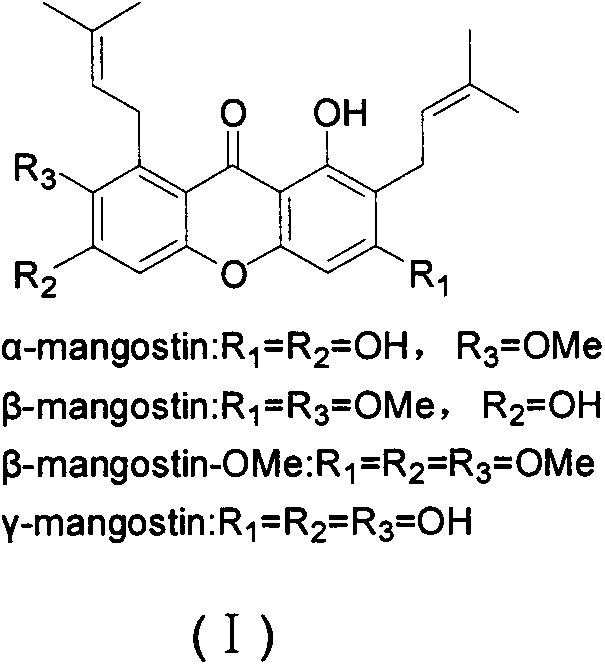Patents
Literature
75 results about "Claisen rearrangement" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
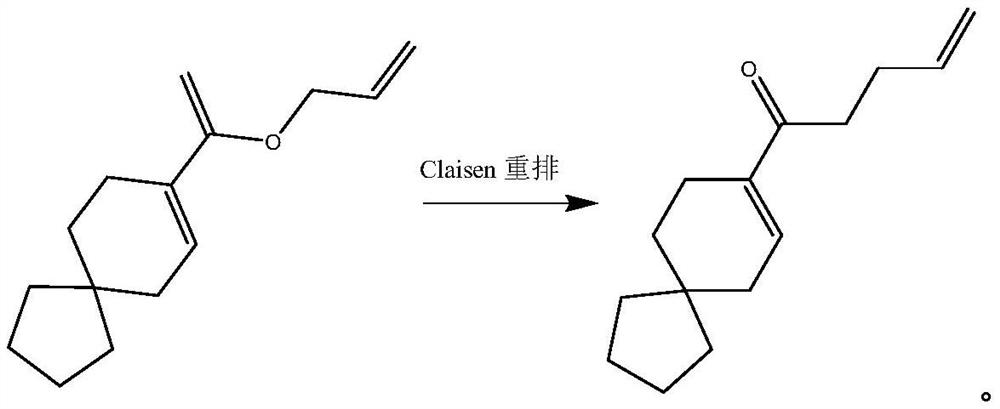
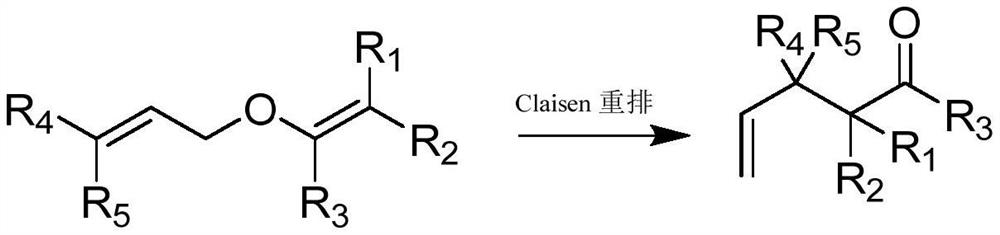
The Claisen rearrangement (not to be confused with the Claisen condensation) is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl.
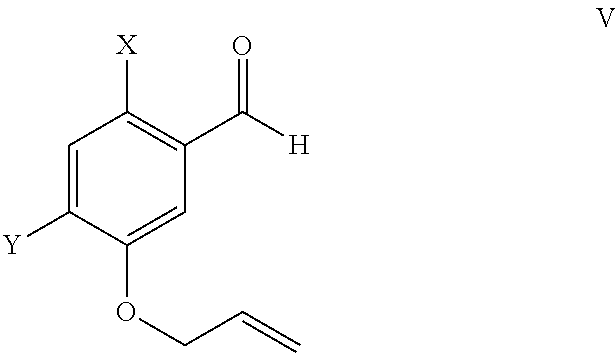
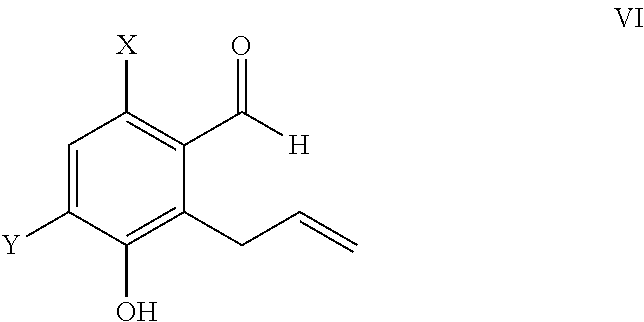
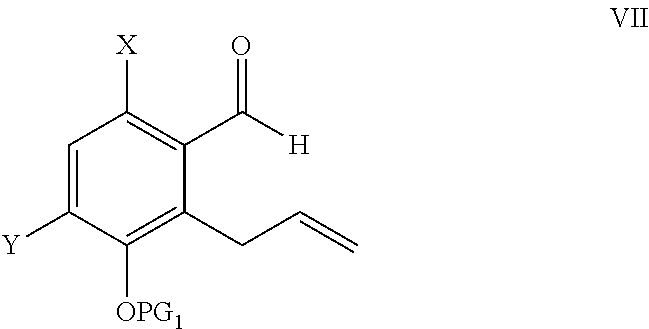
Process for the preparation of treprostinil and derivatives thereof
ActiveUS9346738B2Group 4/14 element organic compoundsOrganic compound preparationTreprostinilBenzaldehyde
A method for the preparation of treprostinil and its derivatives is described. In contrast to prior art, this method utilizes an easily scalable enzymatic resolution of a key intermediate for making these compounds. Another significant improvement of the described method over prior methods is the regioselective Claisen rearrangement of a 5-allyloxy-benzaldehyde precursor, which is facilitated by a bromo substituent in 2-position.
Owner:SCIPHARM SARL
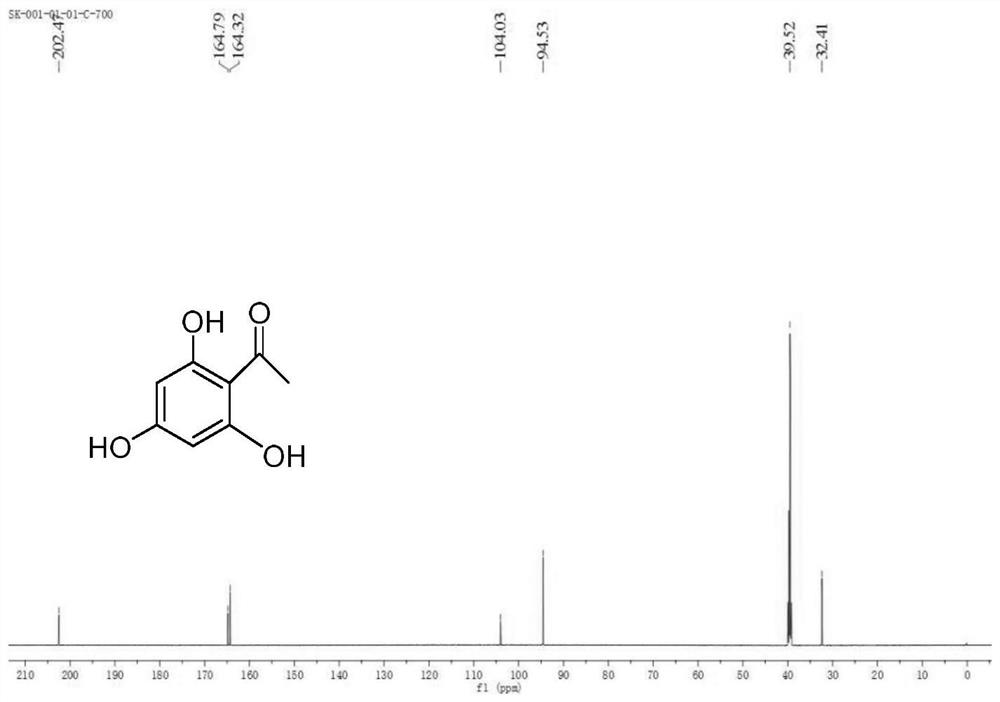
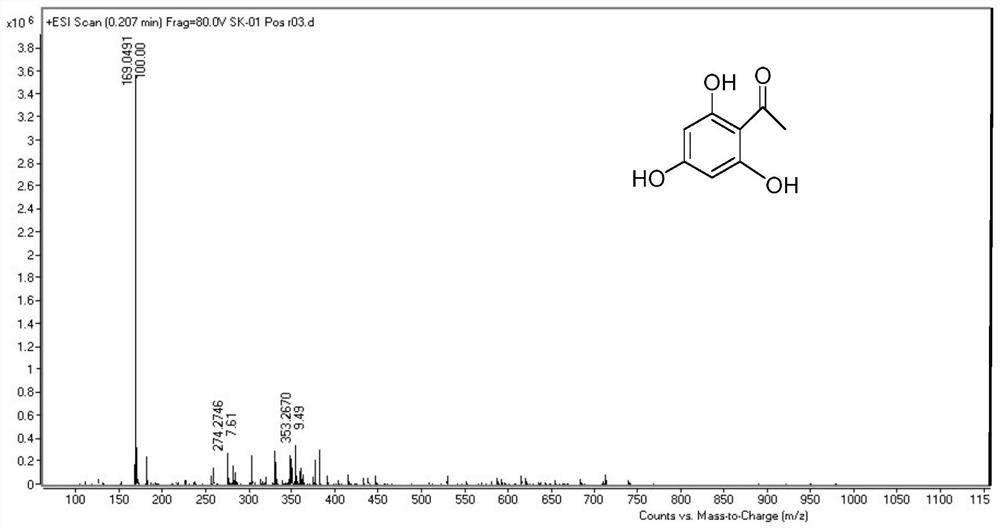
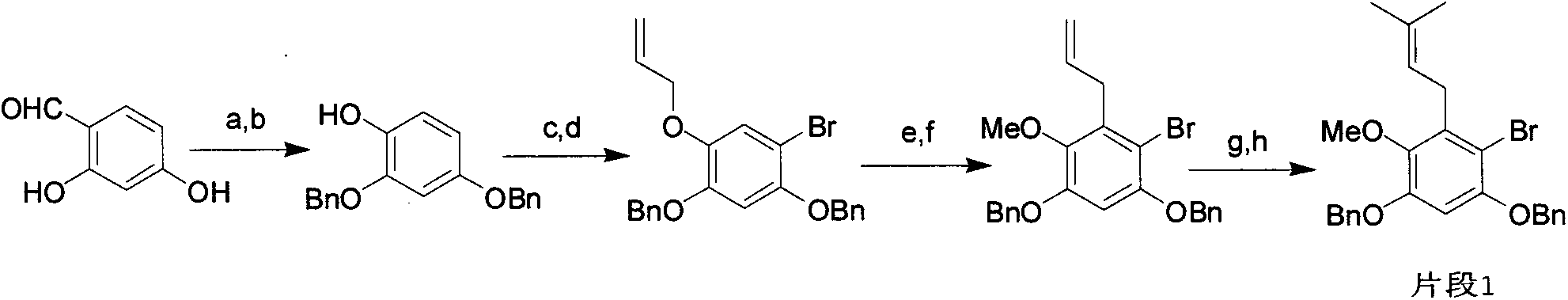
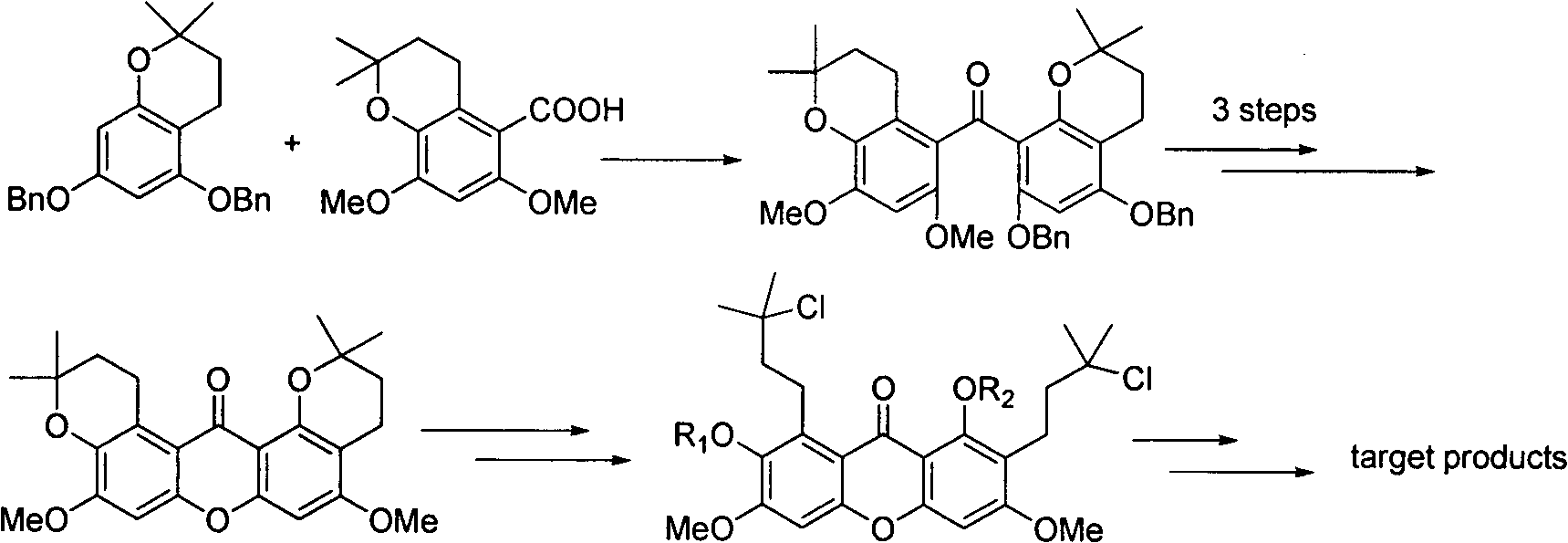
Total synthesis method of mangostin
The invention belongs to the field of chemical synthesis, in particular relates to a synthesis method of a natural product mangostin which is shown in the formula (I) and has good anti-tumor activity, anti-inflammation activity and anti-bacterial activity. The method provided by the invention comprises the following steps of: taking 2,4,5-trialkoxy benzoic acid and 1,3,5-trialkoxy benzene as initial raw materials; carrying out acyl chlorination, Friedel-Crafts acylation and cyclization to prepare a key intermediate 1,3,6,7-tetraalkoxy xanthone; and then, carrying out dealkylation, allylation, Claisen rearrangement, methylation, oxidation reaction, WITTING reaction and dealkylation reaction so as to prepare the mangostin.
