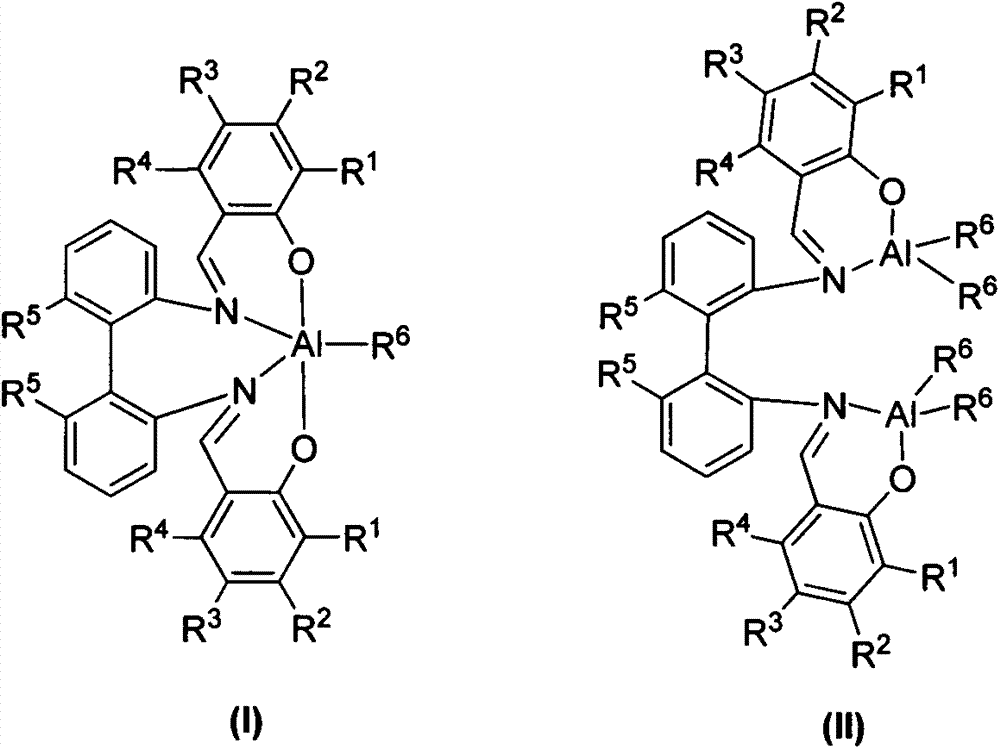
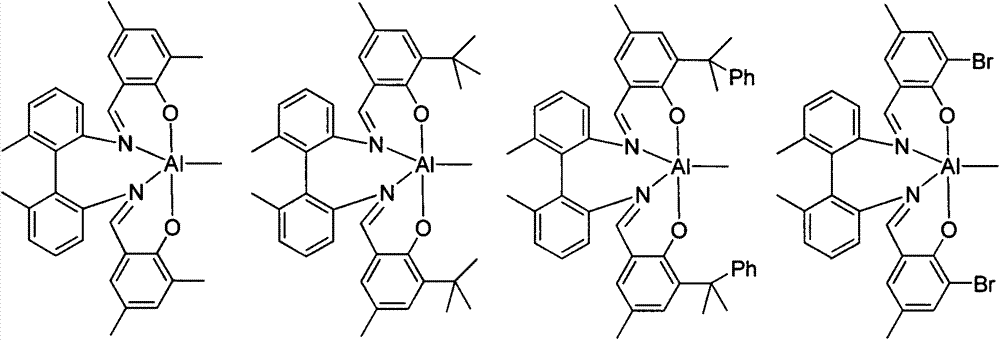
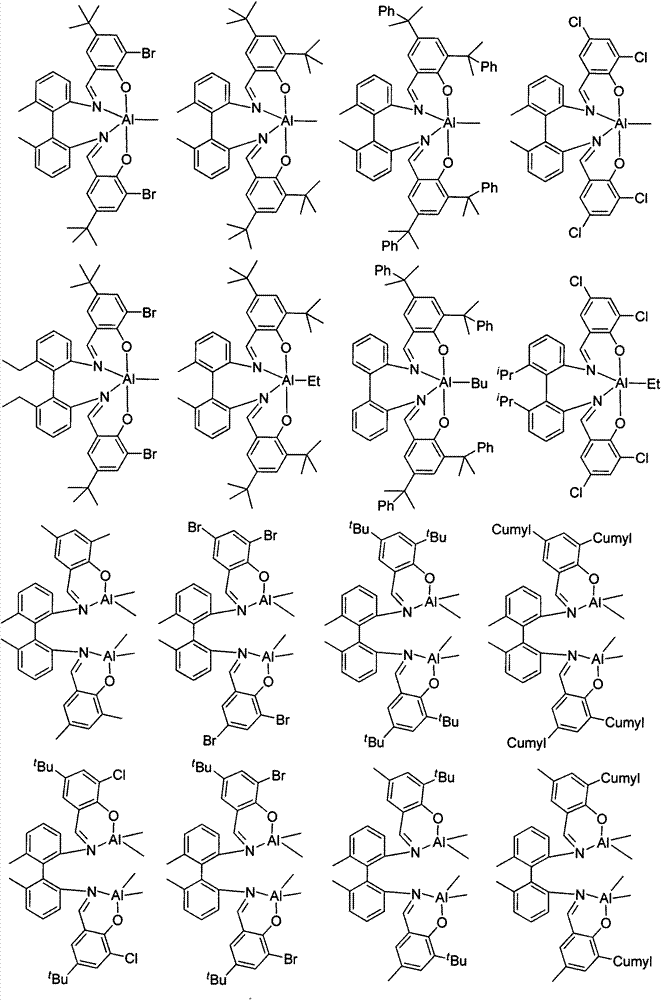
Mononuclear aluminum and binuclear aluminum compounds based on biphenyl skeleton Salen ligand and preparation method and application thereof
An aluminum compound and skeleton technology, applied in the application field of lactone polymerization, can solve the problem of low catalytic activity of catalysts, and achieve the effects of narrow molecular weight distribution, stable properties, high catalytic activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of Ligand L1
[0039] Add 1.79g (8.4mmol) 2,2'-diamino-6,6'-dimethylbiphenyl and 2.53g (10.0mmol) 2,4-dimethyl salicylaldehyde in a 100mL eggplant-shaped bottle, and then Add 30 mL of absolute ethanol to dissolve it. The solution was dark red, heated to ethanol reflux with an oil bath, and reacted for 16 hours. After the reaction was completed, the oil bath was removed, and the reaction solution was slowly cooled to room temperature, and orange-red crystals were precipitated. The reaction solution was filtered, the filter cake was washed with ethanol, and dried to obtain orange-red crystal L1 (3.56 g, yield: 88.5%).
