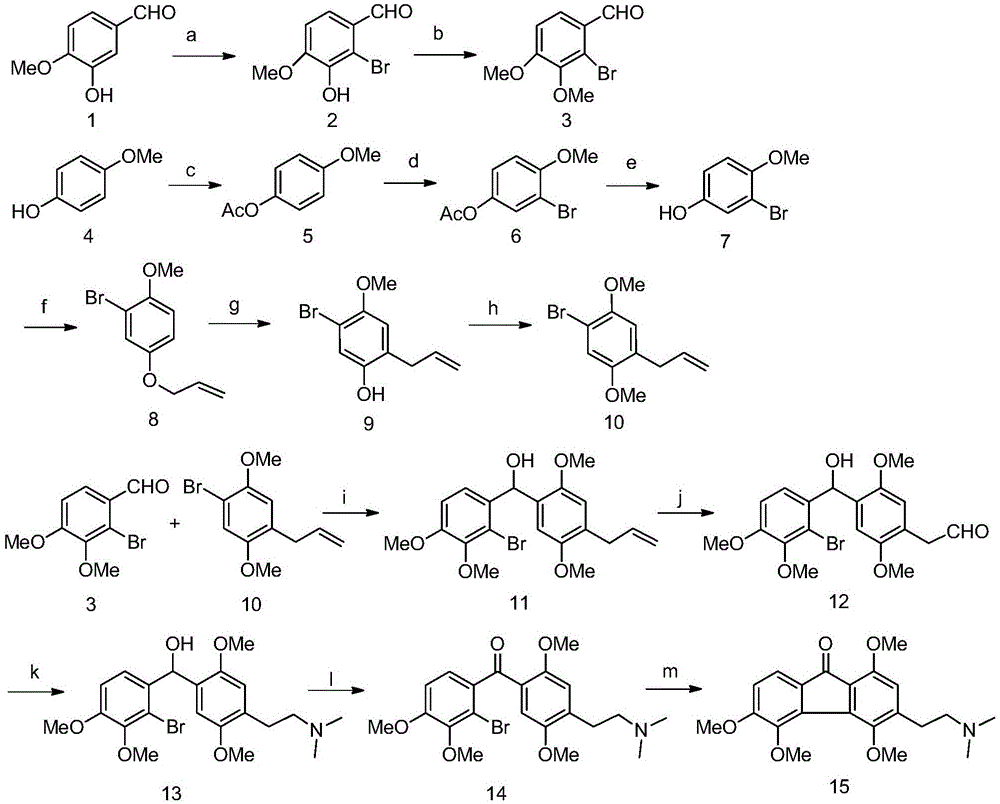
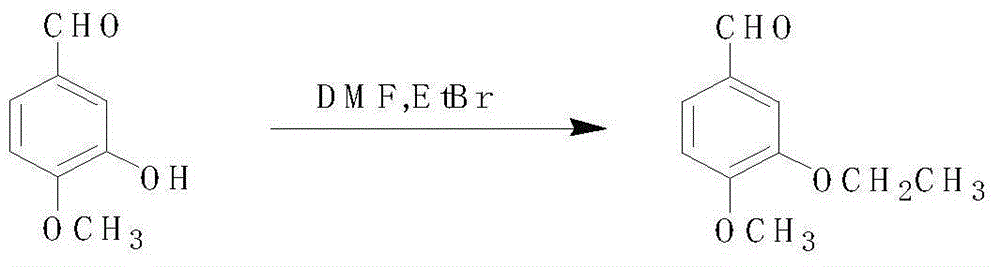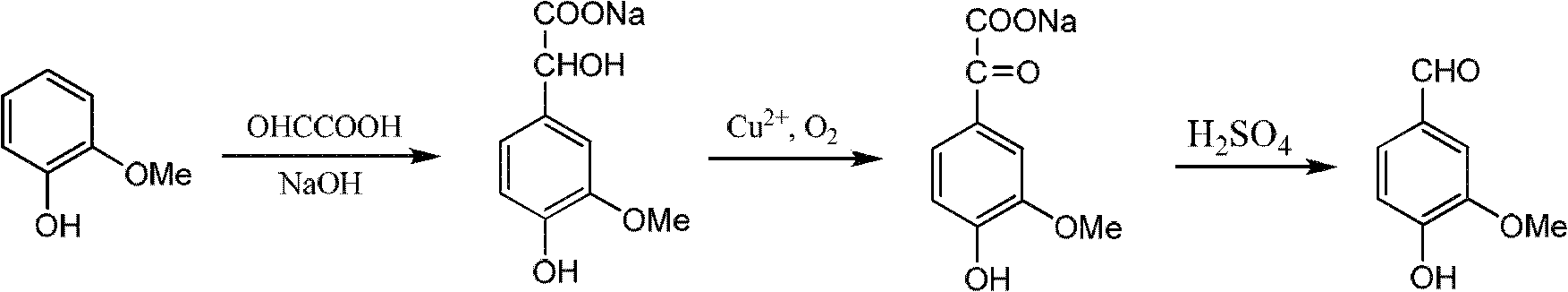Patents
Literature
85 results about "Isovanillin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
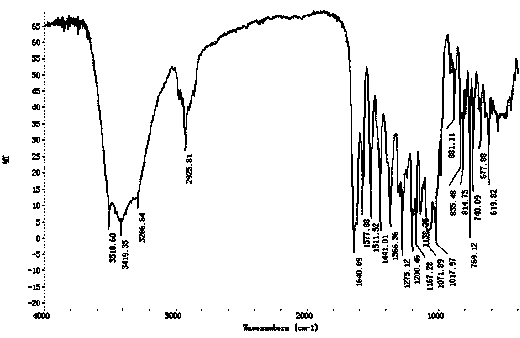
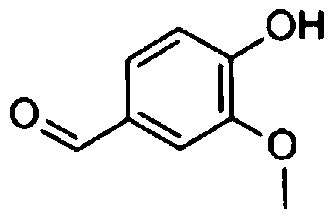
Isovanillin is a phenolic aldehyde, an organic compound and isomer of vanillin. It is a selective inhibitor of aldehyde oxidase. It is not a substrate of that enzyme, and is metabolized by aldehyde dehydrogenase into isovanillic acid. Isovanillin can be used as a precursor in the total synthesis of morphine.