Owner:CHINA PHARM UNIV
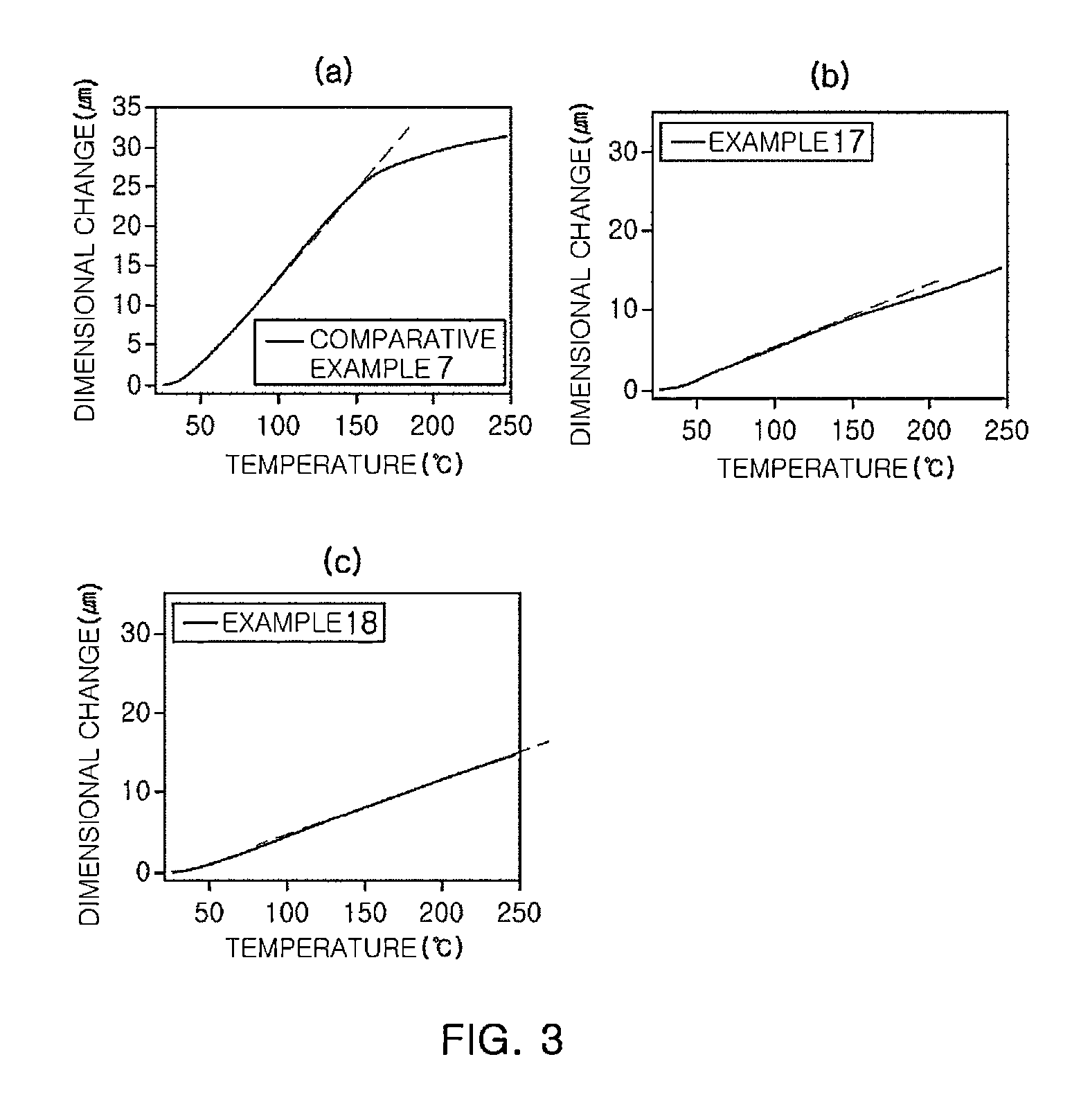
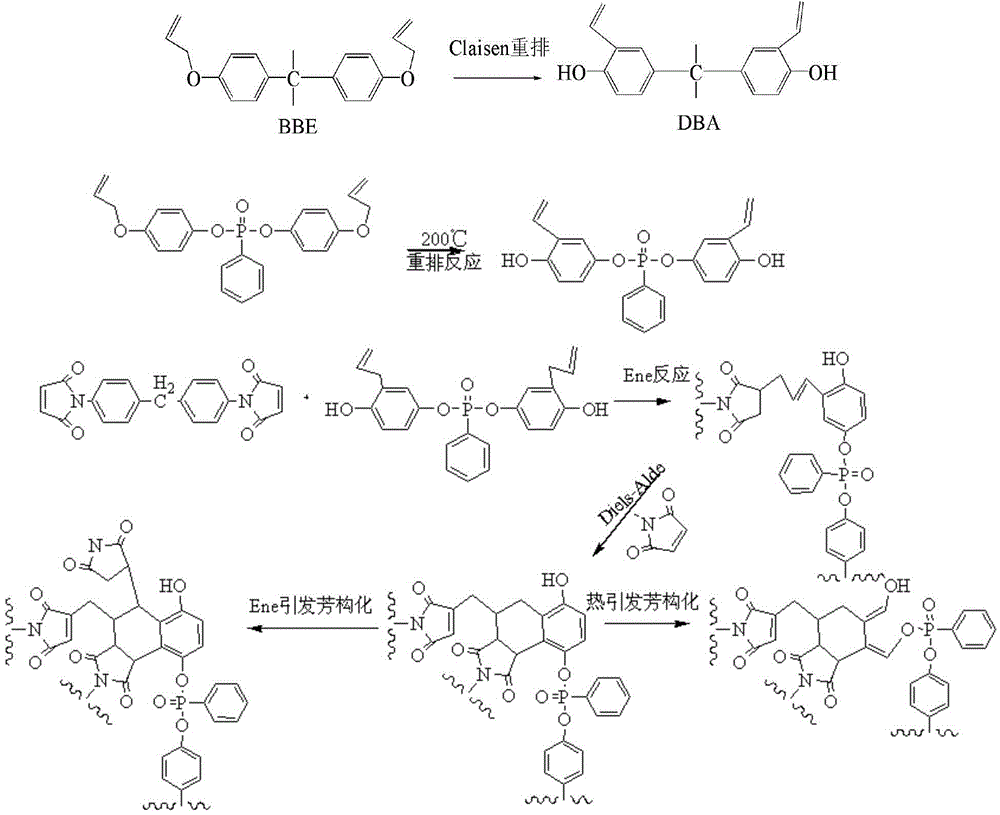
Heat convertible resin composition and its preparing process
ActiveCN101104655AIncrease polarityOvercome the defect of poor binding performanceVitrificationPolymer science
The invention discloses a thermosetting resin combination and a preparation method of the thermosetting resin combination. The thermosetting resin combination is prepared by prepolymerization reaction between Claisen rearrangement allylated novolac resins whose weight percentage is of 66 to 90 percent and bismaleimide monomers whose weight percentage is of 10 to 34 percents in the temperature of 100 to 150 DEG C. The Claisen rearrangement allyl novolac resin is made through a Claisen rearrangement reaction of allyl etherified novolac resins. The bismaleimide monomers are provided with a formula I structure, wherein, R1 and R2 are H; R is (see above graph). The invention is not only in high glass transition temperature, but also in fine resistance to heat deterioration performance. Fiber reinforced composites of the invention has high temperature strength / modulus retention, which can be used for preparing high temperature resistant composites that is used temporarily under the temperature of 350 DEG C, and can be applied widely in manufacturing the high temperature resistant composites such as matrix resins for missile radomes in aerospace industry.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Epoxy compound having alkoxysilyl group, method of preparing the same, composition and cured product comprising the same, and uses thereof
ActiveUS20140179836A1Improve flame retardant performanceChemical bonding efficiencySilicon organic compoundsOther chemical processesEpoxyPolymer science
Disclosed are an epoxy compound having an alkoxysilyl group, a composite of which exhibits good heat resistant properties and / or a cured product of which exhibits good flame retardant properties, a method of preparing the same, a composition comprising the same, and a cured product and a use of the composition. An alkoxysilylated epoxy compound comprising at least one of Chemical Formula S1 substituent and at least two epoxy groups in a core, a method of preparing the epoxy compound by an allylation, a claisen rearrangement, an epoxidation and an alkoxysilylation, an epoxy composition comprising the epoxy compound, and a cured product and a use of the composition are provided. The composite of the disclosed exhibits improved chemical bonding, good heat resistant properties, a low CTE, a high glass transition temperature or Tg-less The cured product of the composition exhibits good flame retardant properties.
Owner:KOREA INST OF IND TECH
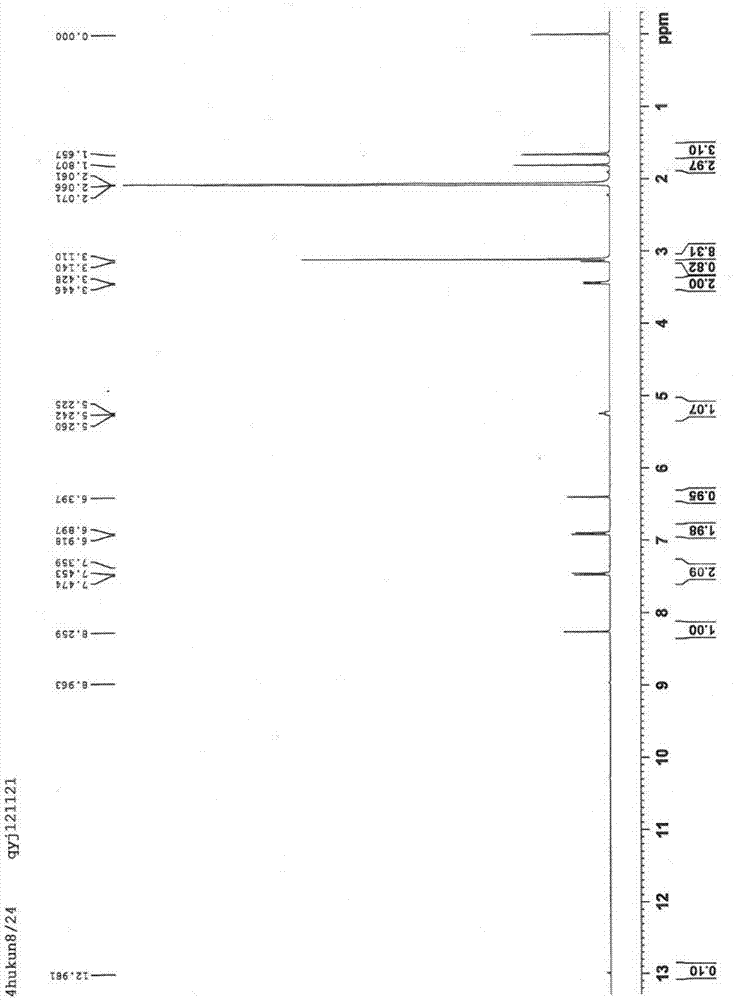
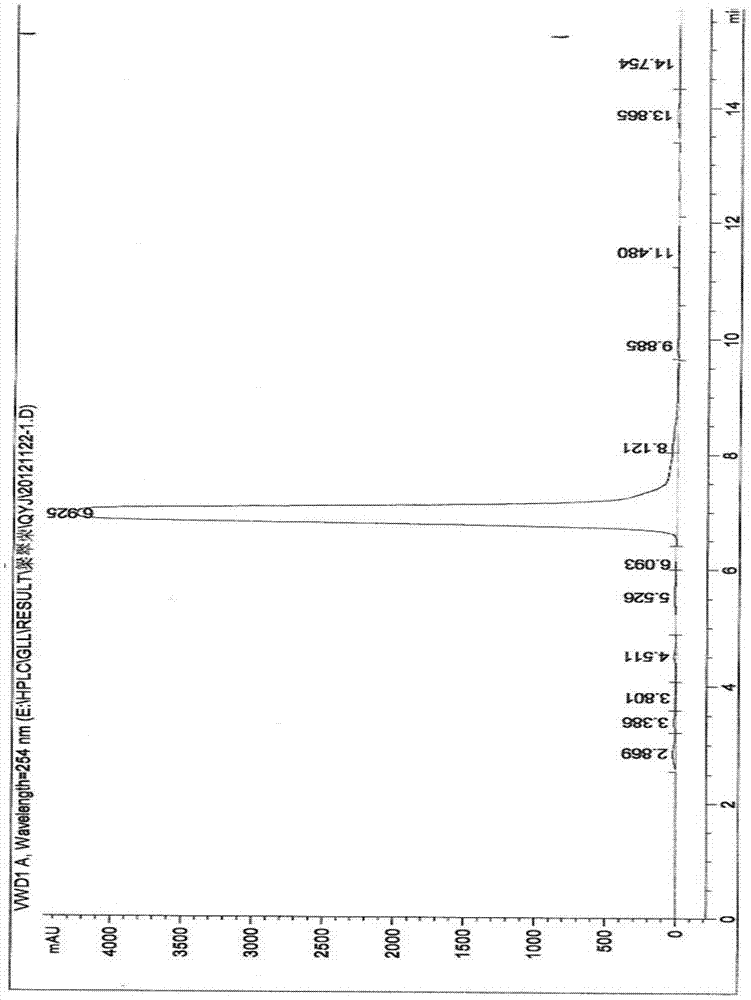
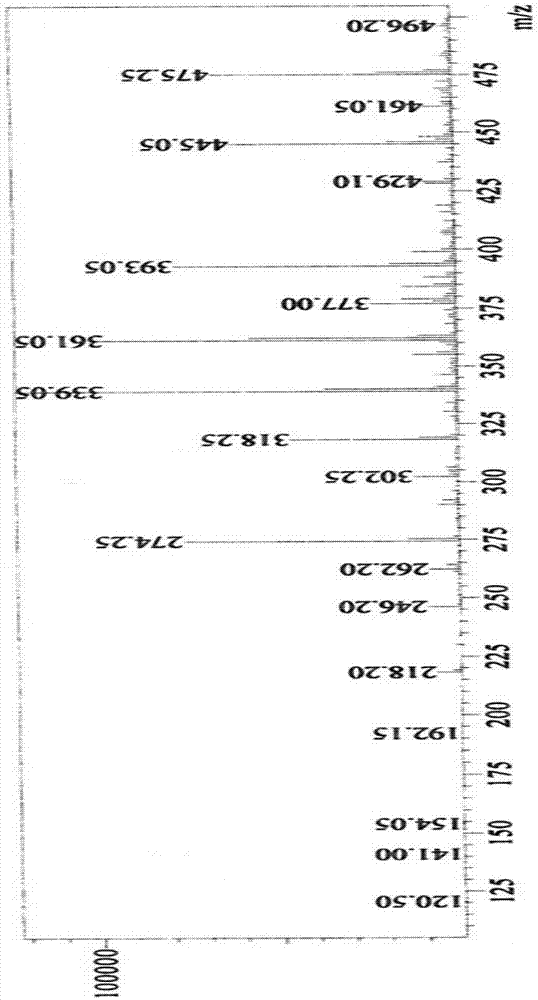
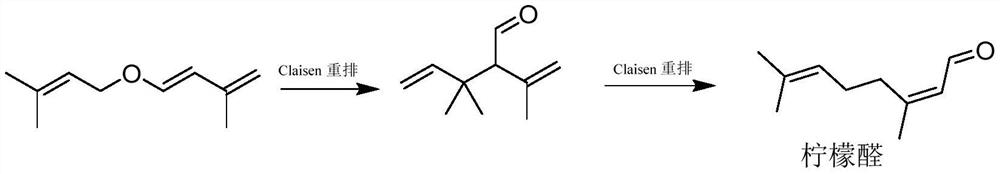
Method for preparation of citral
ActiveCN106977383AHigh selectivityShort reaction timeOrganic compound preparationCarbonyl compound preparationReaction rateEther
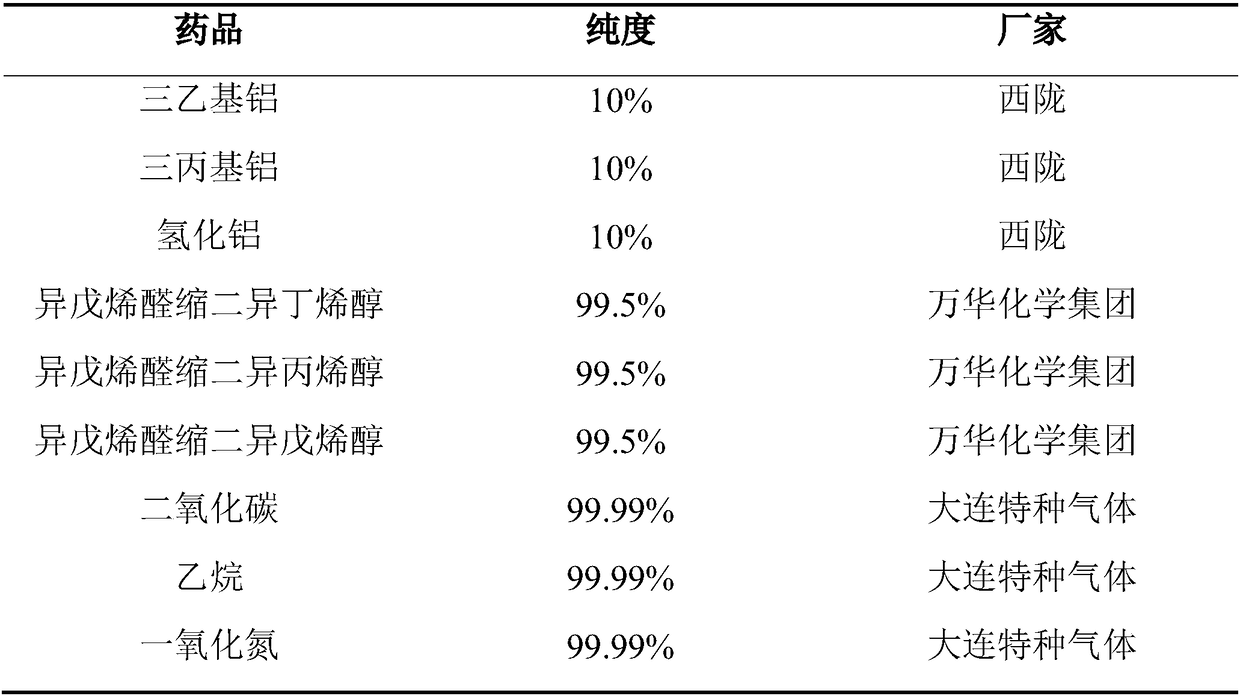
The invention provides a method for preparation of citral. The method comprises that cis / trans-isopentenyl-3-methyl butadiene ether is subjected to Claisen rearrangement to form 2, 4, 4-trimethyl-3-formyl-1, 5-hexadiene, and the 2, 4, 4-trimethyl-3-formyl-1, 5-hexadiene is subjected to Cope rearrangement to form citral (3, 7-dimethyl-2, 6-octadien-1-aldehyde). The method utilizes an ester as a side reaction inhibitor to adjust a reaction rate. The method has high selectivity, a fast reaction rate and whole process residence time of 1-15min.
