Catalyst for catalyzing thioether oxidation as well as preparation method and application of catalyst
A catalyst and oxidant technology, used in the preparation of organic compounds, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve problems such as inapplicability, and achieve mild conditions, good selectivity, The effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1. Synthesis of Salen Ligand
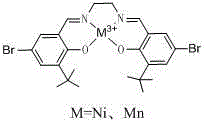
[0020] Into a 250 mL three-necked flask, 2.56 g of the compound, 10.0 mmol of 5-bromo-3-tert-butyl salicylaldehyde, 0.29 g, 4.9 mmol of ethylenediamine (the molar ratio of the two was 1:0.49) and 150 mL of ethanol were added successively. After reacting for 12 h at 85 °C under the protection of an inert gas, it was cooled to 15 °C, and the reaction solution was evaporated at 40 °C by a rotary evaporator, concentrated to 10 mL, and then placed at -5 °C for 3 h. Suction filtration with a Buchner funnel, the filter cake was washed with 10 mL ice ethanol for 3 to 5 times, and the filter cake was collected to obtain 2.50 g of light yellow solid, 4.67 mmol Salen ligand, yield: 95%. 1 H NMR (CD 3 Cl, 400MHz) δ(ppm): 1.43[s, 18H, C(CH 3 ) 3 ], 3.97(s, CH 2 , 4 H), 7.23(s, Ar-H, 2 H), 7.38(s, Ar-H, 2 H), 8.32(s, N=CH, 2 H), 13.84 (s, 1 H, OH) .
Embodiment 2
[0021] Example 2. Synthesis of Ni(Ⅲ)Salen-OAc Catalyst
[0022] Into a 250 mL three-necked flask, 2.00 g, 3.73 mmol of Salen ligand, 1.1 g, 4.48 mmol of Ni(OAc) 2 · 4H 2 O, 60 mL of ethanol and 60 mL of chloroform were reacted at 80 °C for 12 h under the protection of inert gas nitrogen, and reacted with oxygen for 16 h. After the reaction, the solvent was recovered by rotary evaporation at 50 °C, washed twice with water, and reduced Suction filtration and air-drying at room temperature for 5 h gave 2.35 g, 3.61 mmol of Ni(Ⅲ) catalyst. Yield 97%; Elemental analysis (C 26 h 31 N 2 o 4 Br 2 Ni), theoretical values: C, 47.75 %; H, 4.78; N, 4.28 %; O, 9.78 %. Found: C, 47.78 %; H, 4.74 %; N, 4.25 %; O, 9.82 %. High-resolution mass spectrum: calculated value: 591.9865, experimental value: 591.9858.
Embodiment 3
[0023] Example 3. Synthesis of Mn(Ⅲ)Salen-OAc Catalyst
[0024] Into a 250 mL three-neck flask, 2.0 g, 3.73 mmol of Salen ligand, 1.3 g, 4.48 mmol of Mn(OAc) were sequentially added 2 · 6H 2 O, 60 mL of ethanol and 60 mL of chloroform were reacted at 80 °C for 12 h under the protection of inert gas nitrogen, and then reacted with oxygen for 16 h. filtered, and air-dried at room temperature for 5 h to obtain 2.35 g of Mn(Ⅲ) catalyst with a yield of 97%. Elemental analysis (C 26 h 31 N 2 o 4 Br 2 Mn), theoretical values: C,4 8.02 %; H, 4.80; N, 4.31 %; O, 9.84 %. Found: C, 47.78 %; H, 4.77 %; N, 4.28 %; O, 9.82 %. High-resolution mass spectrum: calculated value: 588.9893, experimental value: 588.9876.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com