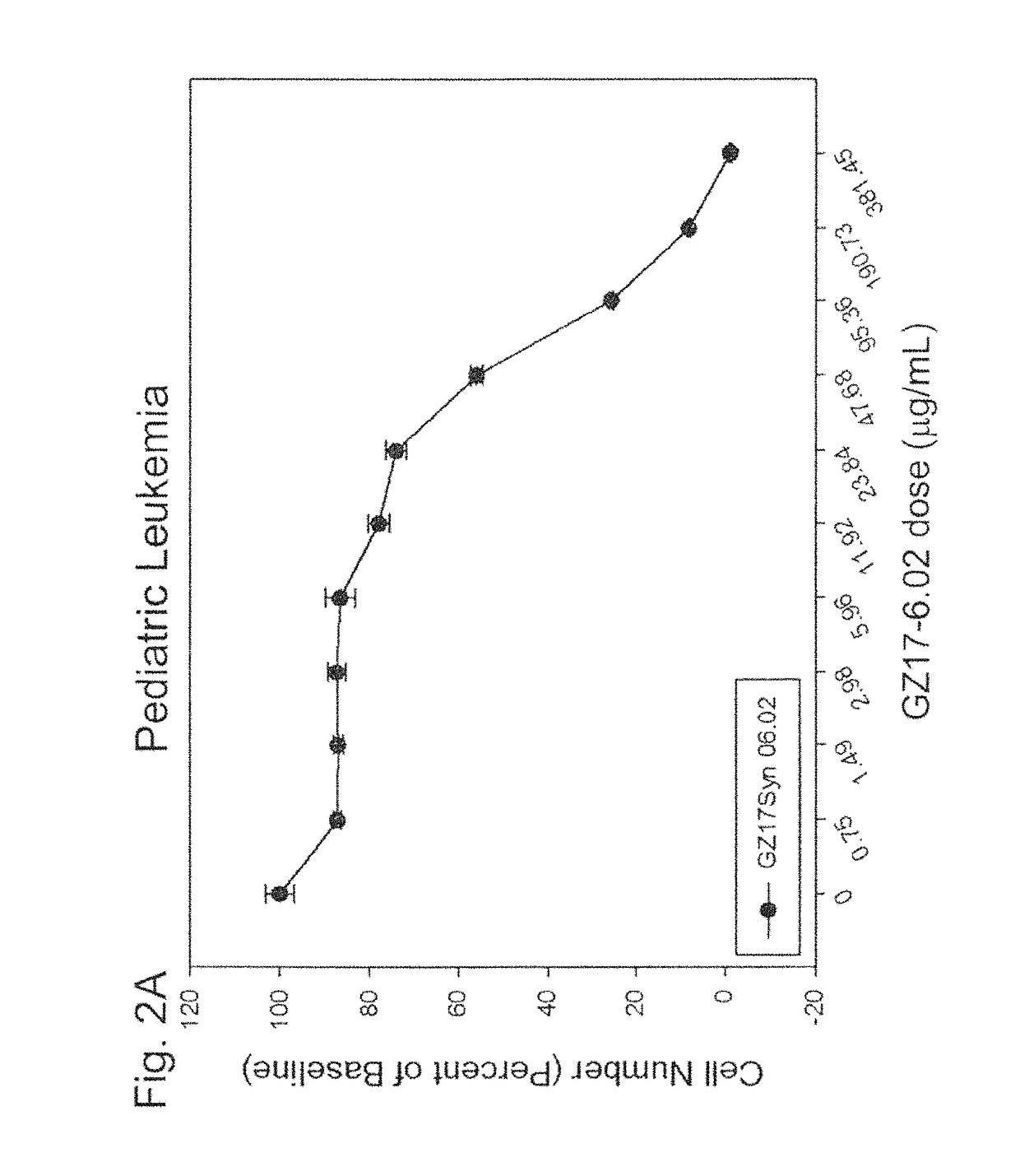
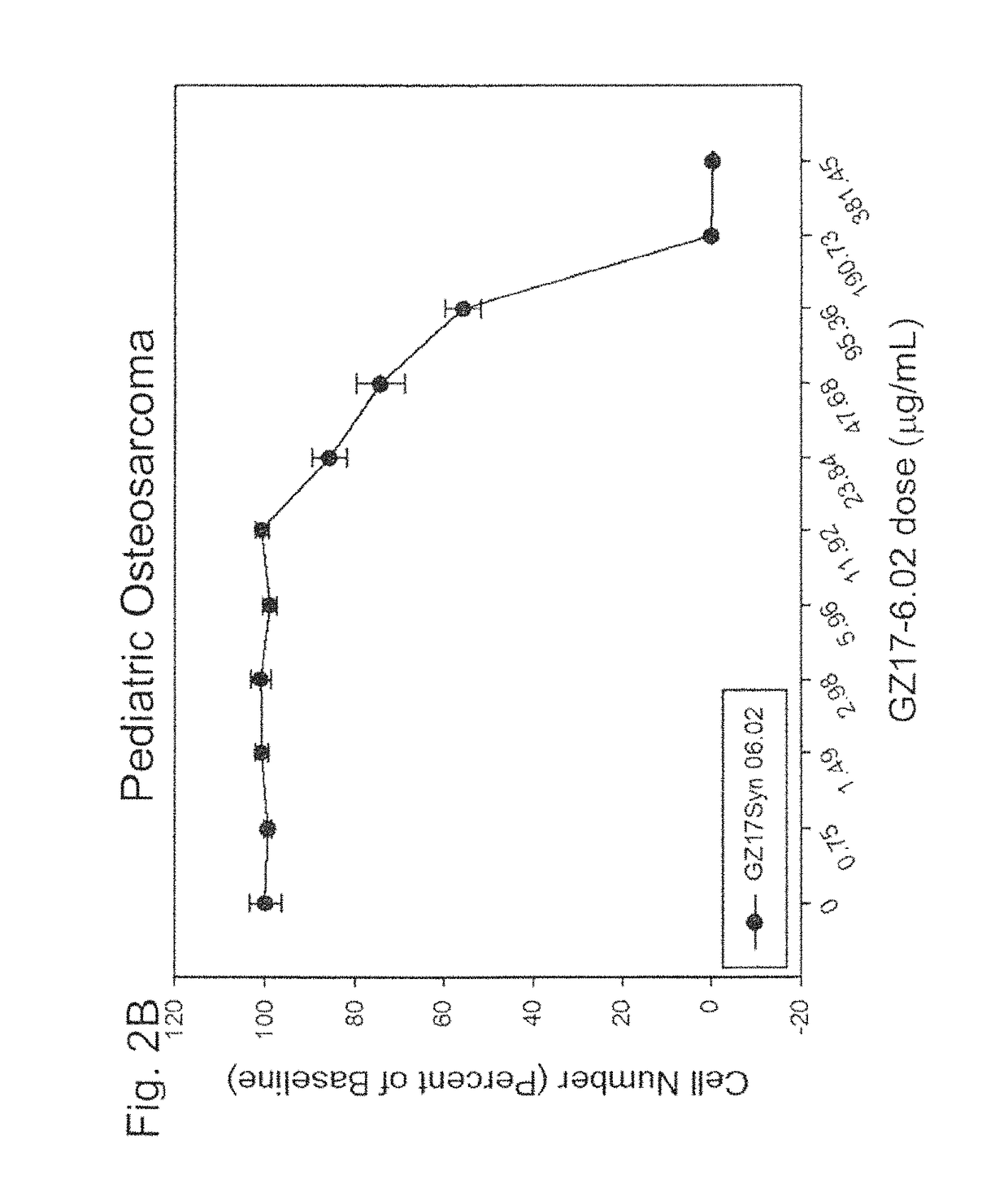
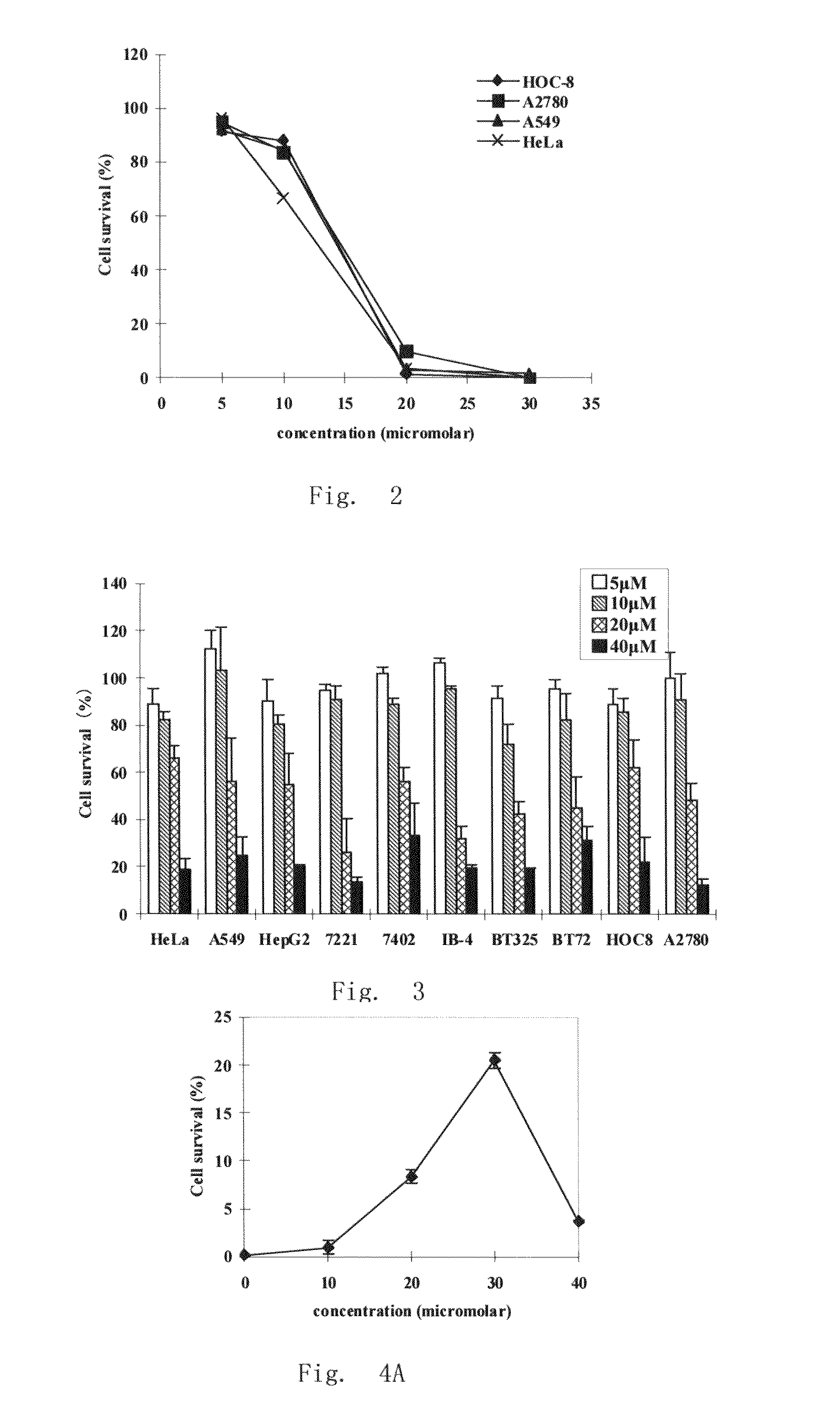
Application of 2-bromide-isovanillin for the manufacture of a medicament for anti-cancer or/and radiation/chemotherapy sensitization
ActiveUS20080221221A1Induce apoptosisHigh sensitivityBiocideAldehyde active ingredientsSide effectTherapeutic effect
Use of 2-bromo-isovanillin in the preparation of an anticancer medicament and / or radio- and chemotherapy sensitizing medicament is disclosed. The medicament for the treatment of cancers and / or for radio- and chemotherapy sensitization comprising 2-bromo-isovanillin as active ingredient provided herein has the following features: (1) low toxicity, without evident adverse effects; (2) significant therapeutic effect, with remarkable proliferation inhibiting and pro-apoptotic effects in tumor cells; (3) a broad-spectrum anticancer activity; (4) suitable to be used in combination with antimetabolites, to enhance the effects and meanwhile lower the toxicity, and also to reduce multi-drug resistance; (5) convenient and safe administration, the main route being oral.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Use of bioflavanoid or polyphenolic substance for treating parkinson's disease
The invention discloses application of bioflavonoids or polyphenol compounds to preventing and treating Parkinson diseases. The bioflavonoids or polyphenol compounds can be selected from kaempferol-3-O-rutinoside, dehydrated carthamin yellow B, 6-hydroxyl kaempferol-3, 6-dioxygen glucoside, (2S)-4', 5-dihydroxyl-6, 