Owner:WANHUA CHEM GRP CO LTD
Preparation method of citral
ActiveCN108117484ATake advantage ofShort reaction timeOrganic compound preparationCarbonyl compound preparationClaisen rearrangementCracking reaction
The invention provides a preparation method of citral. The method comprises the following steps: in a supercritical condition, carrying out thermal cracking reaction on one or more of isoprenaldehydediisobutenol, isoprenaldehyde diisopentenol and isoprenaldehyde diisopentenol as raw materials to obtain cis / trans-isopentenyl-3-methyl-butadiene; and carrying out Claisen rearrangement and Cope rearrangement on the cis / trans-isopentenyl-3-methyl-butadiene to obtain the citral. The method provided by the invention is characterized in that the thermal cracking process is carried out in the supercritical condition, the cis / trans-isopentenyl-3-methyl-butadiene is separated in a flash manner, and a trialkyl aluminum catalyst is introduced in a flash tank to direct rearrange the cis / trans-isopentenyl-3-methyl-butadiene in the flash process to obtain the citral, so that the reaction time is shortened greatly and the product yield is increased.
Owner:WANHUA CHEM GRP CO LTD
Benzenesulfonic acid catalyst supported on silica gel, as well as preparation and application thereof
InactiveCN102302948AHigh bond energyIncrease loading capacityLactams preparationOrganic compound preparationBeckmann rearrangementChlorosulfuric acid
The invention discloses a benzenesulfonic acid catalyst supported on silica gel, which is prepared by adopting silica gel to react with thionyl chloride, phenol and chlorosulfonic acid sequentially. In the catalyst, the silica gel and benzenesulfonic acid are bonded in a covalent bond mode, so that the supporting capacity is stable, and the supporting capacity of the benzenesulfonic acid is about 2.25mmol / g in the benzenesulfonic acid catalyst supported on silica gel through analysis and calculation. Experiments prove that: the prepared catalyst has efficient catalytic activity to two kinds of atom economic reactions, namely Beckmann rearrangement reaction and Claisen rearrangement reaction, is non-toxic, harmless and noncorrosive, is easily separated, can be recycled, and accords with green and environmentally-friendly national industrial policy.
Owner:NORTHWEST NORMAL UNIVERSITY
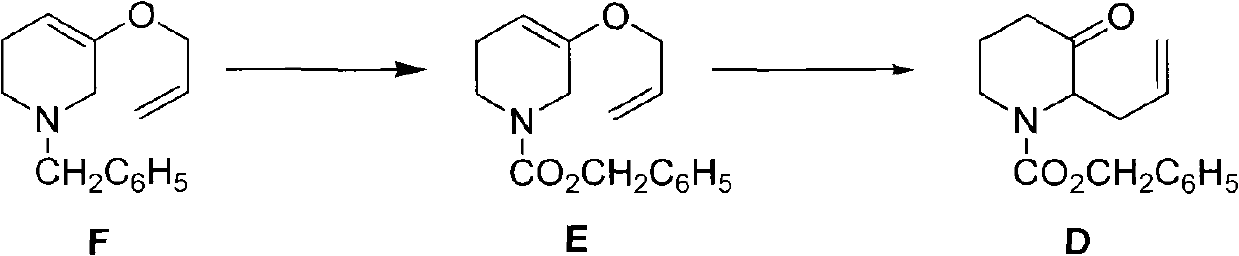
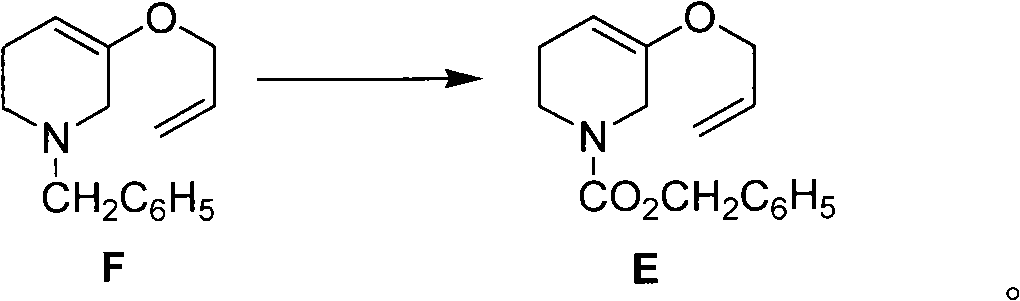
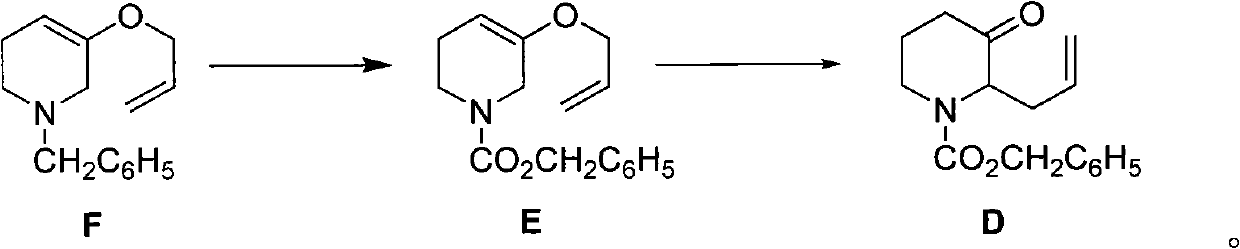
Method for preparing halofuginone intermediate
The invention discloses a method for preparing a halofuginone intermediate shown in a formula D or E. The method comprises the following steps of: (1) dropwise adding solution of a compound F into benzyl chloroformate solution, and performing Van Braun reaction to obtain a compound E; and (2) performing Claisen rearrangement reaction on the compound E obtained in the step (1). By the method, the Van Braun reaction can be completely performed, the using amount of benzyl chloroformate is reduced, the efficiency of the reaction is improved, and a 2-bit product can be selectively obtained in the subsequent Claisen rearrangement reaction. In the better embodiment of the invention, the operation process is simplified by a one-pot method, the using amount of solvents is reduced, the selectivity is improved, the cost of the reaction is reduced, the pollution to the environment is reduced, and good conditions are created for mass production of halofuginone.
Owner:南通远航医药化工有限公司 +1
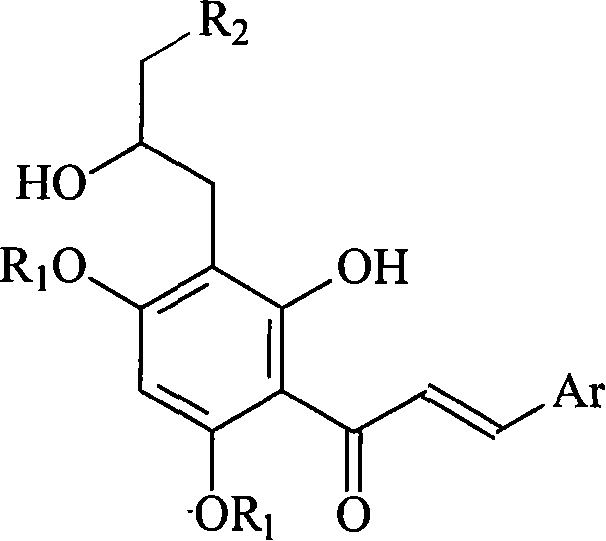
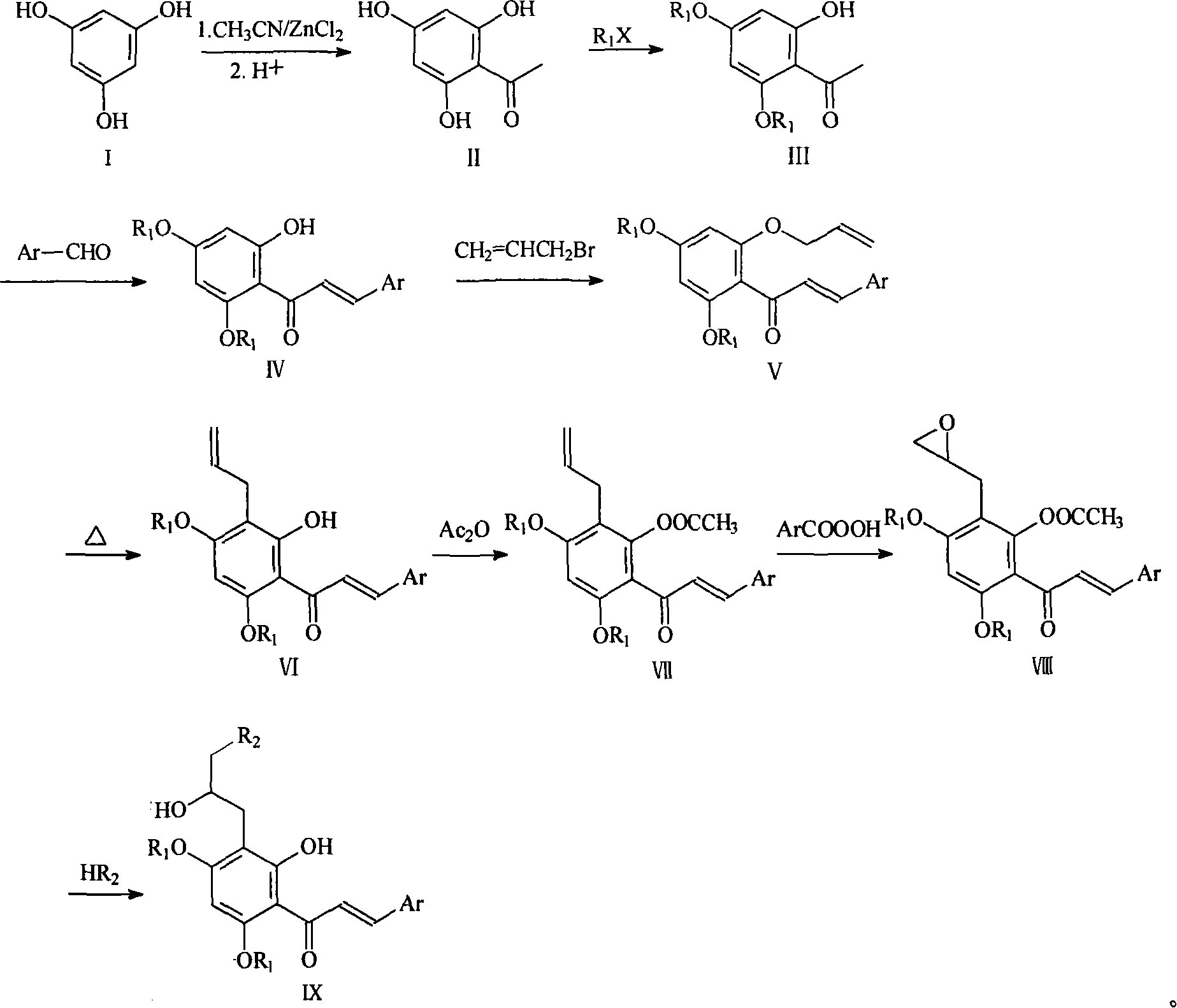
Preparation method and usage for nitrogen-containing chalcone derivatives
InactiveCN101041646AWide variety of sourcesEasy to manufactureOrganic chemistryAntineoplastic agentsNitrogenClaisen rearrangement
The invention discloses a making method of 2'-hydroxy-3'-alkylamino propyl-4', 6'-disubstituted chalcone derivant, which comprises the following steps: adopting 2'-hydroxy chalcone as leading material; proceeding allyl etherifying reaction on the mother core of 2'-hydroxy chalcone; proceeding Claisen rearrangement; epoxidising; additioning; obtaining the product; fitting for industrial manufacturing; improving the receiving rate obviously.
