Metal coordination compounds with zinc (II) and platinum (II) in different doping proportions of based on poly-Salen ligands as well as preparation methods and applications thereof
A technology of metal complexes and ligands, applied in chemical instruments and methods, luminescent materials, etc., to achieve high yield, broad application prospects, and easy molecular design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Wherein the n=2-1000, the average molecular weight of a1, a2, a3 or a4 is 3000-13000
[0037] Under nitrogen protection, 1 mmol (1 eq) of bridged dialdehyde based on bisphenol A structure, 1.1 mmol (1.1 eq) of o-phenylenediamine, 3 mL of 1,2-dichloroethane, 3 mL of 1,2-dichloroethane were added to a Schlenk tube, 80°C Reflux reaction for 24 h. After the reaction, 2 mL of absolute ethanol was added, and a solid was precipitated, which was centrifuged (or filtered), washed with absolute ethanol and ether in turn, dried and weighed. get a 1 , a 2 , a 3 , a 4 .
[0038] 1 yield 84.7%. IR (KBr tablet): n (cm -1 ) 3058, 2966, 1616, 1496, 1424, 1387, 1282, 1176, 1106, 976, 887, 827, 743; 1 H NMR (CDCl 3 , 300 MHz): δ (ppm) 12.96 (s, 2 H), 8.55 (s, 2 H), 7.25 ~ 7.21 (m, 8 H, ), 6.93 (d,J = 7.8, 2 H), 1.64 (s, 6 H); 13 C NMR (CDCl 3 , 300 MHz): δ (ppm) 196.41, 163.38, 158.00, 142.30, 140.65, 131.82, 129.78, 127.29, 119.09, 118.16, 116.85, 41.22, 30...
Embodiment 2
[0043]
[0044] Wherein the n=2-1000, the average molecular weight is 3000-13000
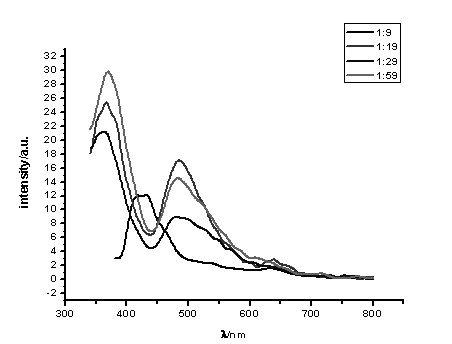
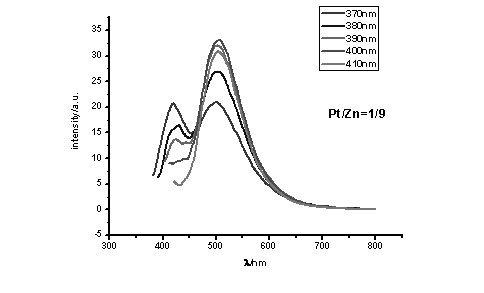
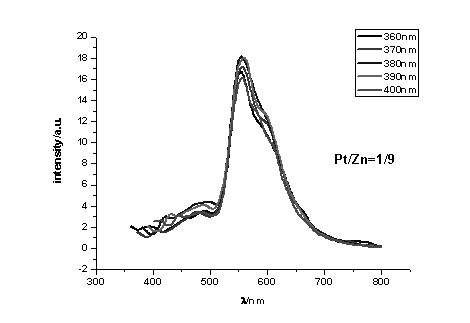
[0045] To a solution of 0.1 mmol (1 eq) of polySalen ligands a1, a2, a3 or a4 in dimethyl sulfoxide under nitrogen protection, 0.11 mmol (1.1 eq) of a mixture of zinc acetate and potassium chloroplatinite was added (Molar fraction of platinum(II): 1 / 10, 1 / 20, 1 / 30 … 1 / 100), then add anhydrous sodium carbonate 0.1 mmol (1 eq), reflux at 70 °C for 24 h, cool to room temperature, 2 mL of absolute ethanol was added to the reaction solution, and a precipitate was precipitated, which was centrifuged (or filtered), and washed with absolute ethanol and ether in turn. Dry and weigh. Metal complexes with different doping ratios were obtained.
Embodiment 3
[0047]
[0048] Wherein the n=2-1000, the average molecular weight of b1, b2, b3 or b4 is 5000-18000
[0049] Under nitrogen protection, 1 mmol (1 eq) of bridged dialdehyde based on bisphenol A structure, 1.1 mmol (1.1 eq) of binaphthalene diamine, 3 mL of 1,2-dichloroethane, 3 mL of 1,2-dichloroethane were added to a Schlenk tube, 80°C Reflux reaction for 24 h. After the reaction, 2 mL of absolute ethanol was added, and a solid was precipitated, which was centrifuged (or filtered), washed with absolute ethanol and ether in turn, dried and weighed. get b 1 , b 2 , b 3 , b 4 .
[0050] 1 yield 87.3%. IR (KBr tablet): n (cm -1 ) 3054, 2965, 1617, 1488, 1428, 1386, 1285, 1222, 1177, 1072, 1026, 975, 826, 749, 624, 527; 1 H NMR (CDCl 3 , 300 MHz): δ (ppm) 11.74 (s, 2 H), 8.54 (s, 2 H), 8.06 ~ 7.31 (m, 13 H), 7.10 ~ 7.01 (m, 3 H), 6.53 (d, J = 8.4, 2 H), 1.55(s, 6 H).
[0051] b 2 yield.64.5%.IR (KBr tablet): n (cm -1 ) 3055, 2968, 1610, 1505, 1456, 1388, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com