7-dioxygen flavonone glucoside, rutin, quercetin, vanillina or isovanillin. Through large amount of experiments, the bioflavonoids or polyphenol compounds have activity of interacting with protein DJ-1 related to the Parkinson diseases. Through further experiments, the bioflavonoids or polyphenol compounds have pharmaceutical activities for resisting oxidative stress, preventing apoptosis of PC12 cells or primary nerve cells induced by the oxidative stress, inhibiting active oxygen generation in cells, improving activity of tyrosine hydroxylase, and the like, and can be used for preventing or treating the Parkinson diseases.
Owner:PEKING UNIV
Method for preparing Roflumilast
InactiveCN102336704AShort stepsRaw materials are cheap and easy to getOrganic chemistryBenzaldehydeEthyl acetate
The invention discloses a method for preparing Roflumilast. The method comprises the following steps of: performing cyclopropyl methylation on isovanillin to obtain 3-cyclopropylmethoxy-4-methoxybenzaldehyde; performing demethylation to synthesize an important intermediate of the Roflumilast, namely 3-cyclopropylmethoxy-4hydroxyl-benzaldehyde; and further synthesizing a key intermediate in a formula (5) according to American patent US5712298 and finally synthesizing the Roflumilast in a formula (7). A crude product of the Roflumilast is treated by isopropanol and water, and is recrystallized by ethyl acetate and petroleum ether. The preparation method has a few steps, raw materials are readily available and cheap, the reaction selectivity is high, the yield is high and the post treatment is simple.