Owner:ZHEJIANG UNIV
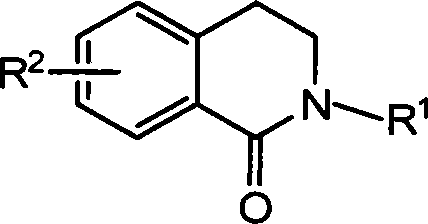
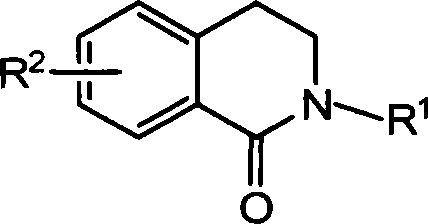
Method for synthesizing 2-substituted-3,4-dihydro-1-isoquinoline ketones and use thereof for preparing cardiovascular agents
The invention discloses a synthesis method of 2-substituted-3, 4-dihyidro-1-isoquinoline ketone compound and a relative application in the drug preparation for cardiovascular disease, wherein the compound is represented as above, R1 is gaseous normal alkyl and aromatic group or various substituted alkyl and aromatic group of C1-C19, R2 is OH, OCH3, CH3, NO2 and halogen or the like and can be one or more substituents. The preparation of 2-substituted-3, 4-dihyidro-1-isoquinoline ketone compound uses isovanillic acid ester as raw material, via six-step reaction as allyl etherified, Claisen rearrangement, oxidation, reaction with primary amine (generating Schiff base), reduction and aminolysis of molecular lactone, to synthesize the product. The isolated arterial ring tension test proves that the compound can relax vessel effectively. The 2-substituted-3, 4-dihyidro-1-isoquinoline ketone compound is a new potential cardiovascular drug, with wide application for preparing the drug of cardiovascular disease.
Owner:XI AN JIAOTONG UNIV
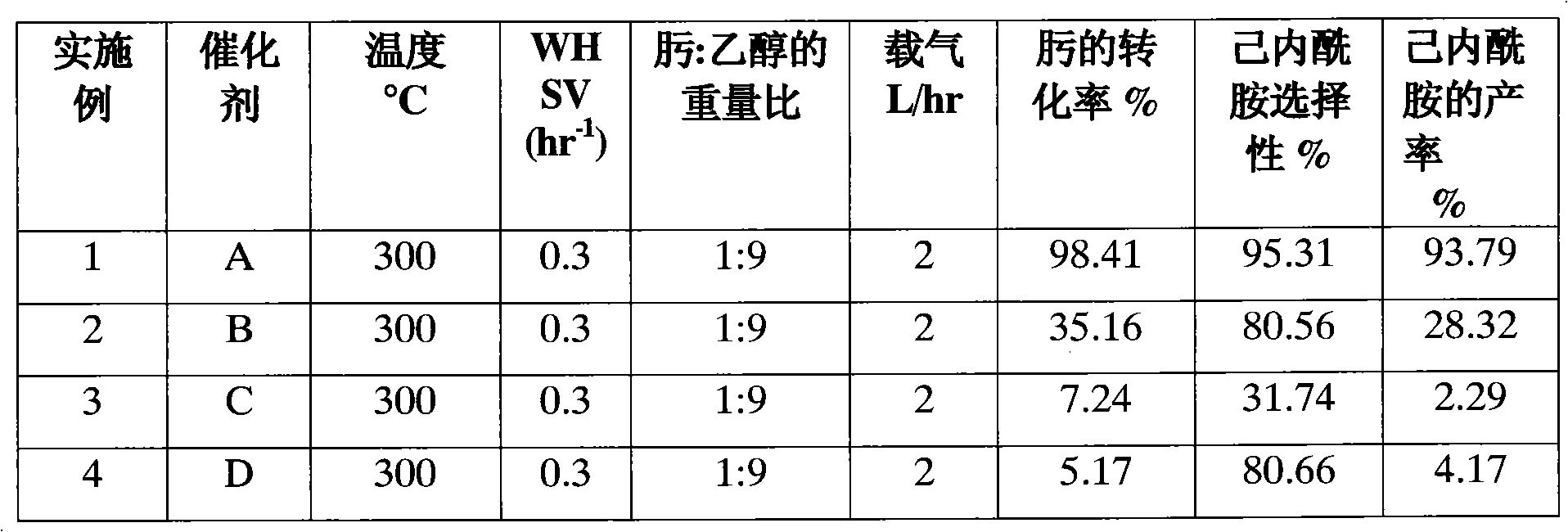
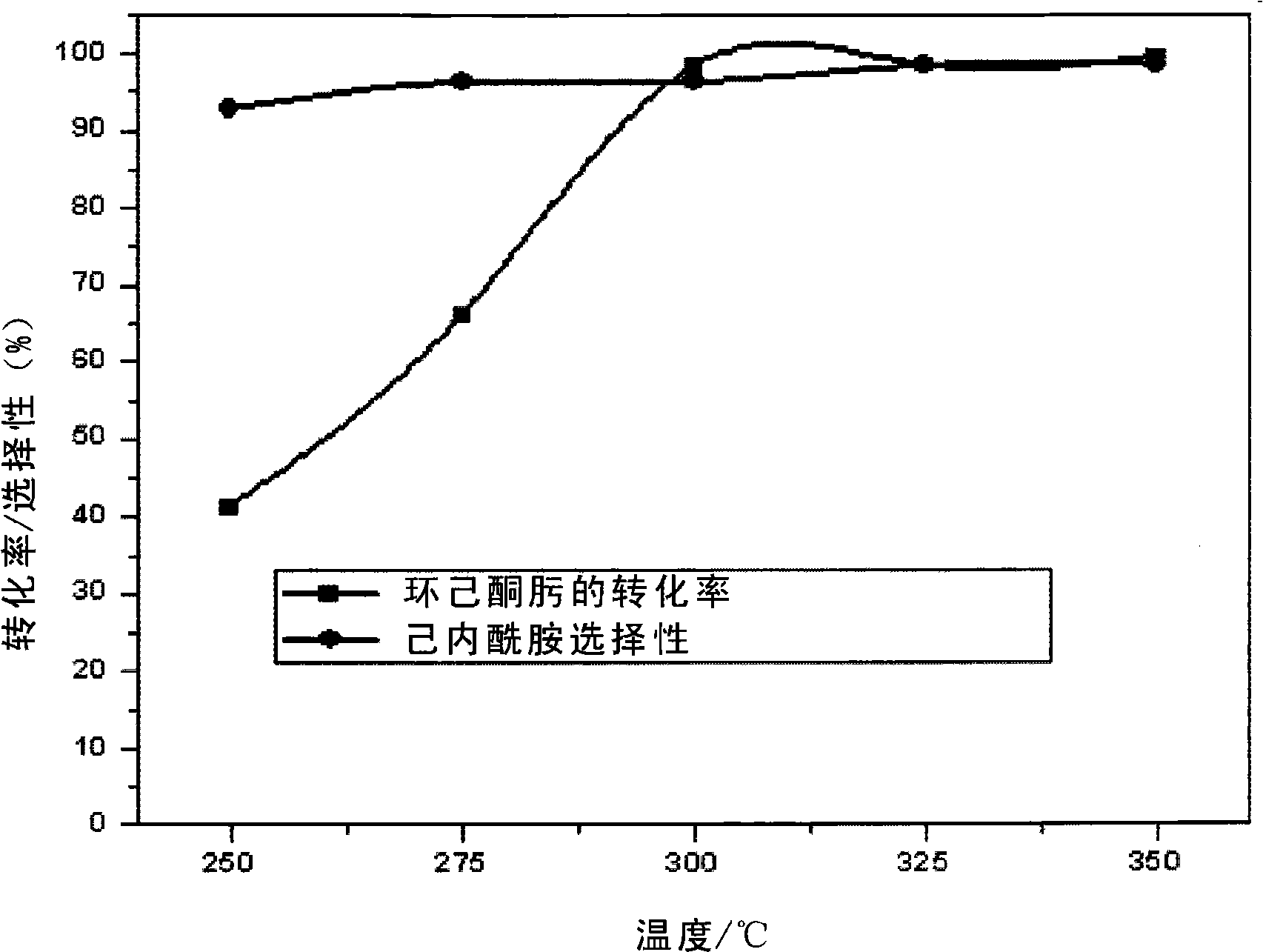
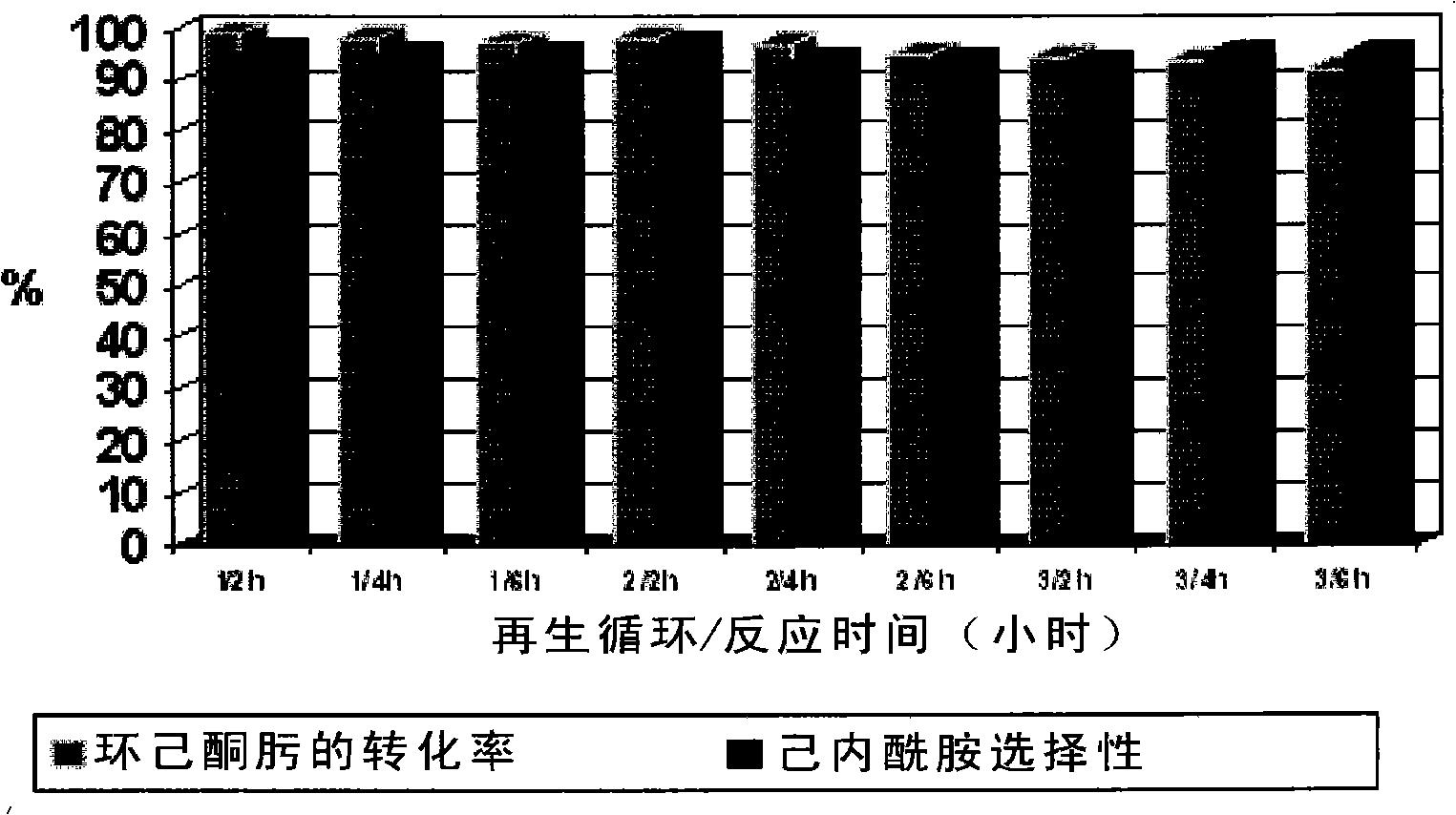
Method for preparing caprolactam by phase rearrangement of cyclohexanone oxime liquid
InactiveCN101250148AEasy to separateHigh selectivityLactams preparationBulk chemical productionBeckmann rearrangementSolid acid
A method for rearranging the liquid phase of cyclohexanone oxime to prepare caprolactam comprises dissolving cyclohexanone oxime indehydrated organic solvent, using dried solid acid as catalyst, in a liquid-solid reaction system, catalyzing cyclohexanone oxime to generate Beckmann rearrangement to prepare caprolactam. The invention has simple operation, normal pressure process, mild reaction conditions, easily separated reaction product, catalyst and solvent, completed converted cyclohexanone oxime and high selectivity of caprolactam.
Owner:XIANGTAN UNIV
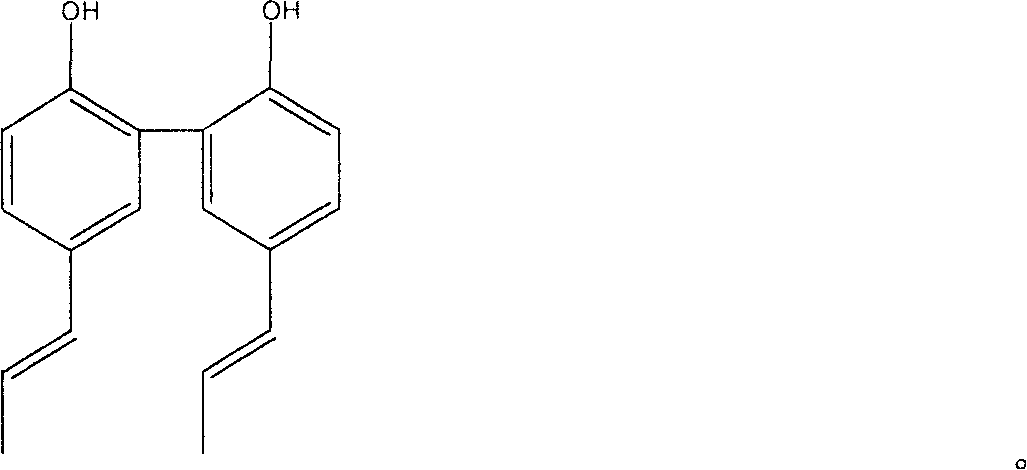
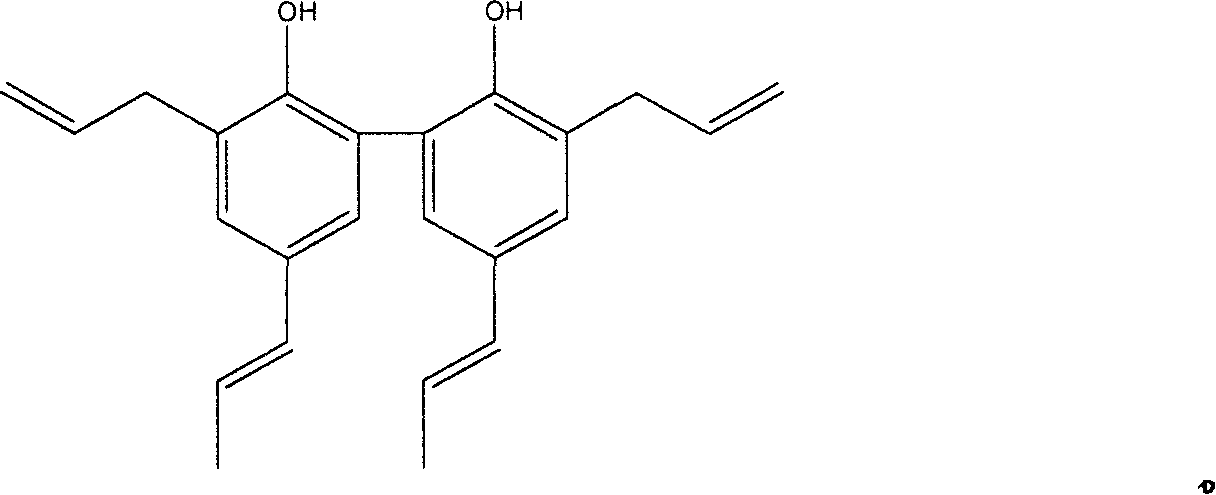
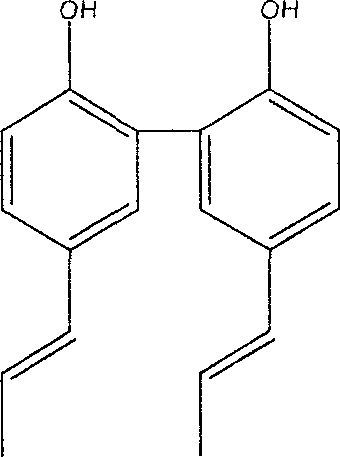
Preparation method of magnolia bark phenol derivative
InactiveCN1948249AImproves antioxidant activityGood symmetryOrganic chemistryHydroxy compound active ingredientsHonokiolIsomerization
The present invention relates to two magnolol derivatives and their preparation methods. In the concrete, one is compound 5,5'-dipropenyl diphenyl diphenol and its preparation method, said preparation method is characterized by making magnolol undergo the process of isomerization reaction to obtain said compound; and another derivative is compound 3,3'diallyl-5,5'-dipropenyl diphenyl diphenol and its preparation method, said method is characterized by including the following steps: making 5,5-dipropenyl diphenyl diphenol and allyl bromide undergo the process of Williamson reaction to synthesize allyl ether, then making Claisen rearrangement so as to obtain said derivative.
