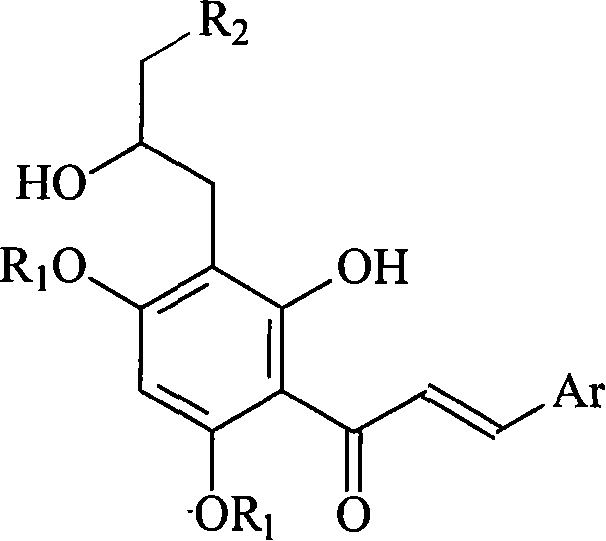
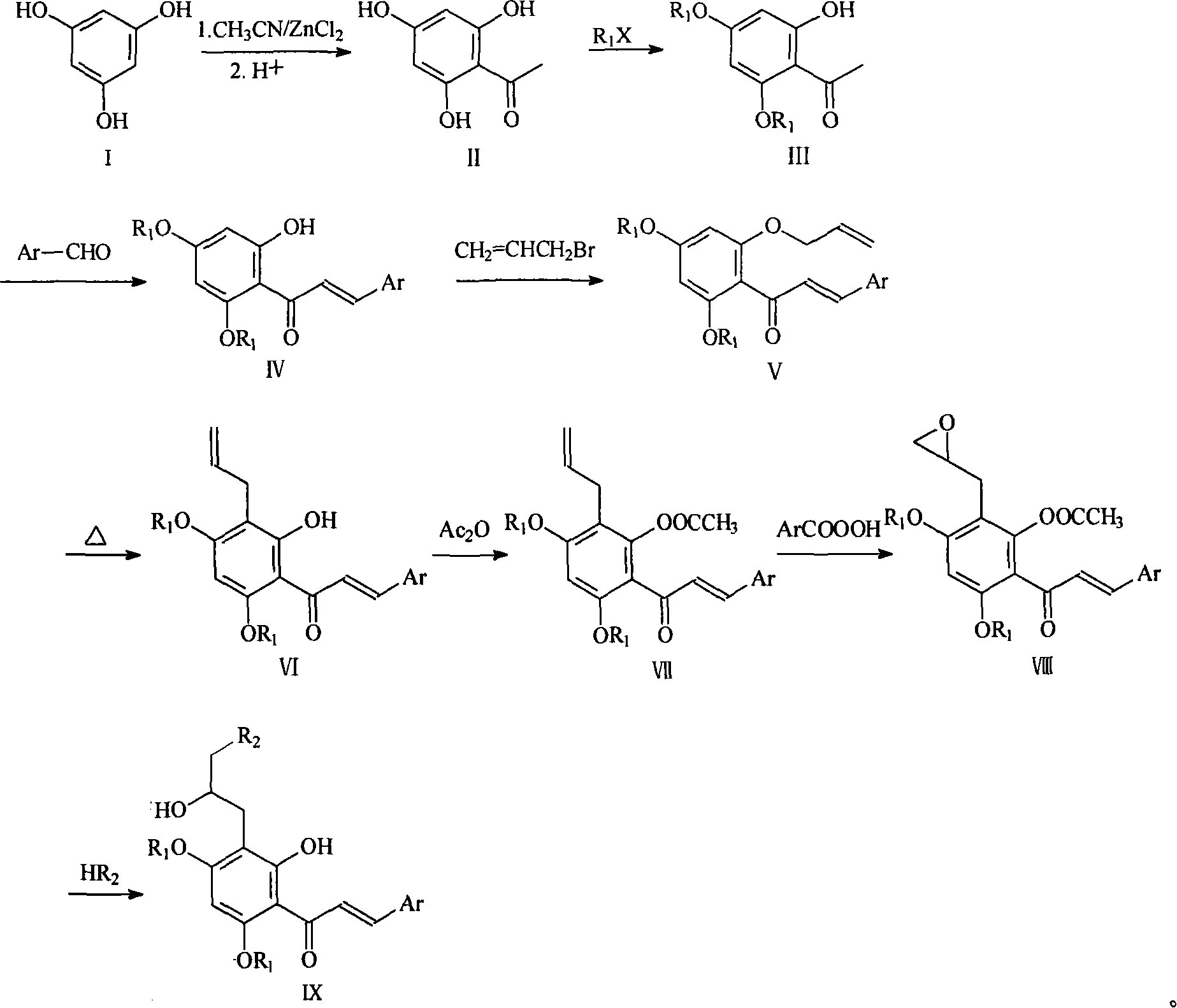
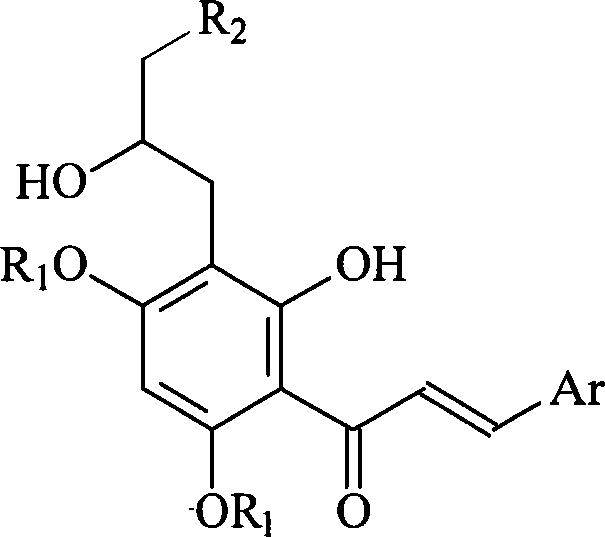
Preparation method and usage for nitrogen-containing chalcone derivatives
A technology of chalcone derivatives and compounds, which is applied in the field of synthesis of organic compounds, can solve problems such as in-depth research on the mechanism of anti-tumor effects, and achieve the effects of easy preparation, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1, 2-hydroxy-4,6-dimethoxy-acetophenone (compound III): prepared according to the literature method (I.Kazuhiko et al, J Nat.Prod., 1988, 51, 906-914) have to.
[0027] M.p.77~78℃.
[0028] 1 H-NMR (δ, CDCl 3 ): 14.06(s, 1H), 6.05(d, 1H, J=2.4Hz), 5.92(d, 1H, J=2.4Hz), 3.90(s, 3H), 3.62(s, 3H), 2.63(s , 3H).
Embodiment 2
[0029] Example 2, 2'-hydroxyl-4', 6'-dimethoxy-2-chloro-chalcone (compound IVa): refer to literature method (X.Y.Bu et al, Synthesis, 1997, 11, 1246-1248 )be made of.
[0030] M.p.123~124℃.