Owner:SHANDONG RUIHE PHARMA R&D CO LTD
Human therapeutic agents
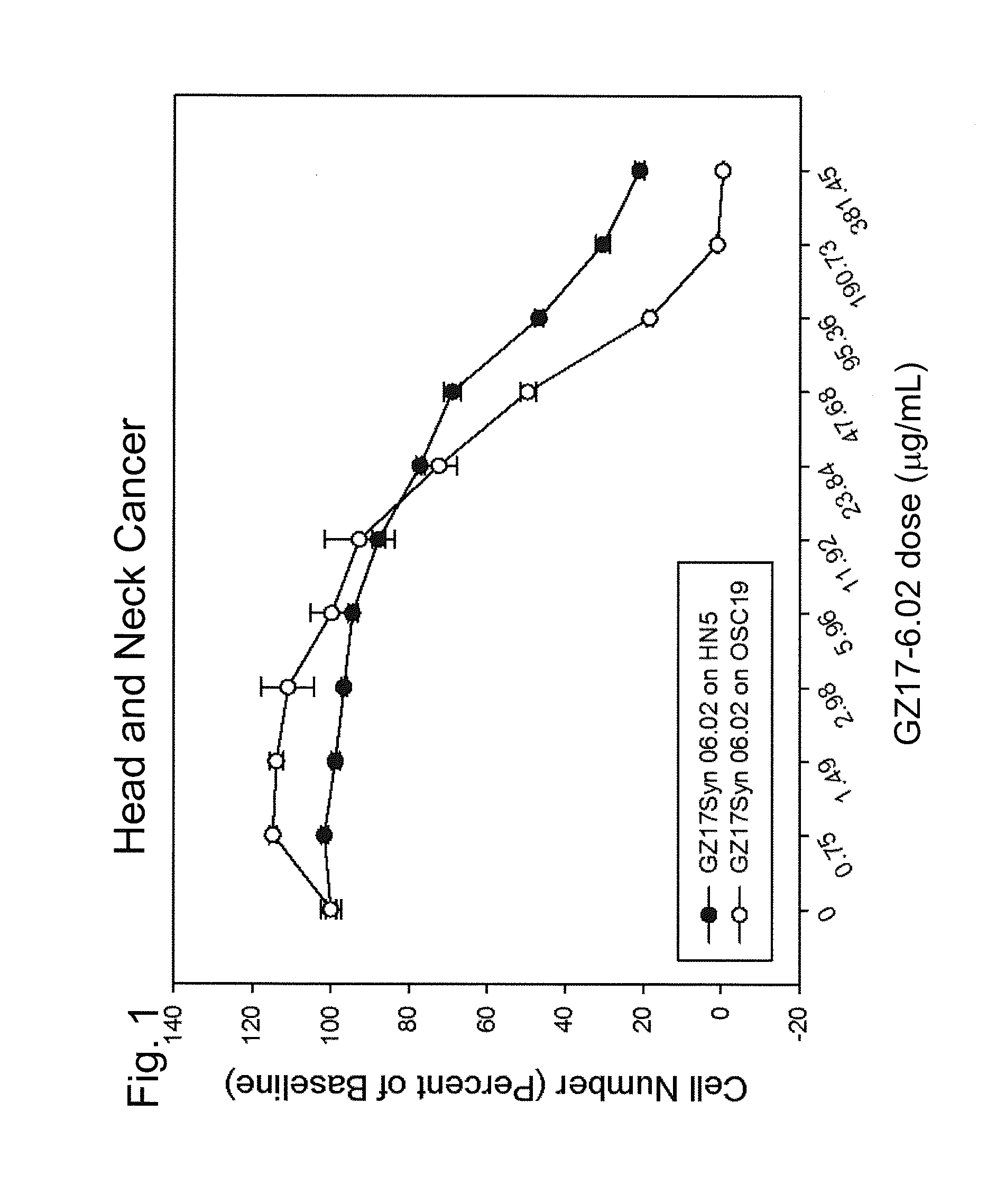
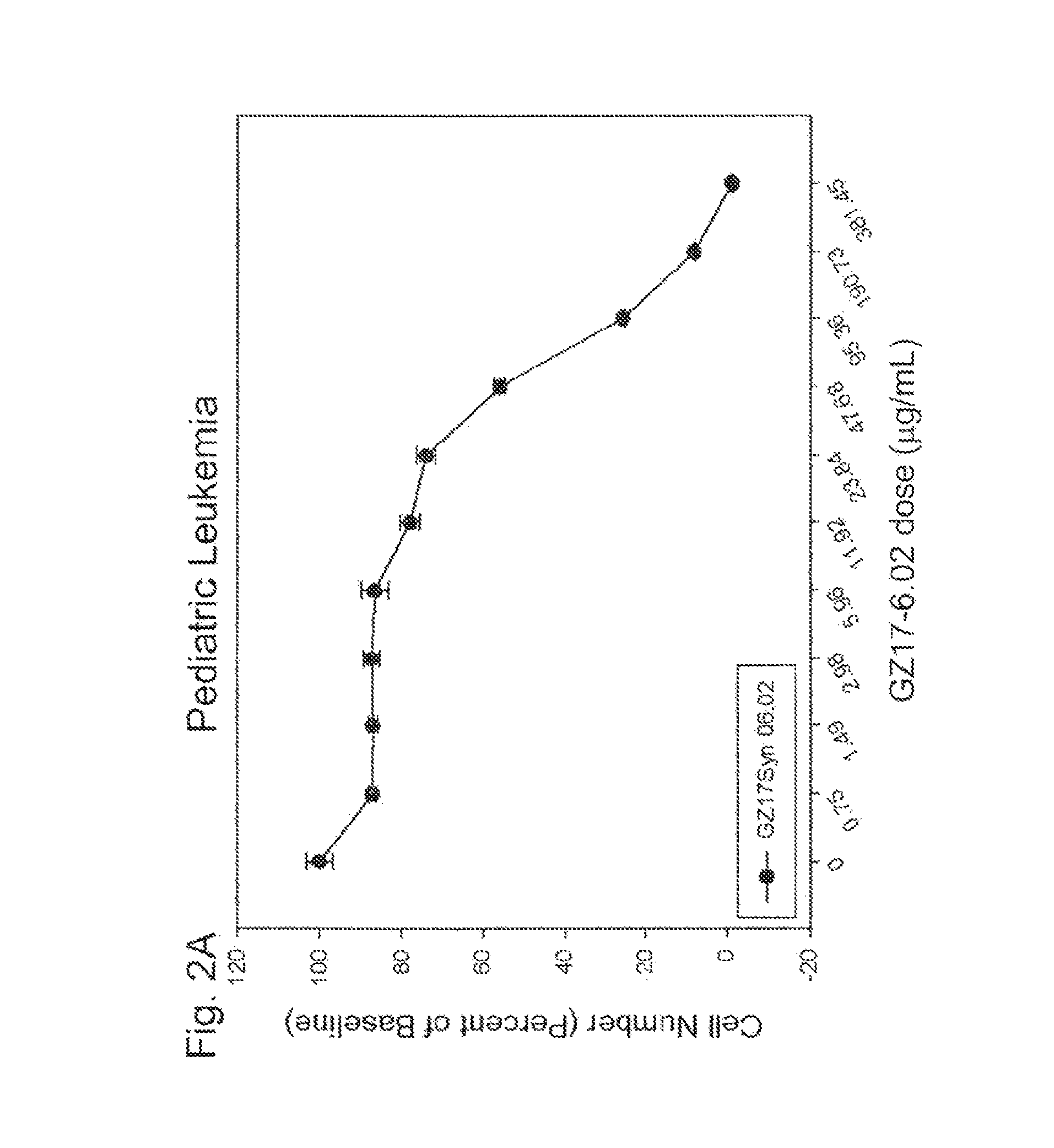
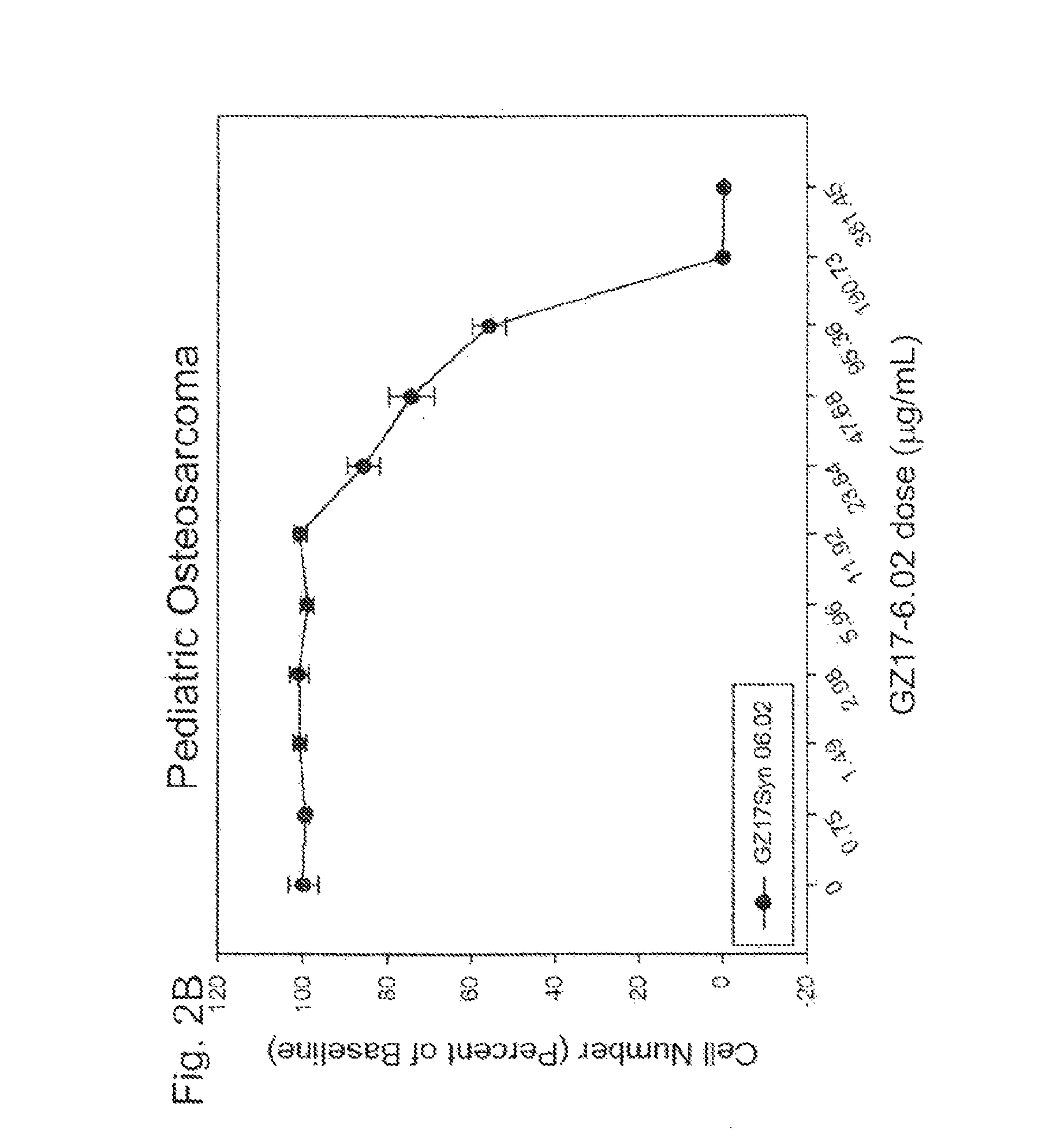
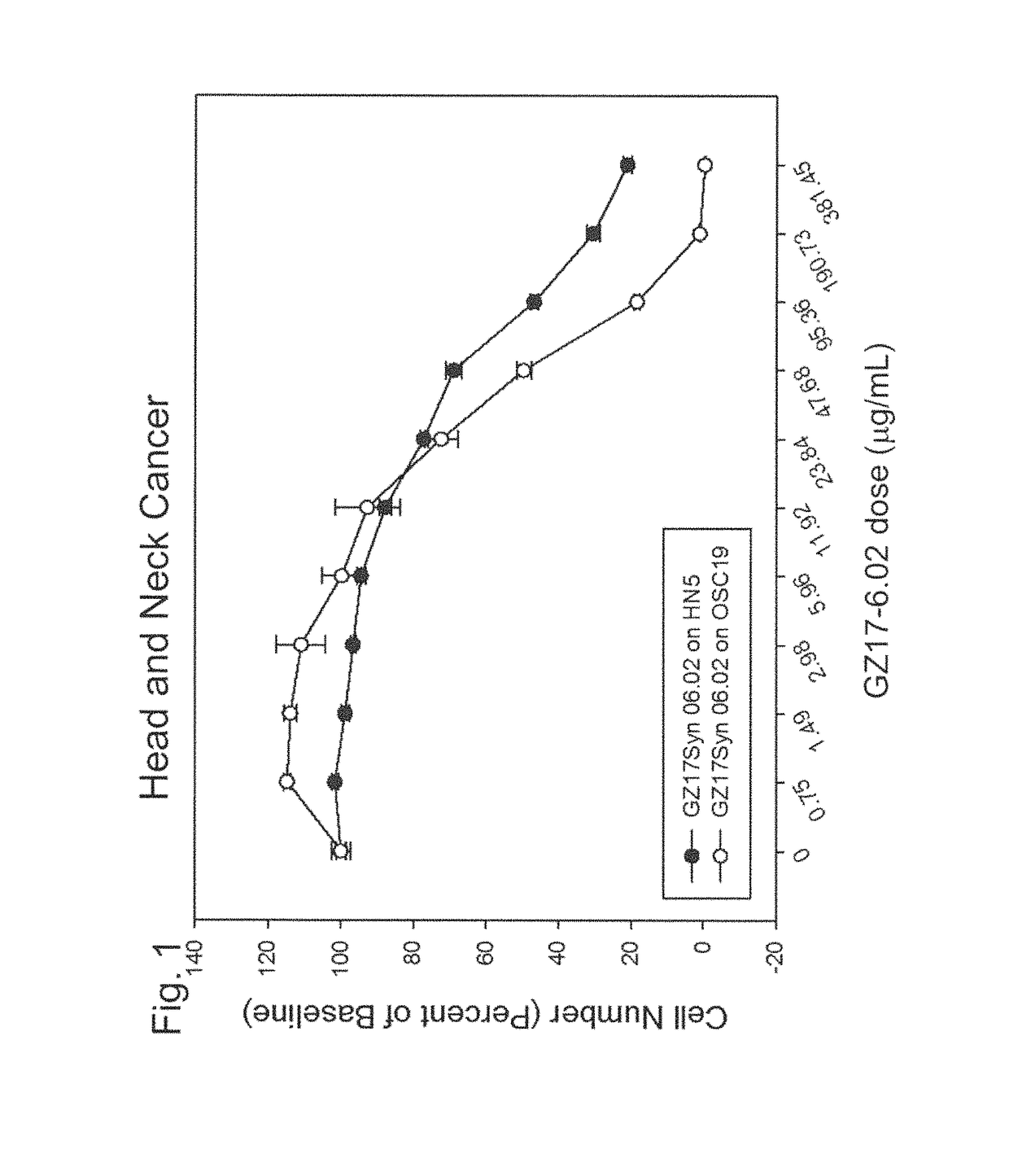
Human therapeutic treatment compositions comprise at least two of a curcumin component, a harmine component, and an isovanillin component, and preferably all three in combination. The agents are effective for the treatment of human conditions, especially human cancers.
Owner:ANKH LIFE SCI LTD
Therapeutic compositions containing curcumin, harmine, and isovanillin components, and methods of use thereof
Human therapeutic treatment compositions comprise at least two of a curcumin component, a harmine component, and an isovanillin component, and preferably all three in combination. The agents are effective for the treatment of human conditions, especially human cancers.
Owner:ANKH LIFE SCI LTD
Neohesperidin synthesis technology
InactiveCN103408620AHigh activityQuick responseSugar derivativesSugar derivatives preparationPhloroacetophenoneChemical synthesis
The invention relates to the field of organic synthesis and discloses a neohesperidin synthesis technology. The neohesperidin synthesis technology comprises the following steps of adding phloroacetophenone-4'-beta-neohesperidin and isovanillin (CAS: 621-59-0) as initial raw materials and a low-carbon alcohol solvent having the content of more than 98wt% into a three-necked flask, carrying out stirring for dissolution, feeding N2 into the three-necked flask to expel air in the three-necked