Owner:SHANGHAI UNIV
High-tenacity flame-retardant bismaleimide resin and preparation method thereof
InactiveCN104530430ALow viscosityImproved molding process typeGroup 5/15 element organic compoundsDiphenylmethaneClaisen rearrangement
The invention relates to a high-tenacity flame-retardant bismaleimide resin and a preparation method of the high-tenacity flame-retardant bismaleimide resin. The resin is composed of 100 parts of diphenylmethane bismaleimide, 1-100 parts of diallyl bisphenol A, 1-100 parts of bisphenol A diallyl ether, 1-50 parts of O,O-bis(4-(allyloxy) phenyl) phenyl phosphonic acid). Both the bisphenol A diallyl ether and the O,O-bis(4-(allyloxy) phenyl) phenyl phosphonic acid) undergo claisen rearrangement at a high temperature to be transformed into derivatives of diallyl and then participate in the curing reaction of the resin, and therefore the bisphenol A diallyl ether can effectively decrease the viscosity of a prepolymer at a low temperature and improve the forming process of the resin; phosphorus in the O,O-bis(4-(allyloxy) phenyl) phenyl phosphonic acid) can restrain combustion of the resin and make the resin generate a small amount of smoke without poisonous or corrosive gas in combustion, meets environmental requirements and has good compatibility with the resin.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
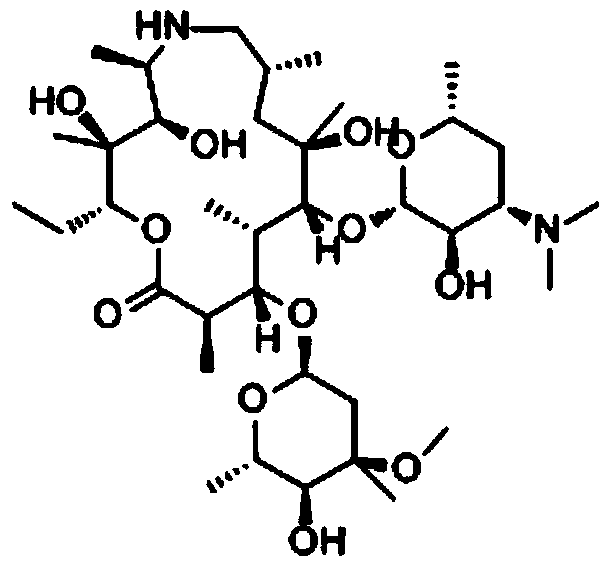
Preparation method of azithromycin amine
ActiveCN103772456AEmission reductionReduce manufacturing costSugar derivativesSugar derivatives preparationClaisen rearrangementSolvent
The invention provides a preparation method of azithromycin amine. The preparation method is characterized by adopting a one-pot reaction method. The preparation method comprises the steps of carrying out Beckman rearrangement for erythrocin A9- oxime, water and rearrangement reagent, adjusting the pH value to be 3.0 to 7.0 after the reaction is ended, adding reducing agent to reduce the reactants, and obtaining the azithromycin amine through acid hydrolysis and crystallization. The one-pot reaction of the Beckman rearrangement and reduction is realized by adopting water as the solvent, so that the time for producing erythrocin A9- oxime or erythrocin A9-oxime thiocyanate and the azithromycin amine can be greatly shortened, the procedures can be reduced, the emission of waste gas, waste water and industrial residue can be reduced, the yield is improved by 1 to 3 percent compared with the original process, and the production cost of the azithromycin is further reduced. The midbody erythrocin A6, 9 imino ether is not extracted, and the reaction condition is moderate; the water is used as the reaction solvent, less waste (waste water, waste gas and industrial residue) is produced, and the environmental friendliness can be realized.
Owner:ZHEJIANG NEXCHEM PHARMA
Production of lactams and carboxylic acid amides by beckman rearrangement of oximes in the presence of nb catalysts
The present invention relates to processes for the production of lactams such as e-Caprolactam, w-Laurolactam, or of carboxylic acid amides such as acetaminophenol and benzanilide by Beckman rearrangement from the corresponding oximes in the presence of Nb-impregnated catalysts, such as Nb on SiO2, preferably in the gaseous phase but also in the liquid phase. The reactions in the gaseous phase can be performed in various reactors, such as fixed bed reactors, plate reactors, fluidized bed reactors, fluidized bed reactors having continuous regeneration in a second fluidized bed at temperatures between 200 DEG C and 500 DEG C and a pressure of 0.01 bar to 10 bar. In the liquid phase, the reactions can take place in different reactors such as autoclaves, stirred reactors, loop reactors, and trickle bed reactors at temperatures from 20 DEG C to 200 DEG C and a pressure between 0.5 and 20 bar. The invention further relates to the method of regeneration of said Nb-containing catalysts in oxidizing and non-oxidizing media at 200 DEG C to 600 DEG C.
Owner:DSM IP ASSETS BV
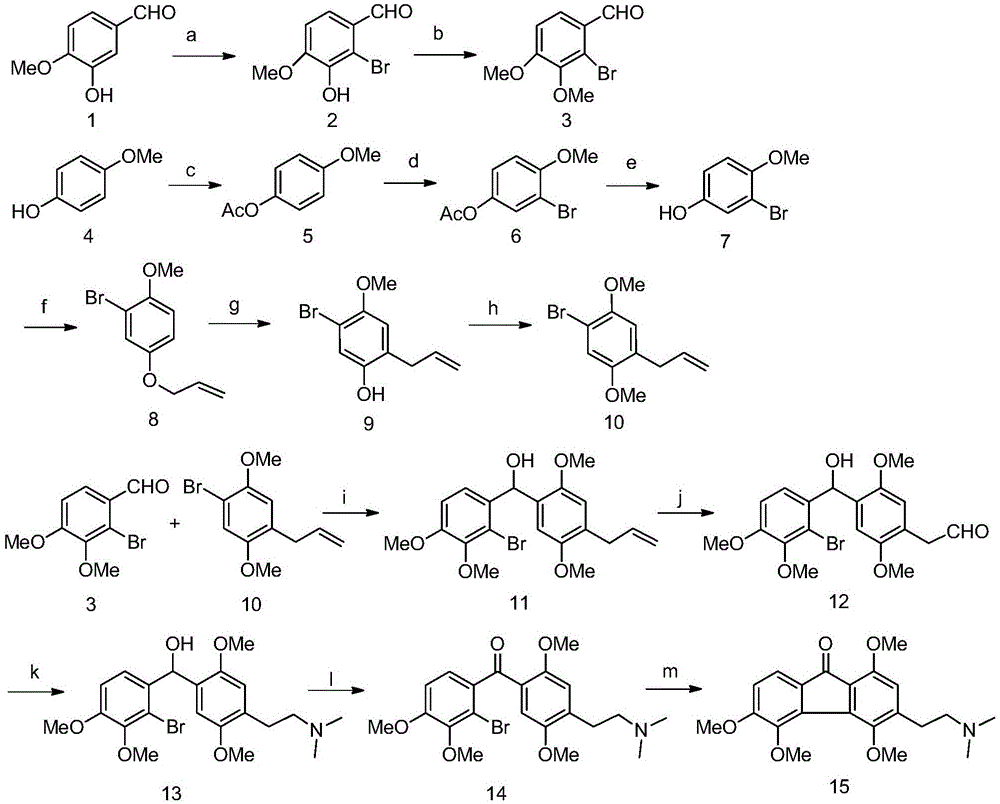
Synthetic method for methyl caulophine
ActiveCN105001107AImprove stabilityMild reaction conditionsOrganic chemistryOrganic compound preparationHydroxybenzoate EthersBenzaldehyde
The invention provides a synthetic method for methyl caulophine. The method comprises the following steps: with isovanillin and p-hydroxyanisole as raw materials, successively subjecting isovanillin to bromination and methylation so as to obtain 2-bromo-3,4- dimethoxy benzaldehyde; successively subjecting p-hydroxyanisole to acetylation, bromination, hydrolyzation, allyl etherification, Claisen rearrangement and methylation so as to obtain 1-allyl-4-bromo-2,5-dimethoxybenzene; successively subjecting the obtained 1-allyl-4-bromo-2,5-dimethoxybenzene and 2-bromo-3,4- dimethoxy benzaldehyde to Grignard reaction, ruthenium chloride / sodium periodate double-bond oxidation, reductive amination, pyridine chlorodichromate oxidation and intramolecular coupling so as to obtain 3-(2-(N,N-dimethylamino)methyl)-1,4,5,6-tetramethoxyl-9-H-fluorene-9-ketone, i.e. the method for synthesizing methyl caulophine is completed. The method in the invention has the advantages of mild reaction conditions, simple operation, easily-available raw materials and reagents, etc., and is suitable for large-scale production of pharmaceutical enterprises
Owner:XI AN JIAOTONG UNIV
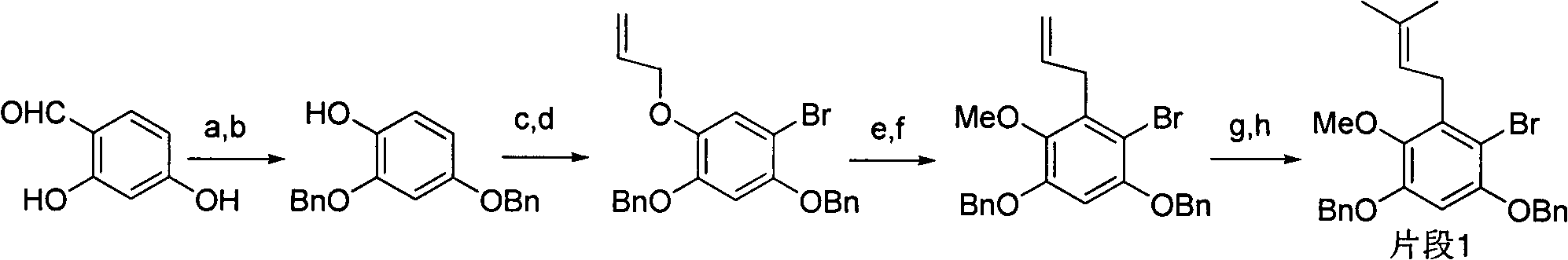
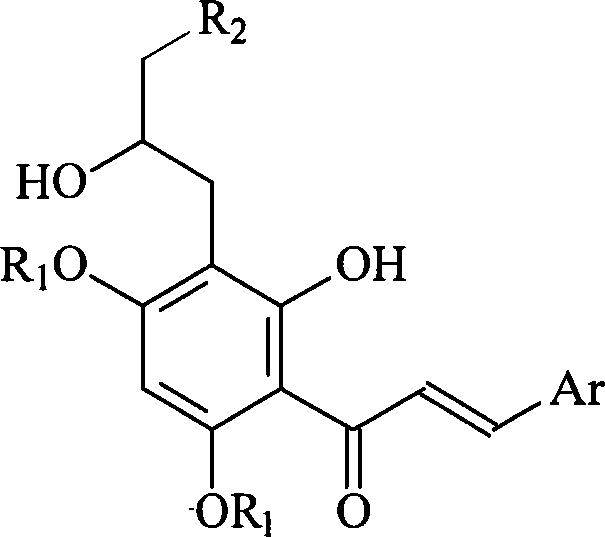
Full synthesis method of 4'',5''-dihydroxyl-5-methoxyl-[6'',6''-dimethyl pyran (2'',3'':7,8)] Hirtellanine A
InactiveCN101792451AAvoid separationReduce consumptionOrganic chemistryIsomerizationSynthesis methods
The invention relates to a chemical full synthesis method of Hirtellanine A 4'',5''-dihydroxyl-5-methoxyl-[6'',6''-dimethyl pyran (2'',3'':7,8)]. the Hirtellanine A with high yield is synthesized from phloroglucinol acetophenone by the following eight steps of selective Claisen rearrangement and cyclization reaction by zones, potful boron esterification and Suzuki coupling, serial cyclization isomerization reaction induced by acid and the like . The invention has the characteristics of easily obtained raw material, simple operation and high yield, provides an effectively approach for greatly synthesizing the Hirtellanine A and is suitable for scale type industrial production.
Owner:BASILEA PHARM CHINA LTD
Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone
The invention provides a method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone. The method comprises the following steps: a, performing the Claisen rearrangement reaction of a compound III in an appropriate solvent to generate a compound IV; b. supplying ozone to the compound IV at a low temperature to perform reaction, adding sodium borohydride or potassium borohydride to perform reduction reaction and obtaining a compound V; c, allowing the compound V to react with methanesulfonyl chloride or p-toluenesulfonyl chloride in pyridine so as to obtain a compound VI and a compound VII; and d, dissolving the compound VI and / or the compound VII in an appropriate reaction solvent, adding appropriate organic base, raising temperature to perform reaction, cooling the obtained product, adding diluted hydrochloric acid for washing, evaporating the solvent and obtaining a compound I, namely the 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone. The method has the advantages ofbrief synthesis route, simple operation, low cost, high yield and convenience for industrial production.
Owner:SHANGHAI PUYI CHEM CO LTD
Epoxy compound having alkoxysilyl group, method of preparing the same, composition and cured product comprising the same, and uses thereof
ActiveUS9150686B2Improve flame retardant performanceImprove bindingGroup 4/14 element organic compoundsSynthetic resin layered productsEpoxyHeat resistance
Owner:KOREA INST OF IND TECH
Preparation method for 5-fluorin-2-hydroxyacetophenone
InactiveCN102557909AOrganic compound preparationCarbonyl compound preparationFries rearrangementClaisen rearrangement
The invention provides a preparation method for 5-fluorin-2-hydroxyacetophenone. The preparation method comprises the following steps of: performing double esterification on amino groups and phenolic hydroxy in one step by taking amino-phenol as a raw material; performing Fries rearrangement under the condition of aluminum chloride / sodium chloride; heating a hydrolyzate after performing fluorine diazotization to obtain finished 5-fluorin-2-hydroxyacetophenone. The preparation method has the advantages of low prices of raw materials, relatively mild reaction conditions, total yield of up to 54.5 percent and industrial production application value.
