Preparation method of chiral Salen-Co(III) catalyst loaded by utilizing Merrifield resin
A catalyst and chirality technology, applied in the field of catalyst preparation, can solve the problems of complex preparation process, easy shedding of active atoms, catalyst deactivation, etc., and achieve the effects of reducing reaction conditions, low price and high load capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The present invention will be further described below in conjunction with the accompanying drawings and specific embodiments, which are not limited by the embodiments.
[0018] Specific implementation plan:
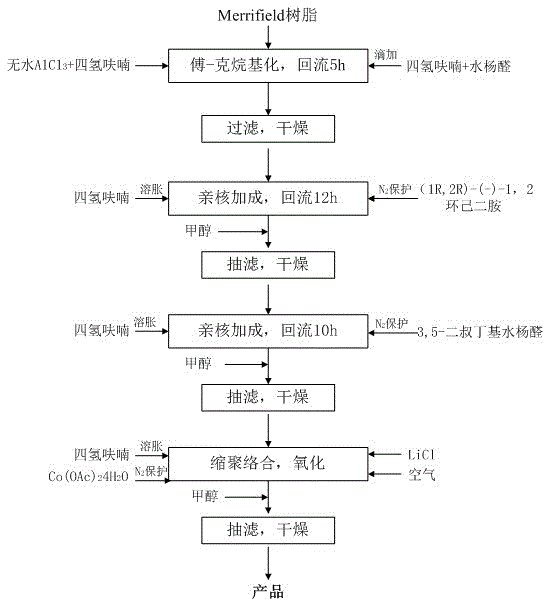
[0019] (1) Preparation of Merrifield resin base-salicylaldehyde (product 1):
[0020] Take 10g (11mmol) Merrifield resin (100~200 mesh, 1.1mmol / g), 10g anhydrous AlCl 3 , put into a three-neck flask containing a mixed solvent of 30ml tetrahydrofuran (THF) and 4ml nitrobenzene and stir, and swell for 30~60min at a temperature of 20°C. After dissolving 1.22g (10mmol) salicylaldehyde in 10ml tetrahydrofuran (THF), slowly add it dropwise to the above mixture, and heat to reflux at 66°C for 5~6h. After cooling to room temperature and filtering, the solid matter was washed successively with 1 mol / L hydrochloric acid, methanol, distilled water and methanol, and dried under normal pressure to obtain product 1.
[0021] (2) Preparation of chiral 4-Merrifield resinyl-2-(1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com