flask, carrying out heating, orderly adding a main catalyst and a cocatalyst into the three-necked flask, carrying out timing at backflow starting time, stopping heating after the reaction lasts for 2-8h, carrying out cooling to a room temperature, carrying out filtration, washing the filter cake by a small amount of hot low-carbon alcohol, and carrying out vacuum drying at a temperature of 50 DEG C to obtain white solid neohesperidin powder. Compared with the existing neohesperidin synthesis technology, the neohesperidin synthesis technology provided by the invention has the advantages of low cost, high yield, high purity and small environmental pollution and is suitable for neohesperidin industrial production.
Owner:SOUTHWEST RES & DESIGN INST OF CHEM IND
Method for artificially synthesizing galanthamine
The invention discloses a method for artificially synthesizing galanthamine, which uses isovanillin and bromine as the raw materials. After the raw materials are subjected to substitution reaction, the reaction and industrial chemicla tyramine are subjected to amination and formylation to obtain formamide, the formamide is subjected to reaction to obtain a derivative of racemic narwedine, the derivative of racemic narwedine is reduced into racemic narwedine, N-methylephedrine is used to reduce unsaturated ketone and tartaric acid is used to carry out resolution to obtain levogyrate galanthamine. The method not only can conveniently and rapidly prepare the galanthamine, but also adopts artificial preparation so as to have little limination in the preparation process.