Owner:SHANGHAI SINOFLUORO SCI
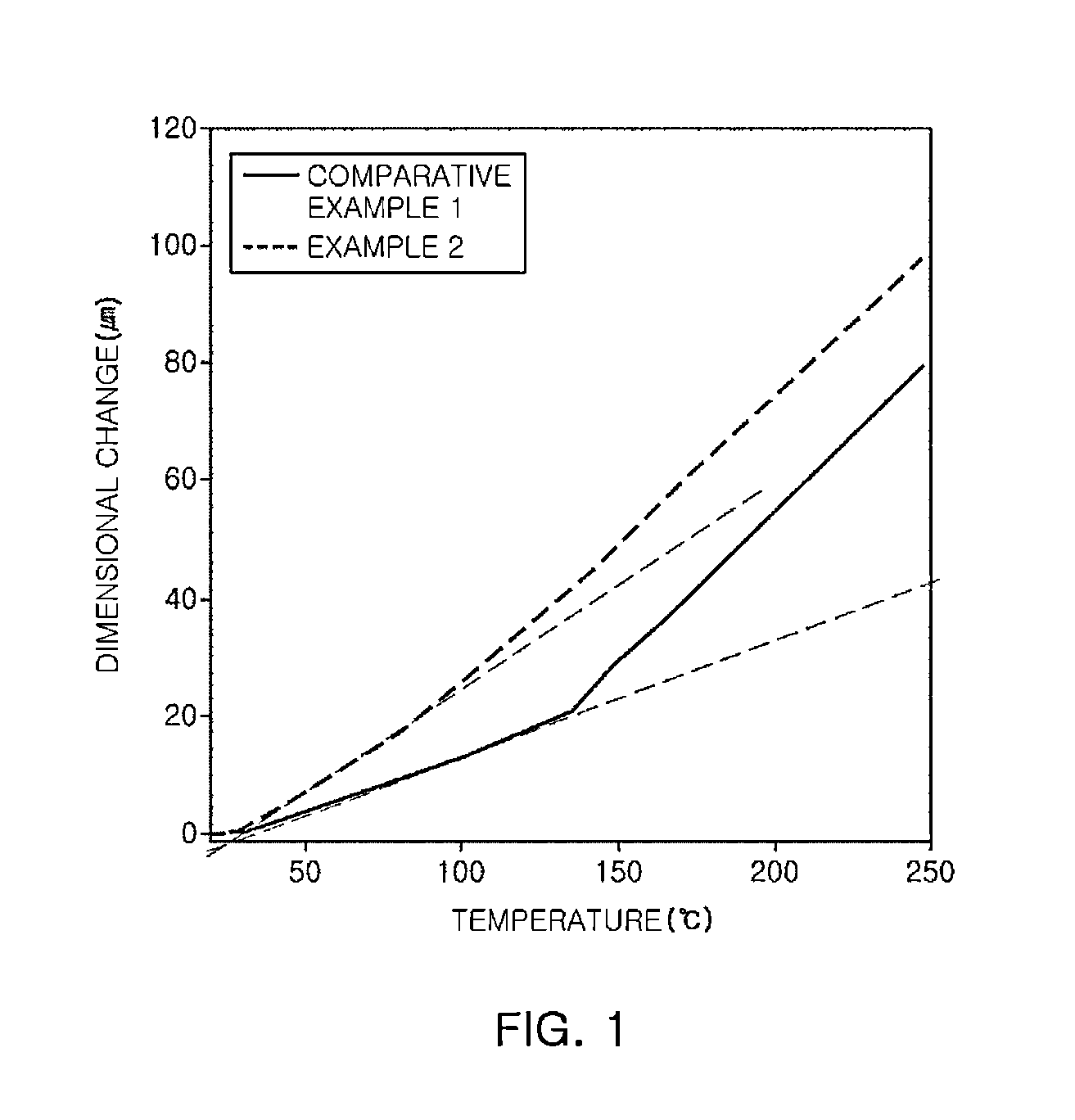
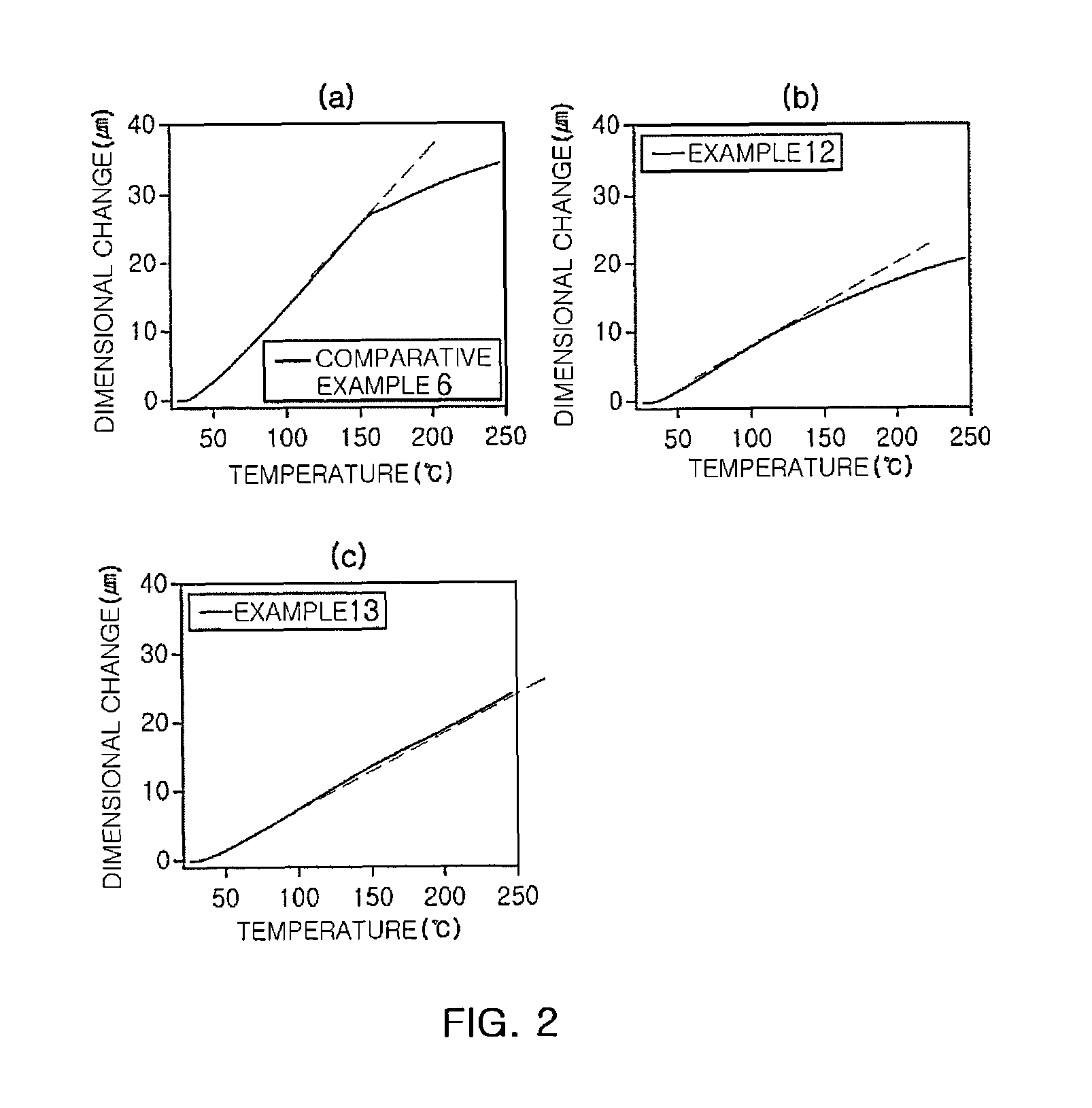
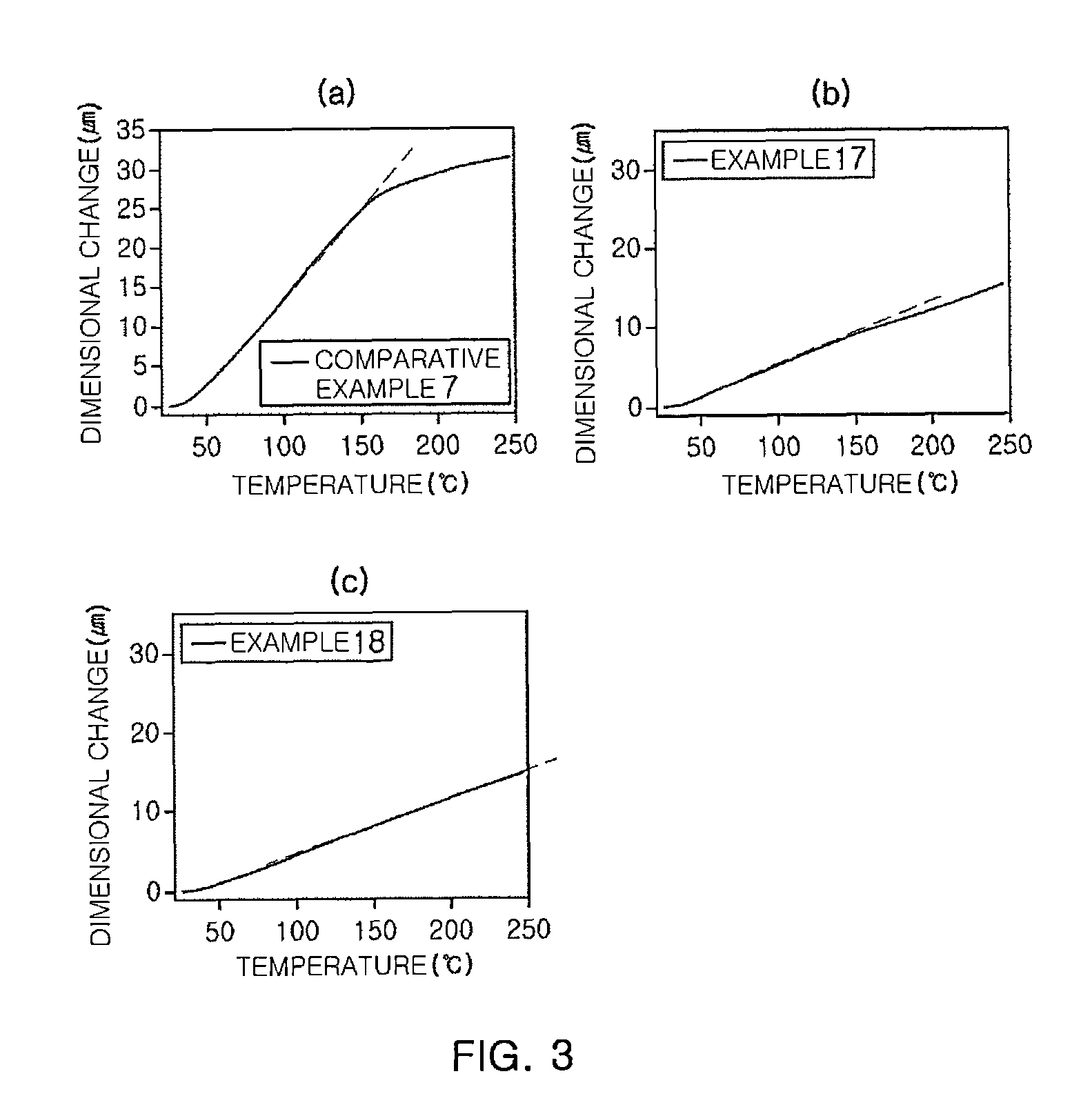
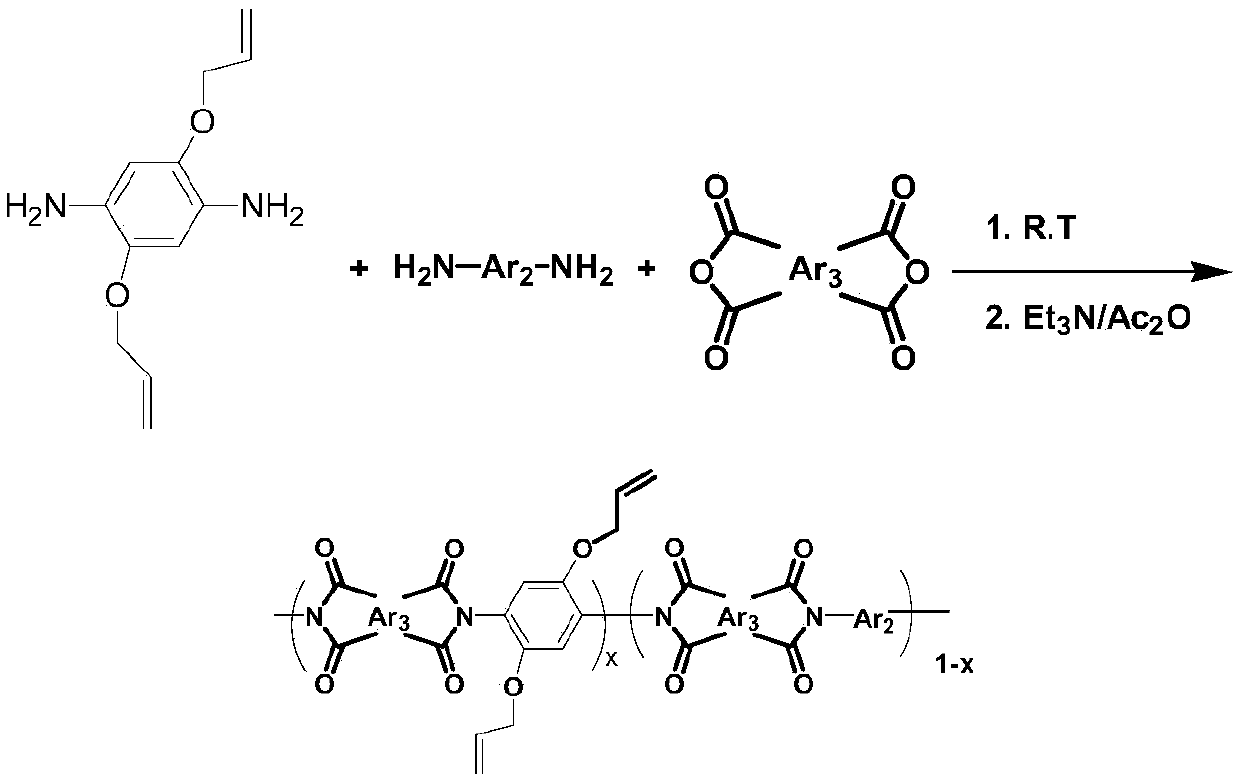
Novel polyimide high polymer material based on 2,5-diallyloxy p-phenylenediamine monomer and preparation method of novel polyimide high polymer material
ActiveCN110591092AImprove thermal stabilityImprove mechanical propertiesClaisen rearrangementAcid anhydride
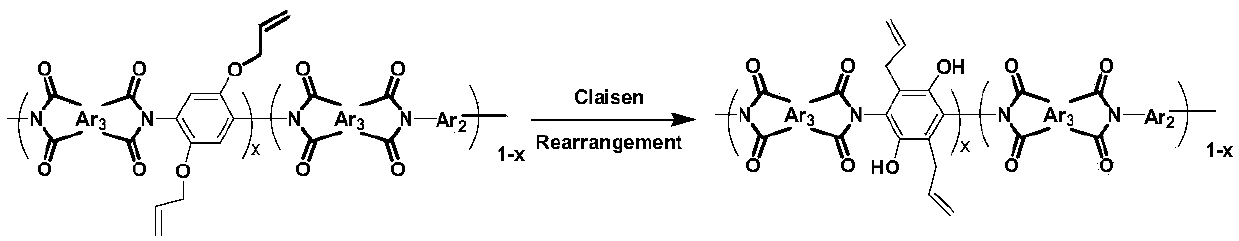
The invention discloses a preparation method and application of allyloxy-containing aromatic polyimide. The allyloxy-containing high polymer compound has a repeated structure unit of a formula I shownin the description. According to the preparation method, a diallyloxy diamine monomer is selected as a basic monomer, the basic monomer is copolycondensed with binary aromatic primary amine and quaternary anhydride according to a ratio, and thus a series of novel aromatic polyimide of different contents of allyloxy is prepared. Hydroxyl and allyl can be obtained from a prepared allyloxy-modifiedpolyimide film through claisen rearrangement. Due to the hydroxyl, the separation selectivity of the film upon gases can be improved, and the allyl can be used as a crosslinking reaction site to improve the plastification resistance of the film. Due to the novel molecular structure designing, a new generation of gas separation films with both separation and plastification resistance can be prepared.
