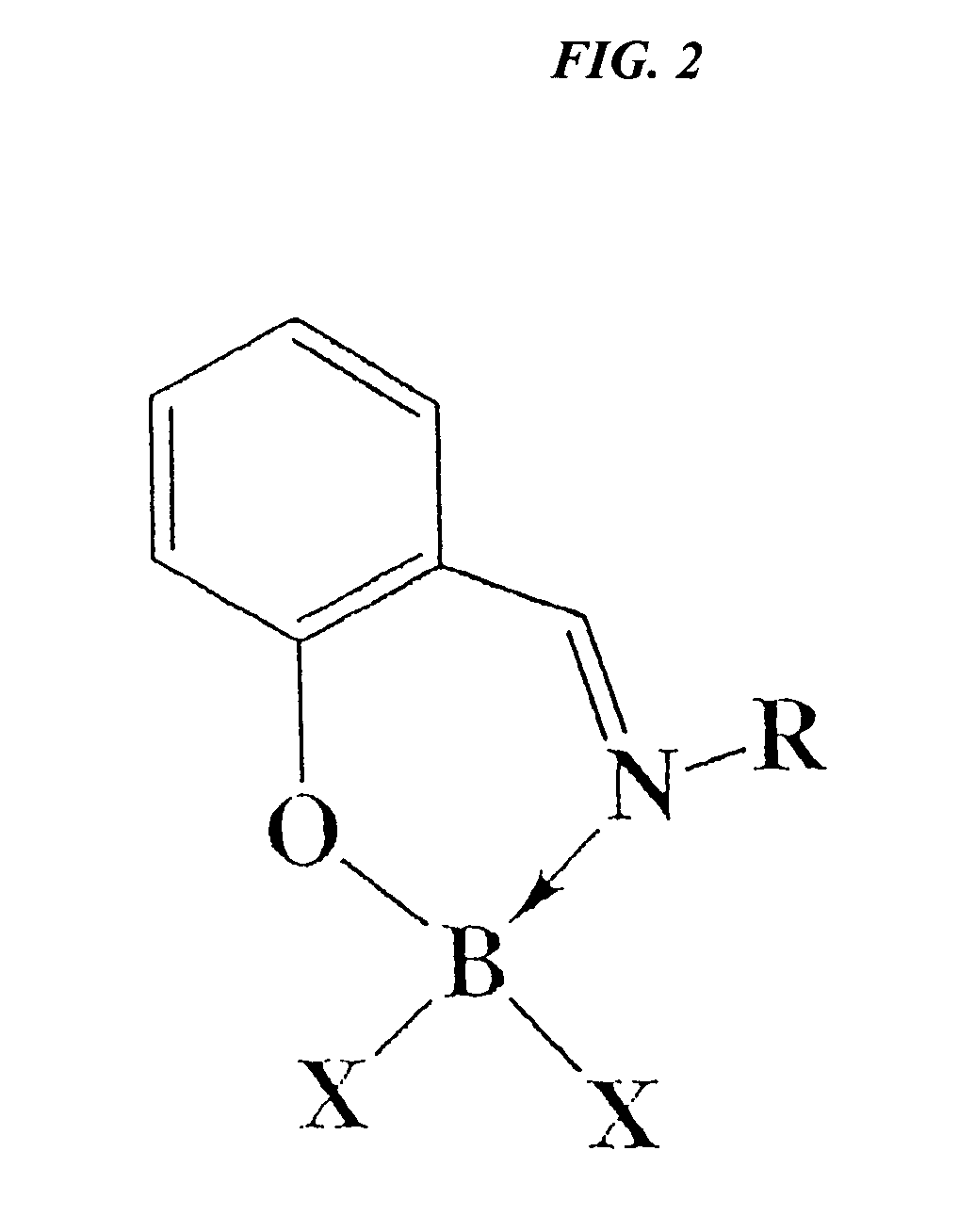
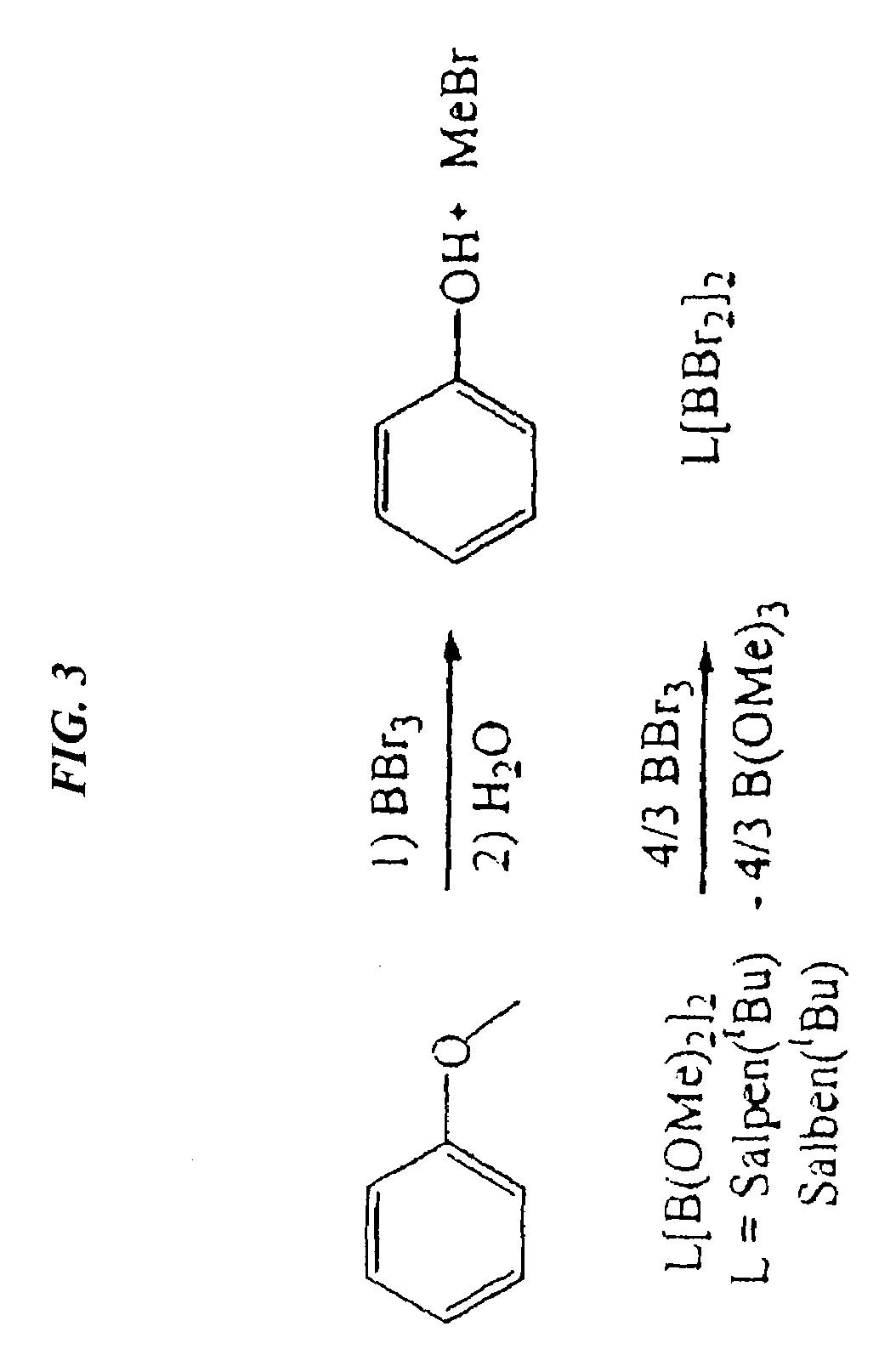
Catalytic cleavage of phosphate ester bonds by boron chelates
Novel chemical compounds are disclosed having the general formula L{YXm}n, wherein X is selected from the Group 13 elements, Y is a halide, and L is a chelating ligand containing at least one binding atom contacting the Group 13 element, the atom being selected from the group consisting of C, N, O, and S, and m and n are integers having a value of at least 1. L may be a Schiff base type ligand, such as a salen ligand. The compositions of the present invention may be bidentate, quadridentate, or greater. The compositions may be used in dealkylation of phosphate esters or ethers. Advantageously, the methods of the present invention may be rendered catalytic.