Owner:泰州市宝嵘新材料有限公司
Method of preparing (Z)-3'-hydroxyl-3,4,4',5-tetramethoxy toluylene
InactiveCN101402555AGood sustainable development abilityCost-effectiveOrganic chemistryOrganic compound preparationPetrochemicalMandelic acid
The invention discloses a method for preparing (Z)-3'-hydroxy radical -3,4,4',5-tetra- methoxyl diphenyl ethylene by the material of renewable natural plant resource. Naturally sourced 3,4,5-tri-methoxybenzaldehyde (a derivative extracted from Chinese gall) is taken as the raw material; and 3,4,5-tri-methoyl mandelic acid is obtained through a dichlorocarbene insertion reaction and is reduced to obtain 3,4,5-tri-methoyl phenylacetic acid. The compound can have a Perkin reaction with naturally sourced isovanillin to construct a cis-form diphenyl ethylene backbone, and the (Z)-3'-hydroxy radical -3,4,4',5-tetra- methoxyl diphenyl ethylene is obtained after a decarboxylic reaction. The method adopts the renewable resource-isovanillin and the 3,4,5-tri-methoxybenzaldehyde rich in China to replace increasingly exhausted petrochemical materials, thereby having a good sustainable development capability and remarkable economic, environmental and ecological benefits.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
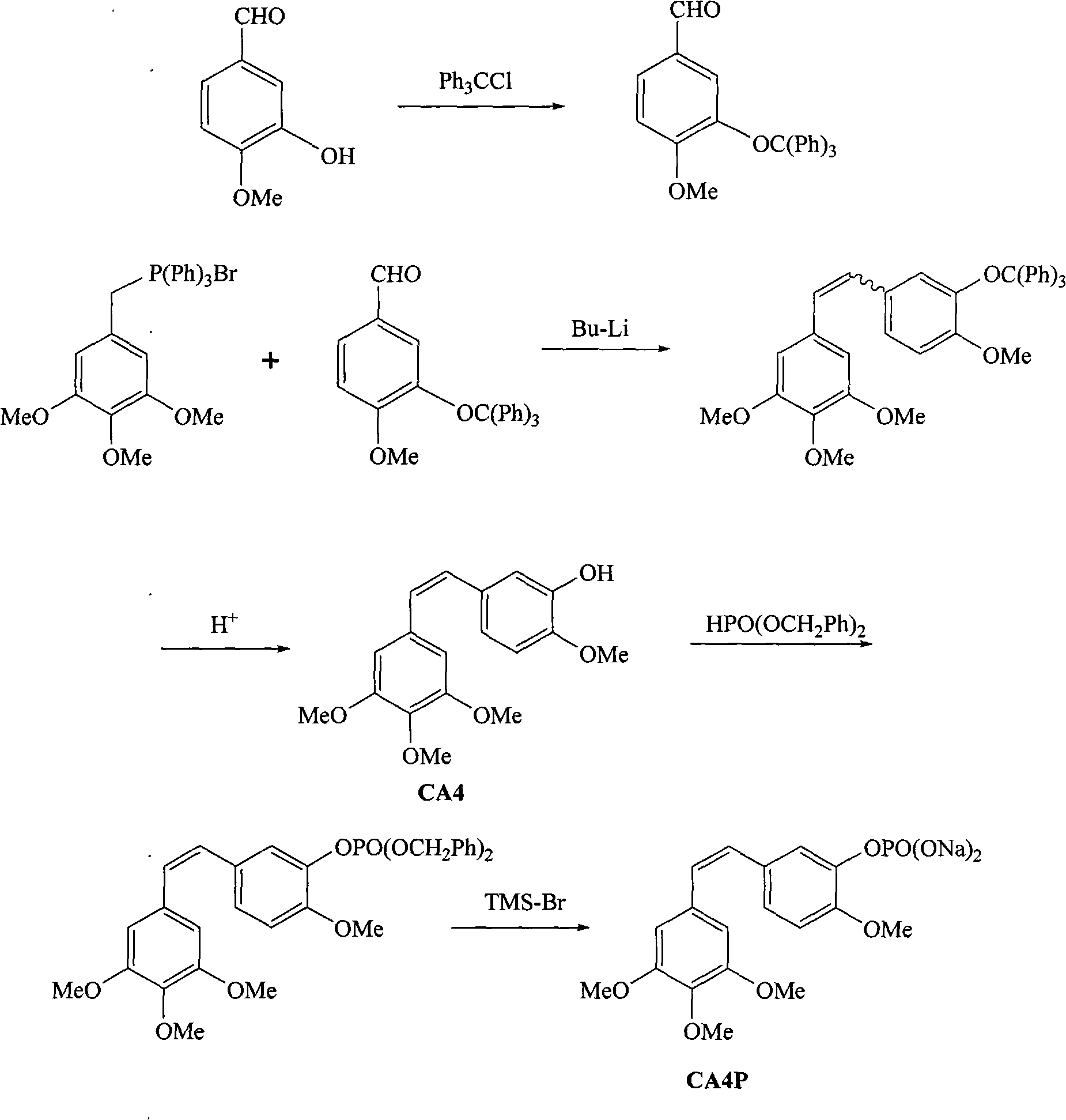
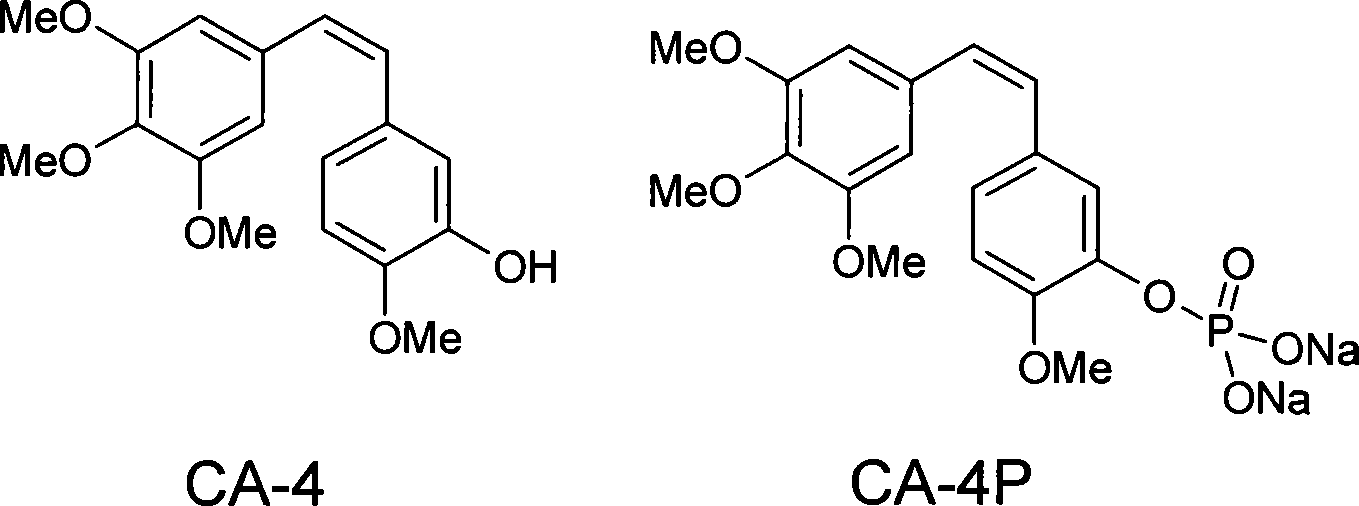
Method for synthesizing CA4P
ActiveCN101885738ARaw materials are cheap and easy to getMild reaction conditionsMethine/polymethine dyesGroup 5/15 element organic compoundsChemical synthesisPhosphate
The invention belongs to the field of chemical synthesis and relates to a method for preparing CA4P, in particular to a method for synthesizing CA4P by the following steps that: isovanillin and trityl chloride, which serve as raw materials, are used to form 3- triphenylmethoxy-4-methoxybenzaldehyde which is an intermediate isovanillin protector; the 3- triphenylmethoxy-4-methoxybenzaldehyde and 3,4,5-trimethoxy-triphenyl benzylidene bromide phosphine salt undergo a Wittig reaction , and the protective group is removed by hydrolysis to obtain CA4; and the CA4 and phosphonic acid bis(phenylmethyl)ester react to form benzyl phosphate, and the benzyl group is removed to form a sodium salt to obtain the target compound, namely CA4P.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD
Therapeutic compositions containing curcumin, harmine, and isovanillin components, and methods of use thereof
ActiveUS20170042865A1Raise countEffective treatmentKetone active ingredientsAldehyde active ingredientsHuman cancerHarmine
Human therapeutic treatment compositions comprise at least two of a curcumin component, a harmine component, and an isovanillin component, and preferably all three in combination. The agents are effective for the treatment of human conditions, especially human cancers.