Owner:PEKING UNIV
Positron emitted computerized tomography imaging agent benzamide derivative synthesizing method
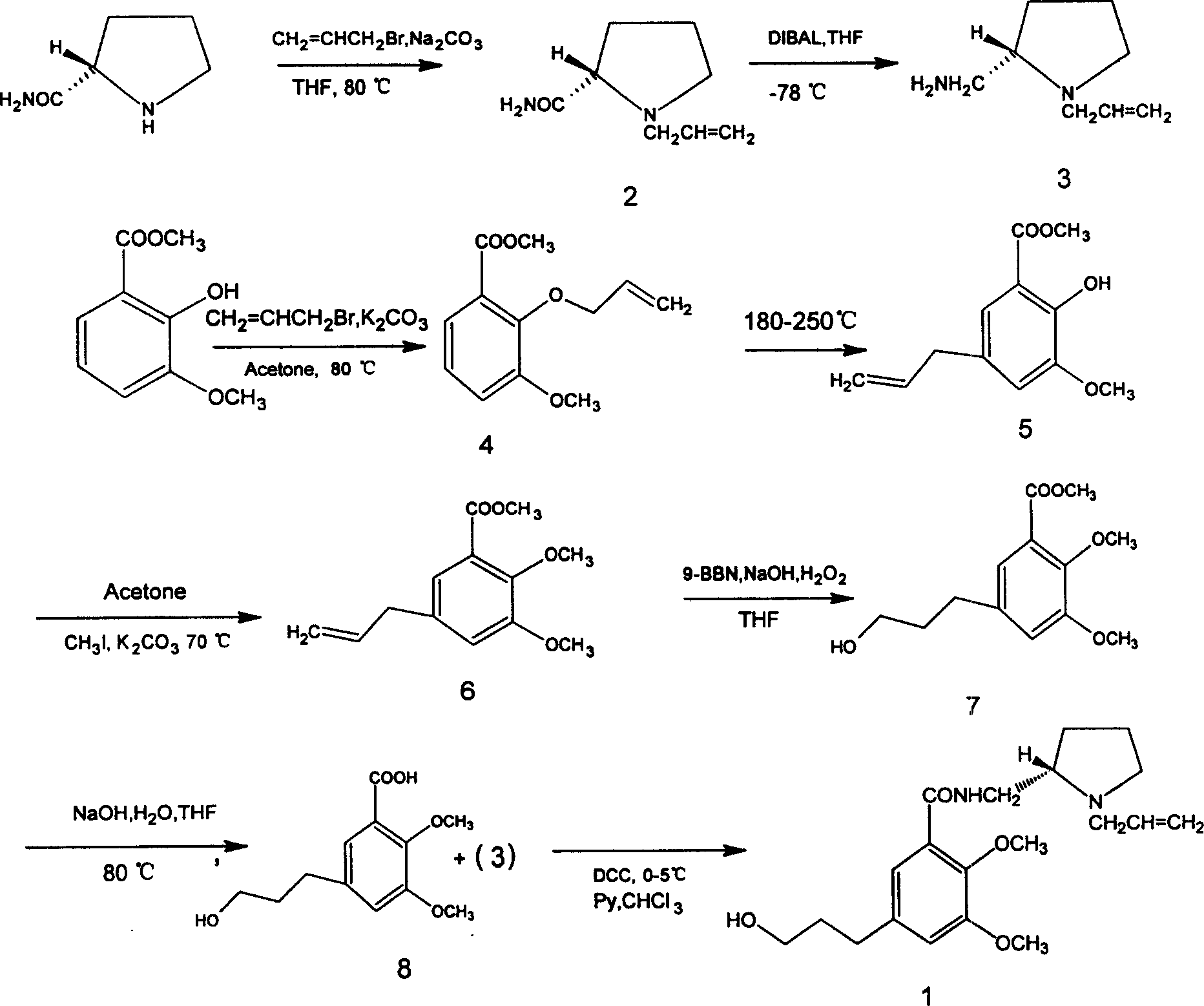
The invention is a method to synthesize (S)-(-)-N-[(1-allyl-2-pyrrolidyl)methyl]-2,3-dimethoxy-5 -(3-hydroxy propyl)benzoic amide, relating to a technique to prepare PET developer. It adopts (S)-(-)-2-formamidopyrrol through allylation and electronation reaction to make (S)-(-)-2- methylamino-N-(allyl)pyrrol alkyl (3); adopts 2-hydroxy-3-methoxy methyl benzoate through allylation, Claisen rearrangement, hydroboration and hydrolyzation reaction to make 2,3-dimethoxy-5-(3-hydroxy propyl)benzoic acid (8); (3) and (8) react to make the aim compound. It improves the synthesis method reported in document, replacing expensive allyl iodide with allyl bromide to make the compound (3). It improves the purifying condition of intermediate.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Synthesis method of isolicoflavonol
PendingCN112047916AEasy to separateEasy to purifyOrganic chemistryBulk chemical productionPtru catalystBenzaldehyde
The invention provides a synthesis method of isolicoflavonol, which comprises the following steps: carrying out condensation reaction on 2,4-O-R1(protective group, the same below)-6-hydroxyacetophenone and 4-O-R2(protective group, the same below)-benzaldehyde to generate 2',4'-O-R1-6'-hydroxy-4-O-R2-chalcone; oxidizing the chalcone to generate flavonol; carrying out selective protection on 3-OH ofthe flavonol to obtain 3,5,7-O-R1-4'-O-R2-flavonol; removing the protecting group R2 from the 3,5,7-O-R1-4'-O-R2-flavonol to obtain 3,5,7-O-R1-4'-hydroxyflavonol; carrying out 1,1-dimethylpropargyl reaction on the 4,4'-OH site to obtain 3,5,7-O-R1-4'-O-(1',1''-dimethyl propargyl)flavonol; carrying out partial hydrogenation on the alkynyl of the 3,5,7-O-R1-4'-O-(1',1''-dimethyl propargyl)flavonolunder the action of a catalyst to obtain 3,5,7-O-R1-4'-O-(1',1''-dimethylpropenyl)flavonol and carrying out Claisen rearrangement on the 3,5,7-O-R1-4'-O-(1',1''-dimethylpropenyl)flavonol to obtain 3,5,7-O-R1-isolicoflavonol, and removing the protecting group R1 from the 3,5,7-O-R1-isolicoflavonol to obtain the isolicoflavonol.
Owner:SUZHOU HIGHFINE BIOTECH
Novel synthesis method of mangostin
The invention belongs to the field of chemical synthesis and particularly relates to a novel synthesis method of mangostin as shown in the formula (I), wherein the mangostin as a natural effective component has favorable anti-tumor activity, cardiovascular activity, antioxidant activity, anti-inflammatory activity, antibacterial activity and other pharmacological activities. The novel synthesis method comprises the steps: with 1, 7-dihydroxyl-3, 6-dialkoxyl-9H-xanthenone as a raw material, sequentially carrying out nucleophilic substitution, Claisen rearrangement, alkylation, deprotection and the like to obtain alpha-mangostin, beta-mangostin, belt-mangostin-OMe and gamma-mangostin. The novel synthesis method is simple in step and suitable for industrial production.
Owner:CHINA PHARM UNIV
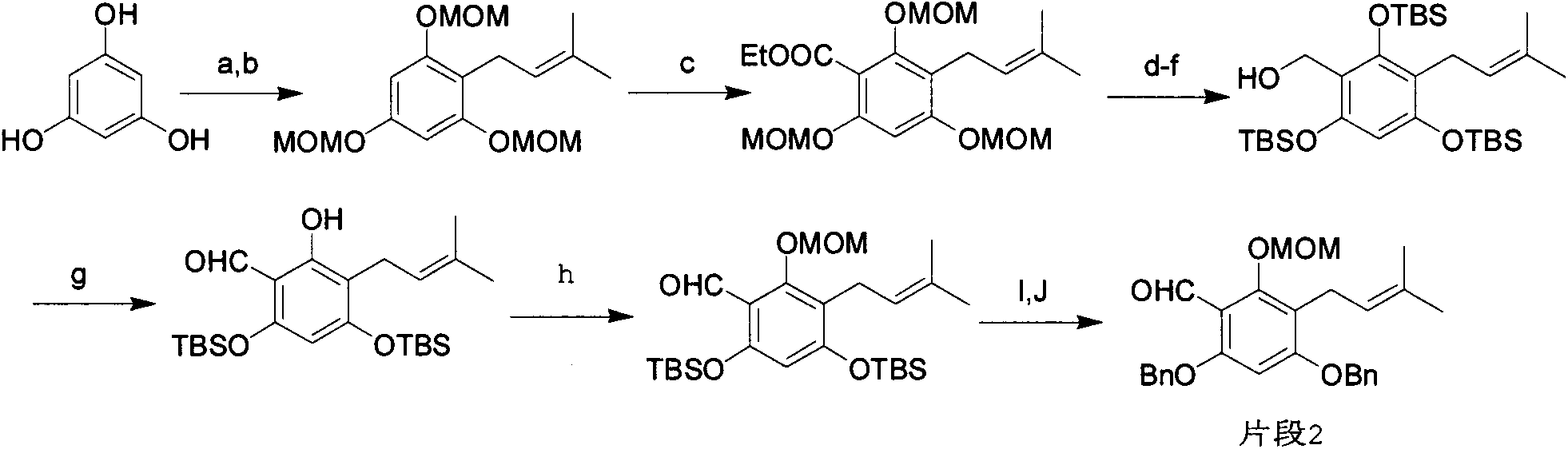
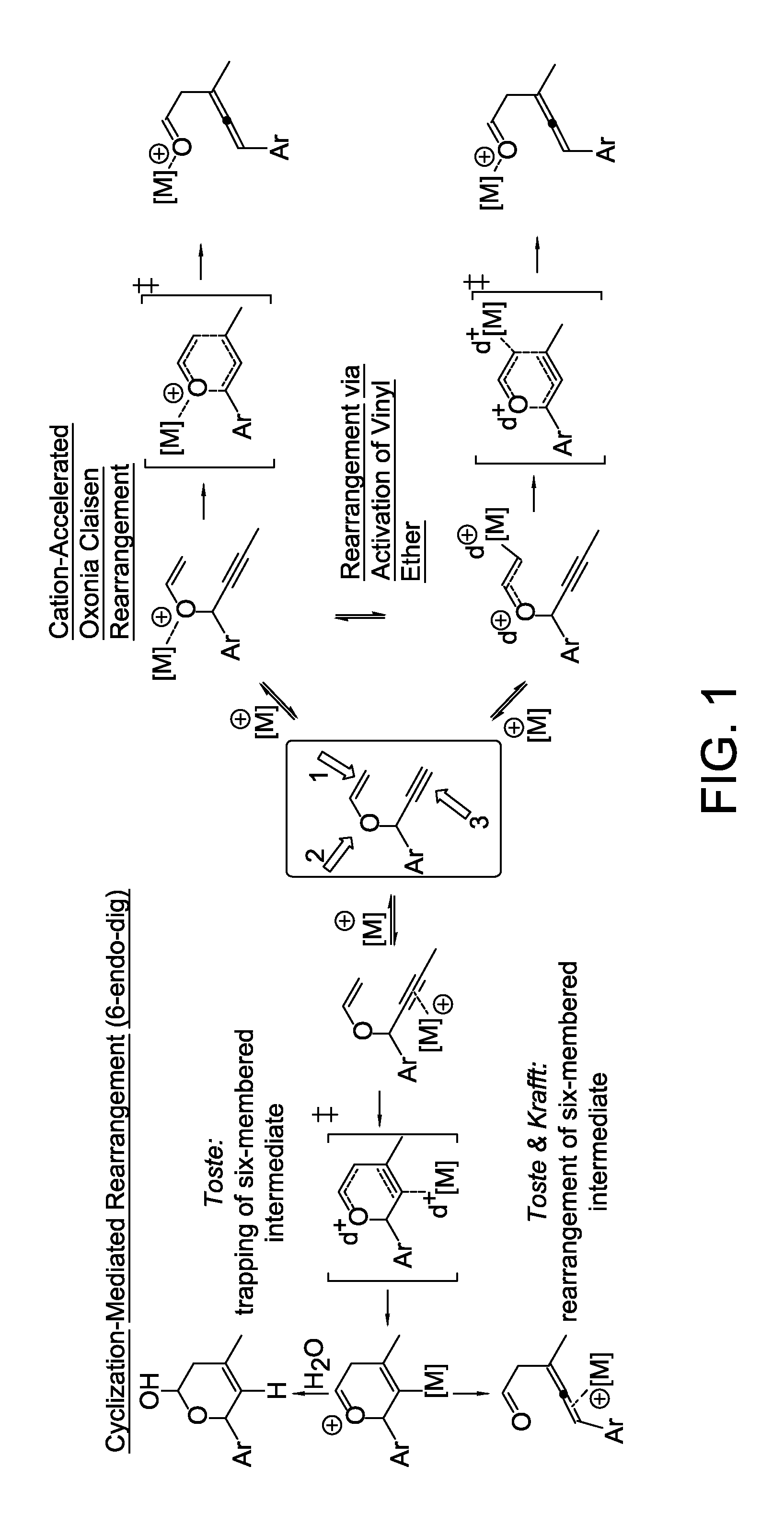
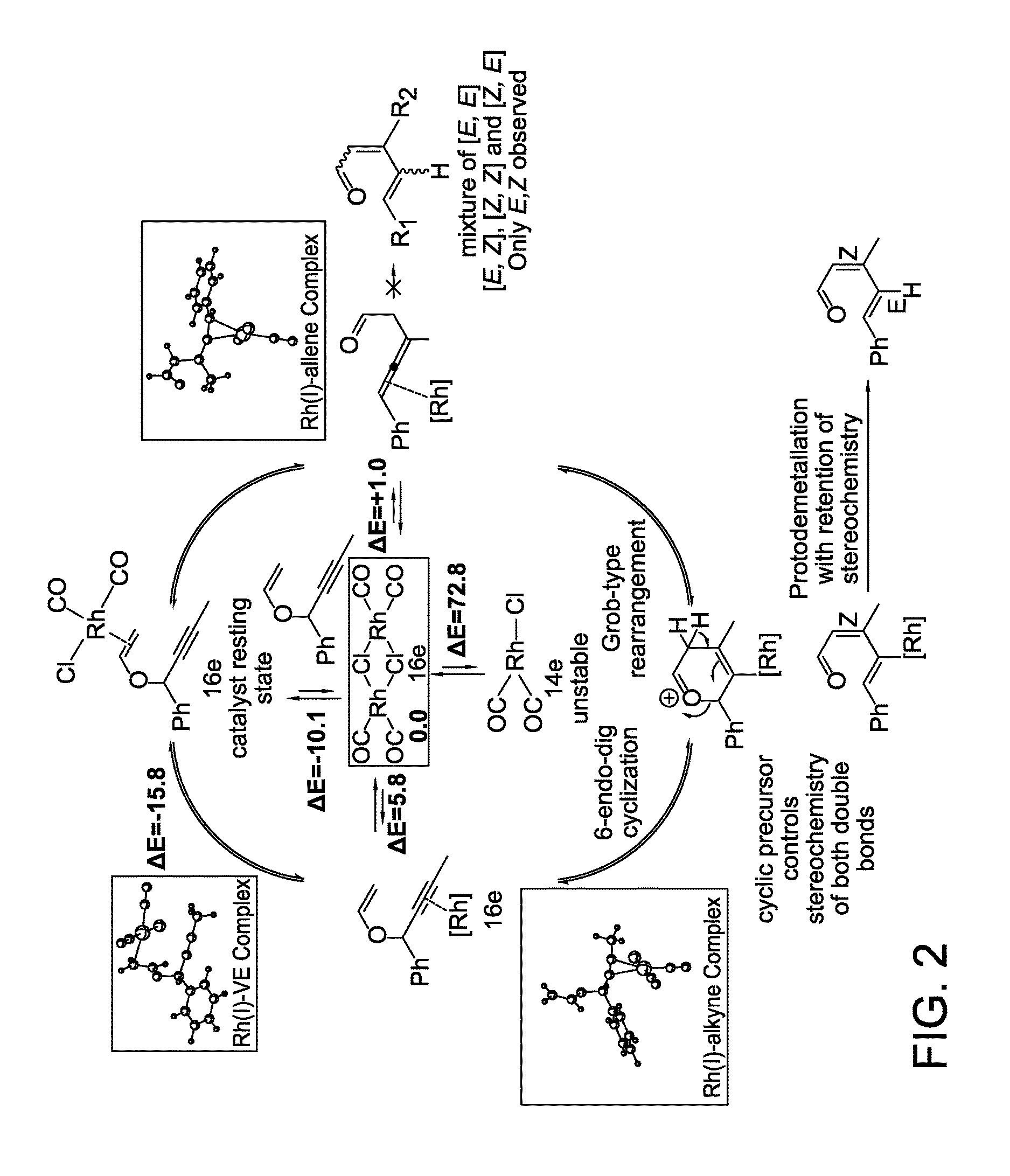
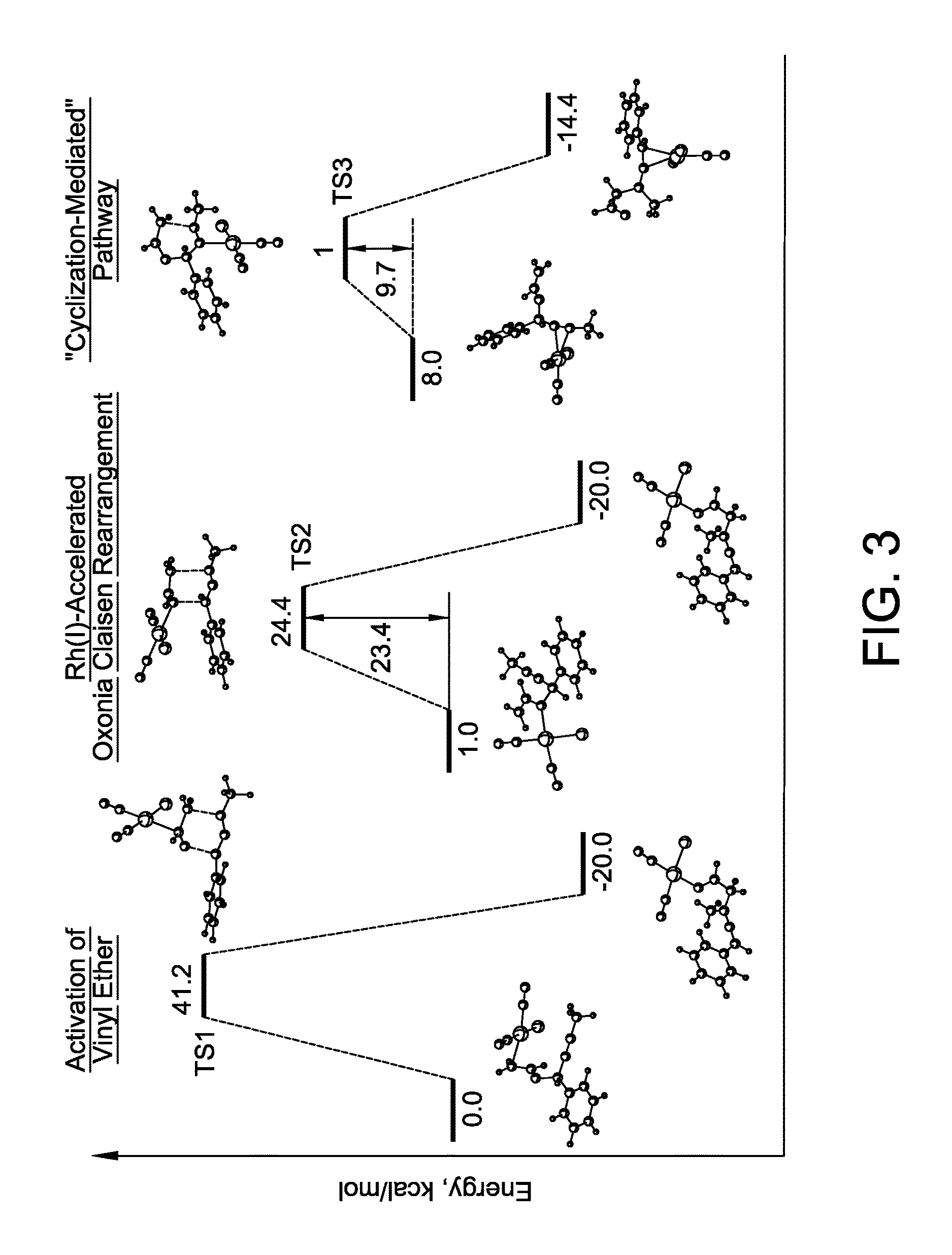
Stereo controlled synthesis of (E,Z)-dienals via tandem Rh(I) catalyzed propargyl claisen rearrangement
ActiveUS9573871B2High stereoselectivityCarboxylic acid nitrile preparationOrganic compound preparationDouble bondClaisen rearrangement
Owner:FLORIDA STATE UNIV RES FOUND INC
Synthesis method of Lupinus luteus wighteone
InactiveCN103936706ALow costEasy post-processingOrganic chemistryDimethylaniline N-oxideSynthesis methods
The invention discloses a synthesis method of Lupinus luteus wighteone. The method provided by the invention toakes genistein as a raw material to undergo four-step reaction so as to obtain Lupinus luteus wighteone. In the second-step reaction of the method, isoamylene bromide, potassium carbonate, and a DMF system are employed to replace isopentenyl alcohol, diethyl azodicarboxylate, triphenylphosphine and a THF system, the cost is greatly reduced, the after-treatment is much more convenient, and the yield is enhanced from 83% to 92%. In the third-step reaction, diethyl aniline, dimethylaniline, decahydronaphthalene and other high-boiling point solvents are adopted as the solvents, and under heating reflux conditions, para Claisen rearrangement of isopentenyl is completed to replace Eu(fod)3 catalyzed rearrangement adopted by original methods, thus saving the precious metal catalyst, greatly reducing the cost, and increasing the yield from original 68% to 87%.
Owner:CHANGZHOU UNIV
Method for catalyzing Claisen rearrangement at lower temperature
PendingCN114105744AWays to Avoid DistillationReduce the rearrangement reaction temperatureOrganic compound preparationCarbonyl compound preparationPtru catalystEther
The invention provides a method for catalyzing Claisen rearrangement at a relatively low temperature, and relates to the field of catalysis, and the method uses a catalyst DAPs to catalyze allyl ether of enol to obtain a Claisen rearrangement product through rearrangement. According to the reaction, the production energy consumption can be reduced, the operation can be simplified, and meanwhile the requirement for equipment is low.
