Preparation method and application of catalyst
A catalyst and solvent technology, applied in the field of preparation of Salen organic polymers and Co catalysts, can solve the problems of difficult recovery and cannot be reused, and achieve the effects of easy recovery, good effect, and maintaining catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057]
[0058] In a 250 mL round bottom flask, add salicylaldehyde (21.2 mL, 200 mmol), potassium carbonate (41.4 g, 300 mmol), DMF (100 mL), and magneton in sequence. With stirring at room temperature, allyl bromide (20.74 mL, 240 mmol) was slowly added dropwise, and reacted at room temperature for more than 12 hours, and was detected by TLC analysis until the reaction was complete. After the reaction was complete, ethyl acetate and water were added to the reaction solution for extraction. The organic phase was first washed 3 times with water to remove DMF, and then washed once with saturated brine. The obtained organic phase was washed with anhydrous Na 2 SO 4 Dry, filter and concentrate under reduced pressure to obtain the product which is phenyl allyl ether.
[0059] structure of the product 1 H-NMR and 13 C-NMR determines that its data are as follows:
[0060] 1 H-NMR (400MHz, CDCl 3 )δppm 7.77(dd, J=7.6,1.6Hz,1H),7.48-7.44(m,1H),6.96(dd,J=8.0,8.0Hz,1H),6.91(d,J...
Embodiment 2
[0063]
[0064] In a 100mL round-bottomed flask, add phenyl allyl ether (1.026g) obtained in Example 1 and magnetons, and heat the round-bottomed flask in an oil bath at 200°C after connecting a condenser to cause Claisen rearrangement reaction . After reacting for 4 hours, the reaction was complete according to TLC analysis, and the yellow oily liquid formed was the Claisen rearrangement reaction product.
[0065] structure of the product 1 H-NMR and 13 C-NMR determines that its data are as follows:
[0066] 1 H-NMR (400MHz, CDCl 3 )δppm 11.3(s, 1H), 9.89(s, 1H), 7.44(d, J=7.6Hz, 1H), 7.42(d, J=7.6Hz, 1H), 6.98(dd, J=7.6, 7.6Hz ,1H),6.06-5.95(m,1H),5.14-5.11(m,1H),5.09-5.08(m,1H),3.45(m,1H),3.44(m,1H);
[0067] 13 C-NMR (100MHz, CDCl 3 ) δppm 196.7, 159.5, 137.1, 135.8, 131.9, 128.8, 120.3, 119.6, 116.2, 33.0.
Embodiment 3
[0069]
[0070] In a 250 mL round bottom flask, add Claisen rearrangement product (1.6 mL, 0.018 mol), ethylenediamine (0.5 mL, 0.0075 mol), ethanol (50 mL) in sequence, and heat and stir in a 75°C water bath for 5 hours. After the reaction solution was cooled to room temperature, ethyl acetate and water were added, the organic phase was washed 3 times with water, and then once with saturated brine, and the obtained organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure afforded the product Salen.
[0071] The structure of the product Salen is used 1 H-NMR and 13 C-NMR determines that its data are as follows:
[0072] 1 H-NMR (400MHz, CDCl 3 )δppm 13.6(s, 1H), 8.37(s, 1H), 7.21(d, J=7.2Hz, 1H), 7.14(d, J=7.6Hz, 1H), 6.84(dd, J=7.6, 7.2Hz ,1H),6.11-6.01(m,1H),5.12(d,J=11.2Hz,1H),5.09(s,1H);
[0073] 13 C-NMR (100MHz, CDCl 3 )δppm 166.7(×2), 158.9(×2), 136.6(×2), 132.7(×2), 129.7(×2), 127.8(×2), 118.4(×2), 118.2(...
PUM
| Property | Measurement | Unit |
|---|---|---|
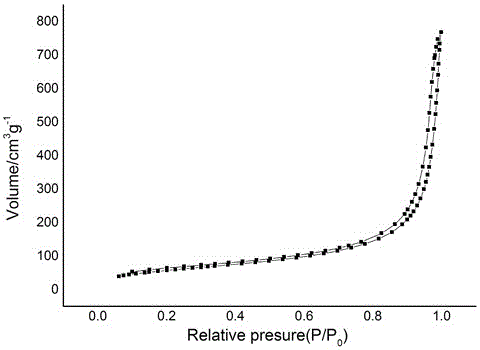
| Specific surface area | aaaaa | aaaaa |
| Mesopore specific surface area | aaaaa | aaaaa |
| Micropore specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com