Owner:UNIV OF KENTUCKY RES FOUND
Cage-shaped supramolecular catalyst for catalyzing thioether oxidation as well as preparation method and application of cage-shaped supramolecular catalyst
ActiveCN112774733AHigh activityStable in natureOrganic chemistryOrganic compound preparationThioetherSalen ligand
The invention discloses preparation and application of a cage-shaped supramolecular catalyst for catalyzing thioether oxidation. The preparation method comprises the following steps: firstly, carrying out amine-aldehyde condensation on a tridentate aldehyde compound and 1, 2-cyclohexanediamine to obtain a covalent cage compound material containing a Salen ligand, then carrying out in-situ synthesis on the ligand and transition metal M (II) to obtain a target cage supramolecular catalyst material, and finally, applying the target cage supramolecular catalyst material to selective oxidation of thioether to generate sulfone or sulfoxide compounds. The catalyst material obtained by the method shows excellent performance in the catalytic thioether oxidation reaction, the conversion rate is up to 95% or above, the catalytic reaction condition involved in the technology is mild, the reaction solvent is greener, and the catalyst has the advantages of high conversion rate, high selectivity and the like, can be recycled for multiple times, the activity and the selectivity of the catalyst are not obviously reduced, and the catalyst has high economic benefits and industrial production application values.
Owner:FUZHOU UNIV
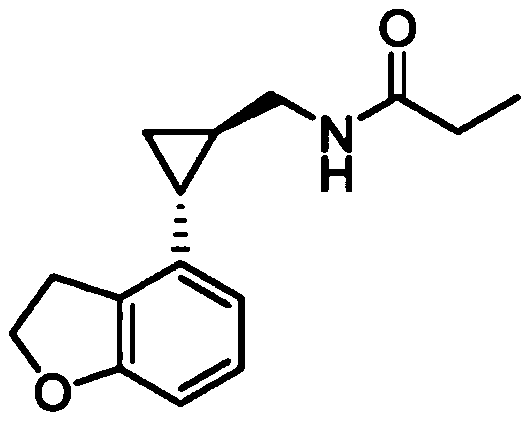
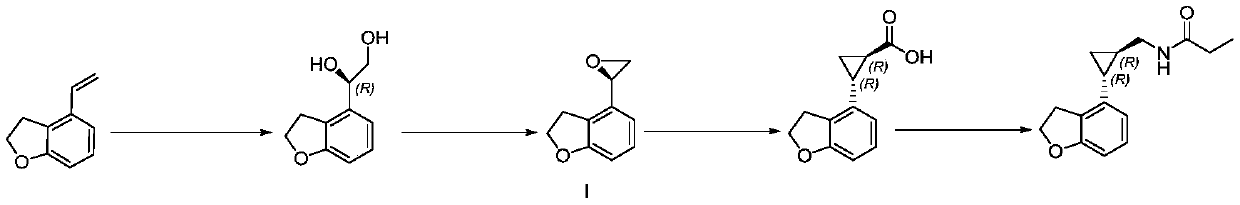
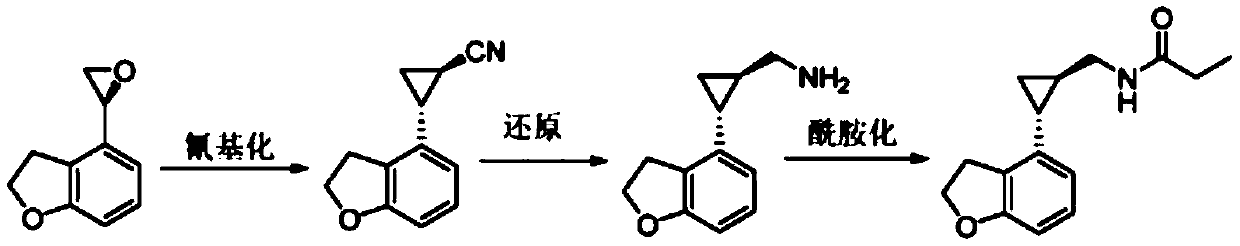
Preparation method of tasimelteon key intermediate
InactiveCN111518062AIncrease profitKey steps shortenedOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsSalen ligandPtru catalyst
The invention discloses a preparation method of a tasimelteon key intermediate. According to the method, 4-vinyl-2, 3-dihydrobenzofuran is used as an initial raw material, (1R, 2R)-2-(2, 3-dihydrobenzofuran-4-yl) cyclopropane-1-ethyl formate is generated through a one-step catalytic reaction, and then (1R, 2R)-2-(2, 3-dihydro-4-benzofuranyl) cyclopropanecarboxylic acid is obtained through hydrolysis and resolution. The key step of asymmetric catalysis of chiral cyclopropane is developed, an adopted salen ligand Ru catalyst can catalyze chiral cyclopropane in one step, the process is simplified, and meanwhile the catalyst is non-toxic, environmentally friendly and high in utilization rate; then hydrolyzing into acid is realized, and chiral resolution is carried out; not only is the opticalpurity improved, but also a reagent for resolution can be recycled. The method is simple in overall process, high in operability, safe and controllable in production process, environment-friendly, energy-saving, low in cost, high in yield and suitable for large-scale production.