Owner:SHANXI WEIQIDA PHARMA IND +1
Synthetic method for 4-(1, 5-dimethyl-1-vinyl-4-hexenyl) phenol
InactiveCN1683297AFew reaction stepsEasy to operateOrganic chemistryOrganic compound preparationMethylvinyl ketoneCyclohexene
The present invention relates to synthesis process of 4-(1, 5-dimethyl-1-vinyl-4-hexenyl) phenol, normally named as antifeedant, and the synthesis process is superior to available ones. The synthesis process has geraniol as main material and includes the reaction with vinyl ethyl ether, Claisen rearrangement reaction to obtain 3-vinyl-rhodinal, reaction with hexahydropyridine, enamiation, conjugate addition with methyl vinyl ketone and cyclization and condensation to 4-(1, 5-dimethyl-1-vinyl-4-hexenyl)-2-cyclohexene ketone, and dehydrogenation to obtain the product 4-(1, 5-dimethyl-1-vinyl-4-hexenyl) phenol. The present invention uses natural product as main material, and has simple reaction process, high selectivity and high yield.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Preparation method and application of catalyst
InactiveCN106563509AImprove catalytic performanceEasy to useCatalyst carriersOrganic compound preparationEthylenediamineSalen ligand
The invention discloses a preparation method and application of a catalyst. The preparation method of the catalyst is characterized by comprising the following steps that (1) in a polar aprotic solvent solution, in the presence of alkali, salicylaldehyde and allyl haloalkane are made to perform a nucleophilic substitution reaction, so that a nucleophilic substitution product is obtained; secondly, the product generated in the step (1) is heated to perform a Claisen rearrangement reaction; (3) the product obtained in the step (2) and ethylenediamine are condensed in an organic solvent, so that a Salen ligand is obtained; (4), in an ether solvent, the Salen ligand and divinyl benzene are copolymerized in the presence of a radical initiator and an auxiliary initiator, so that a Salen organic polymer of a mesoporous structure is obtained; and (5) in a mixed solvent of alcohol and water, the Salen organic polymer is used as a carrier, and mesoporous Salen organic polymer Co catalyst is obtained through preparation. The catalyst prepared through the preparation method is applied to catalytic oxidation of benzoin and has good catalytic activity, and meanwhile, even the catalyst is repeatedly used, the catalytic activity of the catalyst cannot be lowered.
Owner:SHAOXING UNIVERSITY
Synthetic method of 7-(4-ethyl-1-methyl octyl)-8-hydroxyquinoline
InactiveCN102584697AHigh yieldHigh ortho selectivityOrganic chemistryEthyl groupReaction temperature
The invention discloses a synthetic method of 7-(4-ethyl-1-methyl octyl)-8-hydroxyquinoline. The method is characterized by comprising the following steps of: undergoing a Williamson synthetic reaction on 8-hydroxyquinoline and alkenyl halide in a solvent under the catalytic action of an alkali to generate 8-[4-(5-ethyl-2-nonylene)]oxyquinoline, performing Claisen rearrangement on ether allyl phenyl at a reaction temperature, and performing intramolecular rearrangement on ether allyl phenyl to generate 7-(4-ethyl-1-methyl octenyl)-8-hydroxyquinoline, wherein the alkenyl halide is selected from 3-chloro-5-ethyl-2-nonylene, 3-bromo-5-ethyl-2-nonylene and 3-iodine-5-ethyl-2-nonylene; and hydrogenating the 7-(4-ethyl-1-methyl octenyl)-8-hydroxyquinoline under the catalytic action of palladium / carbon to obtain a final product, i.e., 7-(4-ethyl-1-methyl octyl)-8-hydroxyquinoline. The method has the advantages of high product yield, high quality and suitability for industrial production.
Owner:HUAIHAI INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
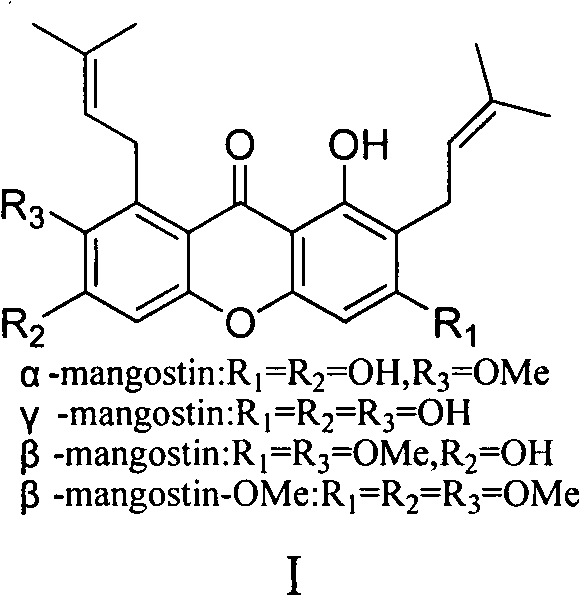
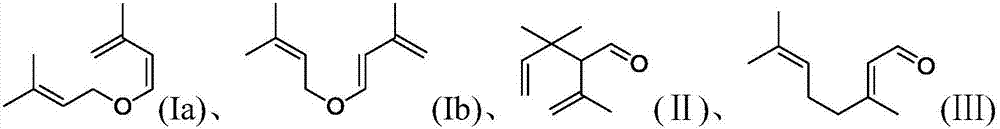
![Full synthesis method of 4'',5''-dihydroxyl-5-methoxyl-[6'',6''-dimethyl pyran (2'',3'':7,8)] Hirtellanine A Full synthesis method of 4'',5''-dihydroxyl-5-methoxyl-[6'',6''-dimethyl pyran (2'',3'':7,8)] Hirtellanine A](https://images-eureka.patsnap.com/patent_img/95af5862-0fec-43e5-b5c7-10d7e34531fc/FSA00000071461000011.PNG)
![Full synthesis method of 4'',5''-dihydroxyl-5-methoxyl-[6'',6''-dimethyl pyran (2'',3'':7,8)] Hirtellanine A Full synthesis method of 4'',5''-dihydroxyl-5-methoxyl-[6'',6''-dimethyl pyran (2'',3'':7,8)] Hirtellanine A](https://images-eureka.patsnap.com/patent_img/95af5862-0fec-43e5-b5c7-10d7e34531fc/GSA00000071461100011.PNG)
![Full synthesis method of 4'',5''-dihydroxyl-5-methoxyl-[6'',6''-dimethyl pyran (2'',3'':7,8)] Hirtellanine A Full synthesis method of 4'',5''-dihydroxyl-5-methoxyl-[6'',6''-dimethyl pyran (2'',3'':7,8)] Hirtellanine A](https://images-eureka.patsnap.com/patent_img/95af5862-0fec-43e5-b5c7-10d7e34531fc/GSA00000071461100031.PNG)
![Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone](https://images-eureka.patsnap.com/patent_img/c0d08b2d-7c0b-48ef-8cfe-d6557b7ab8e7/A2008100427290002C1.PNG)
![Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone](https://images-eureka.patsnap.com/patent_img/c0d08b2d-7c0b-48ef-8cfe-d6557b7ab8e7/A20081004272900041.PNG)
![Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone Method for synthesizing 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone](https://images-eureka.patsnap.com/patent_img/c0d08b2d-7c0b-48ef-8cfe-d6557b7ab8e7/A20081004272900051.PNG)