Owner:ANKH LIFE SCI LTD
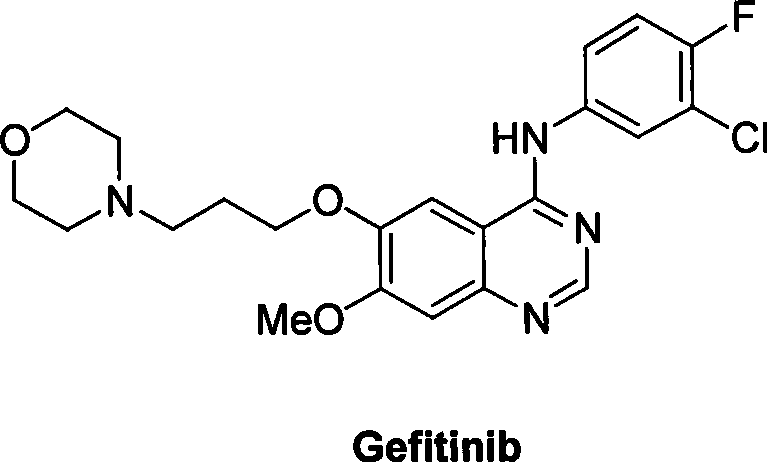
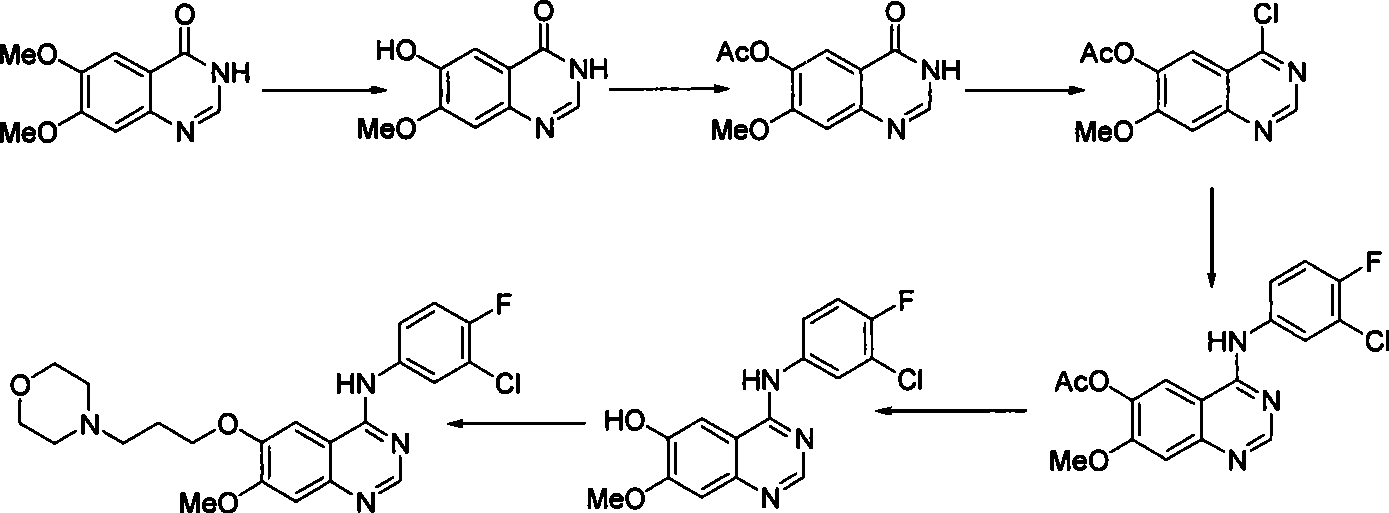
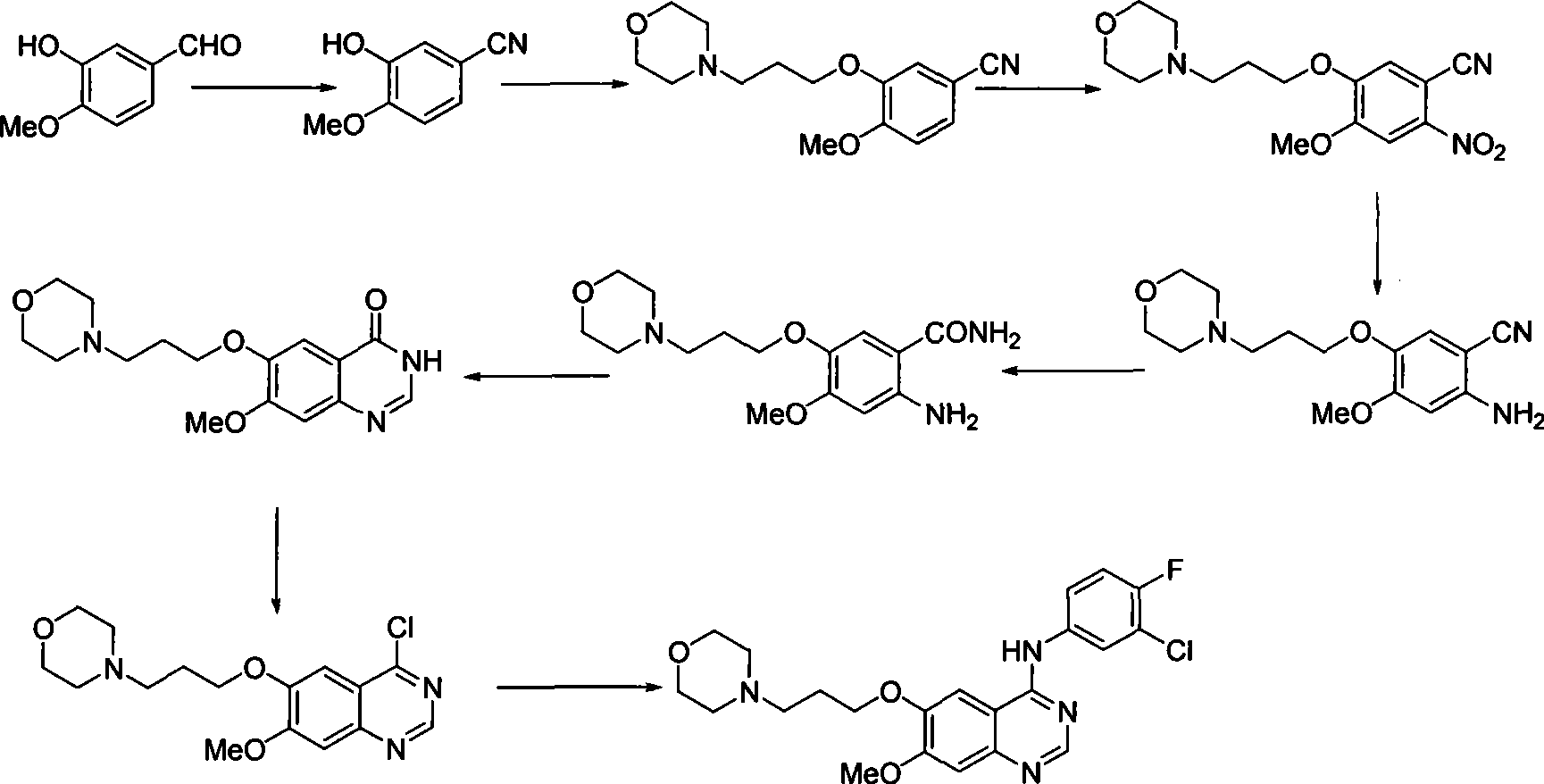
Preparation of gefitinib
ActiveCN101463012AAvoid the risk of overhydrolysisMild reaction conditionsOrganic chemistryMorpholineQuinazoline
The invention discloses a preparation method of gefitinib. In the method, isovanillin is taken as a raw material and synthesized to obtain 7-methoxy-6-(3-morpholine-propoxy)quinazoline-4-one which is directly chloridized to obtain a product, the product is allowed to react with 3-chlorine-4-fluoroaniline to obtain gefitinib hydrochloride which neutralized off hydrochloric acid to obtain the gefitinib. The method has mild reaction condition and is applicable to industrialized production.