[0031] 1 H-NMR (δ, CDCl 3 ): 14.20(s, 1H,), 8.14(d, 1H, J=15.6Hz), 7.87(d, 1H, J=15.6Hz), 7.68-7.70(m, 1H), 7.42-7.44(m, 1H ), 7.26-7.31 (m, 2H), 6.11 (s, 1H), 5.96 (s, 1H), 3.90 (s, 3H), 3.84 (s, 3H).
Embodiment 3
[0032] Example 3, 3'-allyloxy-4', 6'-dimethoxy-2-chloro-chalcone (compound Va):
[0033] 3.2g (10.0mmol) of compound IV, 2.0g (15.0mmol) of anhydrous potassium carbonate and 30mL of anhydrous acetone were put into the reaction flask, and 10.4mL (12.0mmol) of allyl bromide was added dropwise with stirring. After the addition, the temperature was raised to reflux for 4 hours. After cooling the reaction liquid, a small amount of solid precipitated out, which was suction filtered, and the filtrate was recovered under reduced pressure to obtain 3.4 g of light yellow liquid with a yield of 95%.
[0034] 1 H-NMR (δ, CDCl 3): 7.79(d, 1H, J=16.0Hz), 7.66(m, 1H), 7.39(m, 1H), 7.28(m, 2H), 6.93(d, 1H, J=16.0Hz), 6.16(d , 1H, J=2.0Hz), 6.14(d, 1H, J=2.0Hz), 5.95(m, 1H), 5.33(dd, 1H, J 1 =15.8Hz,J 2 =1.2Hz), 5.50(m, 2H), 3.84(s, 3H), 3.78(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com