Owner:杭州拜善晟生物科技有限公司
A Ni(ii)-salen ligand metal-organic framework crystal material and its preparation method and application
ActiveCN111732736BNovel structureUnique structureProductsOther chemical processesEthylenediamineSalen ligand
Ni(II)-Salen ligand metal-organic framework crystal material and its preparation method and application. The chemical formula of the material: {[Zn 4 O(L) 6 ]·DMF·H 2 O} n , where L is the dicarboxylate dicarboxylate of (R,R)-N,N'-bis(3-methyl-5-carboxysalicylidene)-1,2-diphenylethylenediamine nickel(II) Valence anion, n is the degree of polymerization. The metal organic framework crystal material adopts a solvothermal synthesis method, has simple operation, low cost, high yield, and is easy for large-scale industrial production. The prepared MOF crystalline material has high thermal stability (400°C), and the BET specific surface area is 228m 2 / g. For CO at 273K, 1atm 2 The adsorption capacity is 18.8m 3 / g. In the presence of an oxidizing agent, the selective oxidation of styrene is catalyzed in an aqueous phase to generate benzaldehyde with a yield of 99%. The catalyst is recycled five times with little loss of activity. In the presence of tetrabutylammonium bromide, 1 atm, 50 ℃ solvent-free catalysis of epoxy styrene and CO 2 The reaction produces styrene carbonate with a yield of 91%. The catalyst is recycled five times and still maintains its activity. This material is a good heterogeneous catalyst.