[0040]
[0041] 1 H NMR (CDCl 3 , 400MHz): δ12.12(s, 2H, Ar-OH), 8.33(s, 2H, N=C-H), 7.33(t, 2H, J=7.6Hz, Ar-H), 7.22(d, 2H, J=7.6Hz, Ar-H), 7.02(d, 2H, J=7.6Hz, Ar-H), 6.94(s, 2H, Ar-H), 6.83(s, 2H, Ar-H), 2.22( s, 6H, Ar-CH 3 ), 2.12(s, 6H, Ar-CH 3 ), 2.05(s, 6H, Ar-CH 3 ).Anal.Calcd.for:C 32 h 32 o 2 N 2 : C, 80.64; H, 6.77; N,...
Embodiment 2
[0043] Synthesis of Ligand L2
[0044] Add 1.06g (5.0mmol) 2,2'-diamino-6,6'-dimethylbiphenyl, 1.93g (10.0mmol) 2-tert-butyl-4 methylsalicylaldehyde in a 100mL bottle, and add 30mL without Water and ethanol were heated in an oil bath to reflux the ethanol and reacted for 16 hours. After the reaction was completed, the oil bath was removed, and the reaction solution was slowly cooled to room temperature. It was found that needle-shaped yellow crystals were precipitated in ethanol. The reaction solution was filtered,
[0045] The filter cake was washed with ethanol and dried to obtain yellow crystal L2 (1.8 g, yield: 64.3%).
[0046]
[0047] 1 H NMR (CDCl 3 , 400MHz): δ12.80(s, 2H, Ar-OH), 8.35(s, 2H, N=C-H), 7.30(t, 2H, J=7.6Hz, Ar-H), 7.20(d, 2H, J=7.6Hz, Ar-H), 7.06(d, 2H, J=2.0Hz, Ar-H), 7.01(d, 2H, J=8.0Hz, Ar-H), 6.84(d, 2H, J= 2.0Hz, Ar-H), 2.22(s, 6H, Ar-CH 3 ), 2.07(s, 6H, Ar-CH 3 ), 1.30(s, 18H, Ar- t Bu).Anal.Calcd.for: (contains 0.33AcOEt)C 38 h 44 o ...
Embodiment 3
[0049] Synthesis of Ligand L3
[0050] Add 1.06g (5.0mmol) 2,2'-diamino-6,6'-dimethylbiphenyl, 3.305g (10.0mmol) 2,4-dipentyl salicylaldehyde, 30mL anhydrous Ethanol, heated with an oil bath to reflux the ethanol, and reacted for 16 hours. After the reaction was completed, the oil bath was removed, and the reaction solution was slowly cooled to room temperature. It was found that needle-shaped yellow crystals were precipitated in ethanol. The reaction solution was filtered, the filter cake was washed with ethanol, and dried with B to obtain yellow crystal L3 (3.9 g, yield: 84.8%).
[0051]
[0052] 1 H NMR (CDCl 3 , 400MHz): δ12.54(s, 2H, Ar-OH), 8.09(s, 2H, N=C-H), 7.30(d, 2H, J=8.0Hz, Ar-H), 7.24(m, 8H, Ar-H), 7.16(m, 8H, Ar-H), 7.06(m, 8H, Ar-H), 6.95(d, 2H, J=2.0Hz, Ar-H), 6.81(d, 2H, J =8.0Hz, Ar-H), 1.90(s, 6H, Ar-CH 3 ), 1.68(s, 12H, cumyl-CH 3 ), 1.52(s, 6H, cumyl-CH 3 ), 1.50(s, 6H, cumyl-CH 3 ).Anal.Calcd.for:C 64 h 64 o 2 N 2 : C, 86.06; H, 7.22; N, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com