Owner:ZUNYI MEDICAL UNIVERSITY
Preparation method of ni-salen complex and ni-salen complex
ActiveCN108103523BSimple stepsEasy to operateElectrolysis componentsElectrolytic organic productionSalen ligandBiological activation
The preparation method of the Ni-Salen complex and the Ni-Salen complex belong to the technical field of Ni-Salen preparation. After electrodeposition of nickel ferricyanide complexes on the electrode surface, the Salen ligand is modified and electroactivated in lye. It uses electrodes to directly electrochemically synthesize Ni-Salen complexes, with simple steps and convenient operation.
Owner:湖北特腾新材料技术有限公司
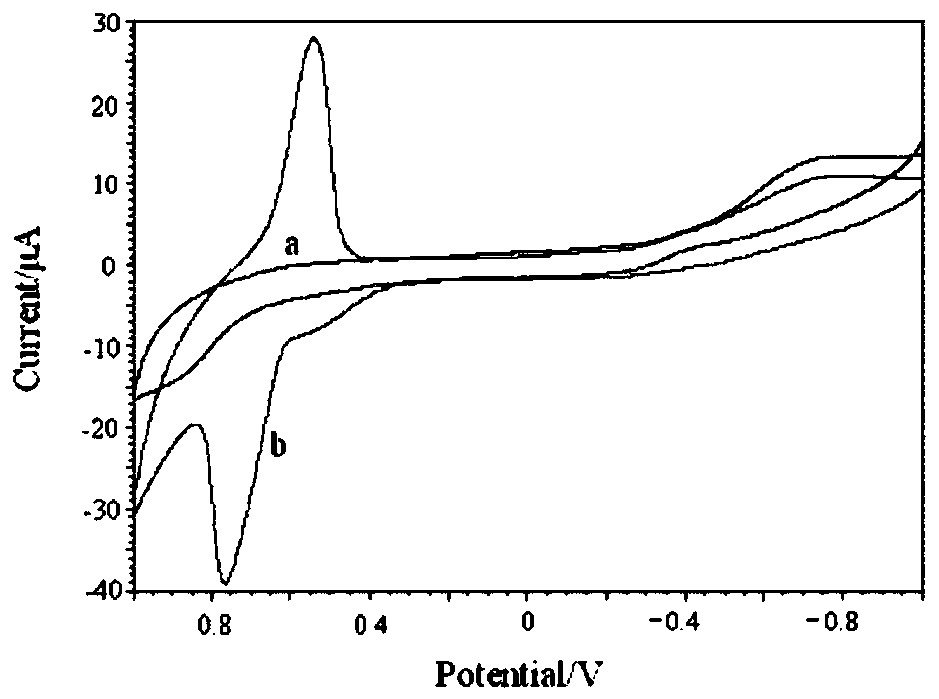
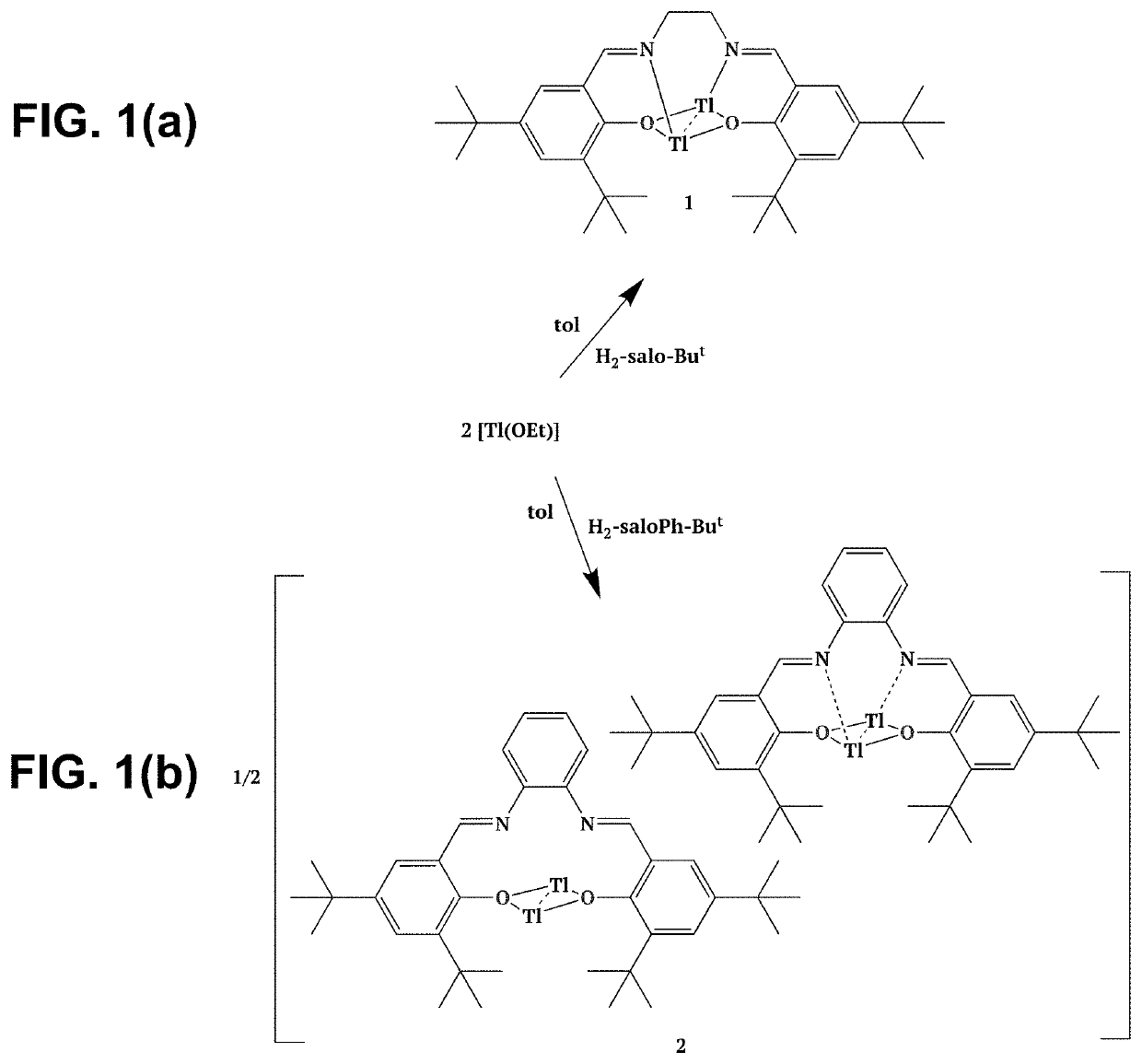
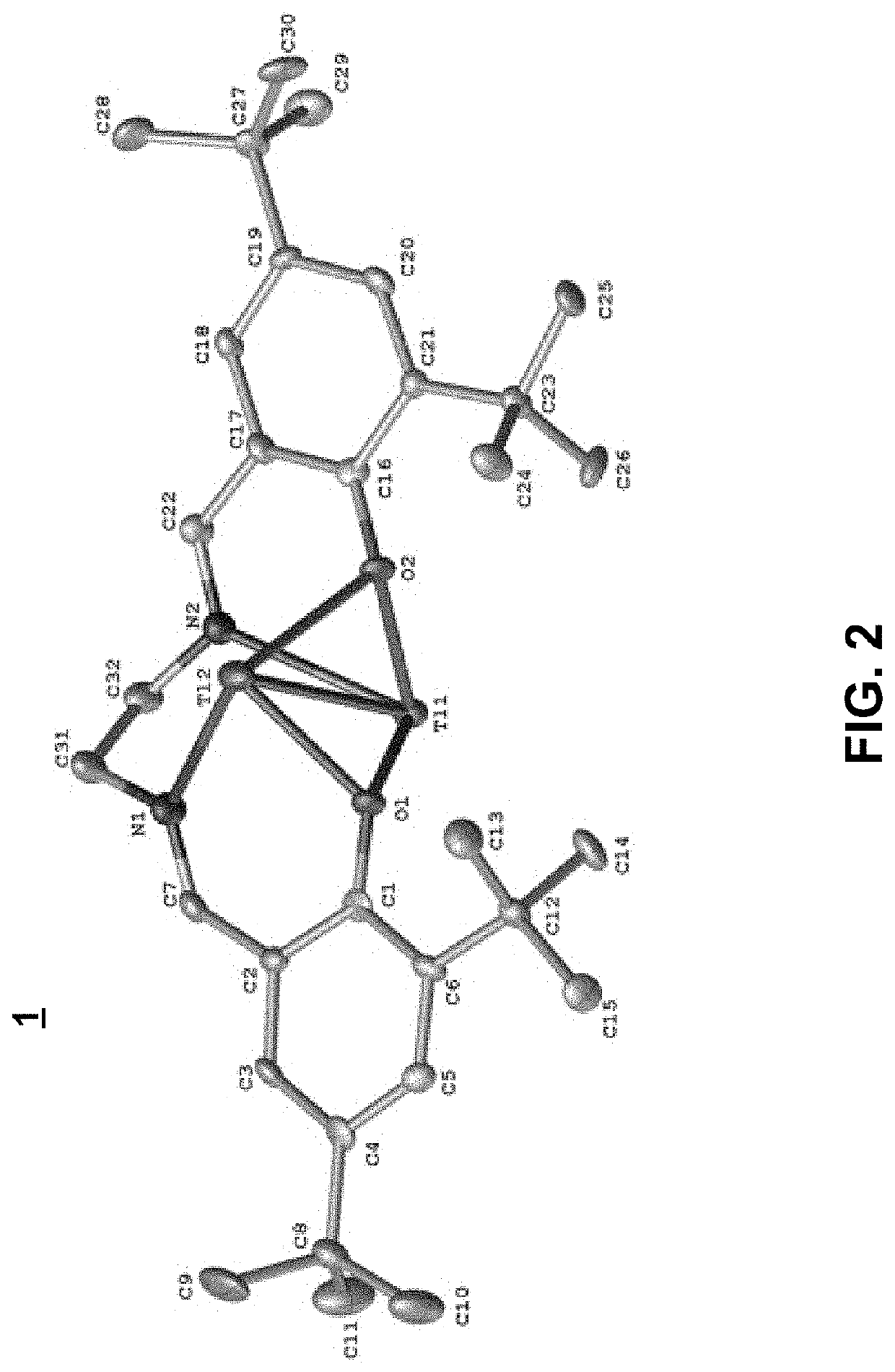
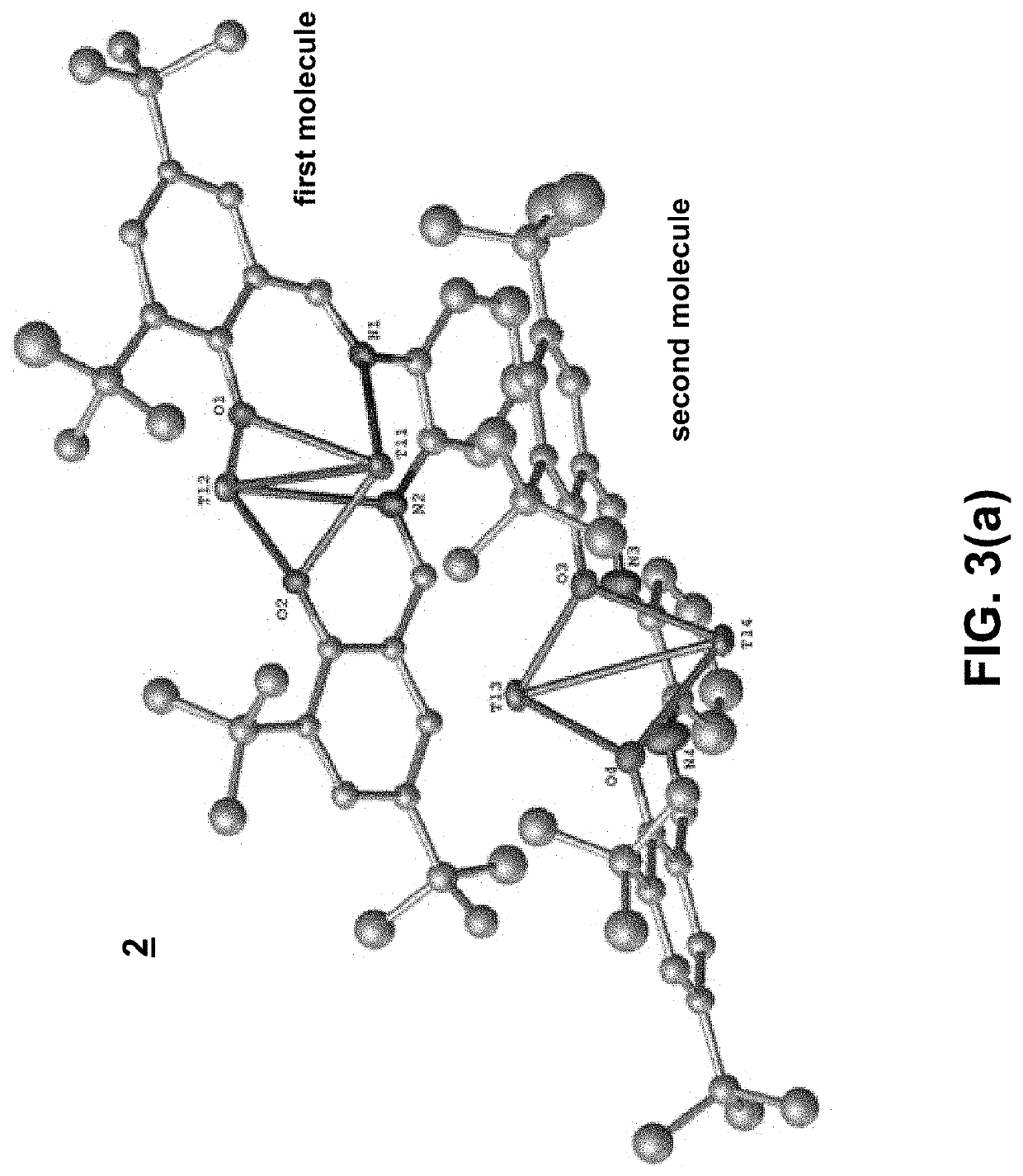
Thallium salen fluorescent tracers
Thallium salens can be synthesized by reacting thallium alkoxide with a salen ligand. As examples of the invention, the dinuclear complexes Tl2-(salo-But) and Tl2-(saloPh-But) were synthesized by the reaction of thallium ethoxide with (H2-salo-But) or (H2-saloPh-But). These thallium salens may have applications as fluorescent tracers (or taggants) for subterranean fluid flows.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com