Owner:FUJIAN SOUTH PHARMA CO LTD
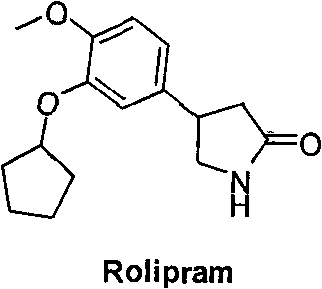
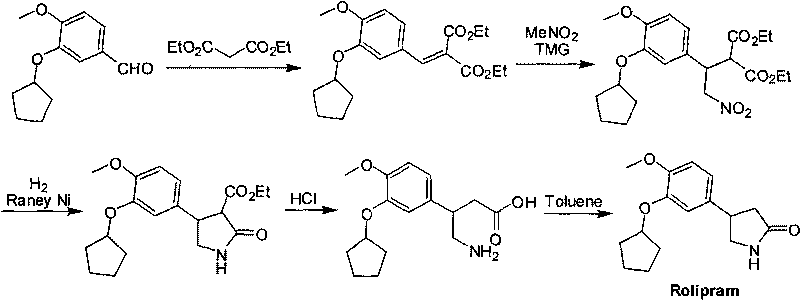
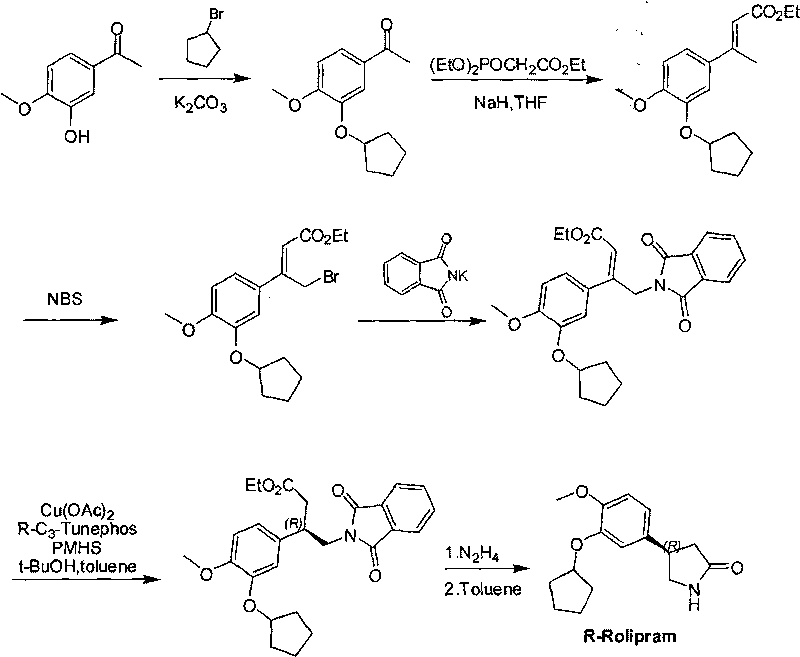
Synthetic method of R-structured Rolipram
InactiveCN101747252AHigh yieldMild reaction conditionsOrganic chemistryEnantioselective synthesisEnantiomer
The invention relates to a synthetic method of R-structured Rolipram which is synthesized by using isovanillin as starting raw materials and through six steps of reactions of cyclopentylation, addition reaction, asymmetric Michael addition reaction, reduction ring closing reaction, decarboxylation and cyclization reaction in a stereoselectivity way. The asymmetric synthetic method of the R-structured Rolipram has the advantages of high yield, high enantiomer excess, mild reaction condition, simple and convenient posttreatment, low cost and the like, thereby being a synthetic route with industrialized prospect.
Owner:JIAXING EPOCHEM PHARMTECH
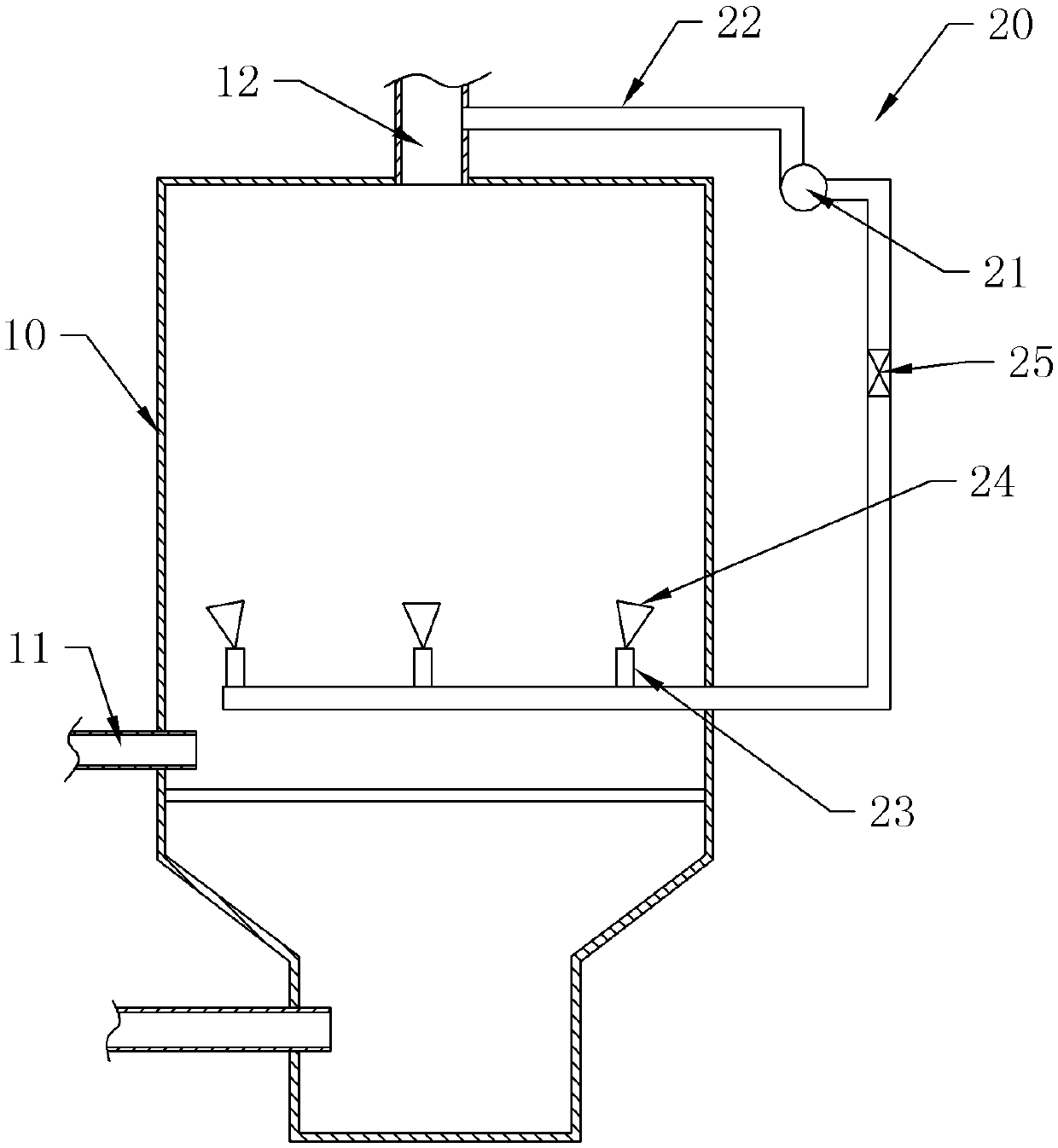
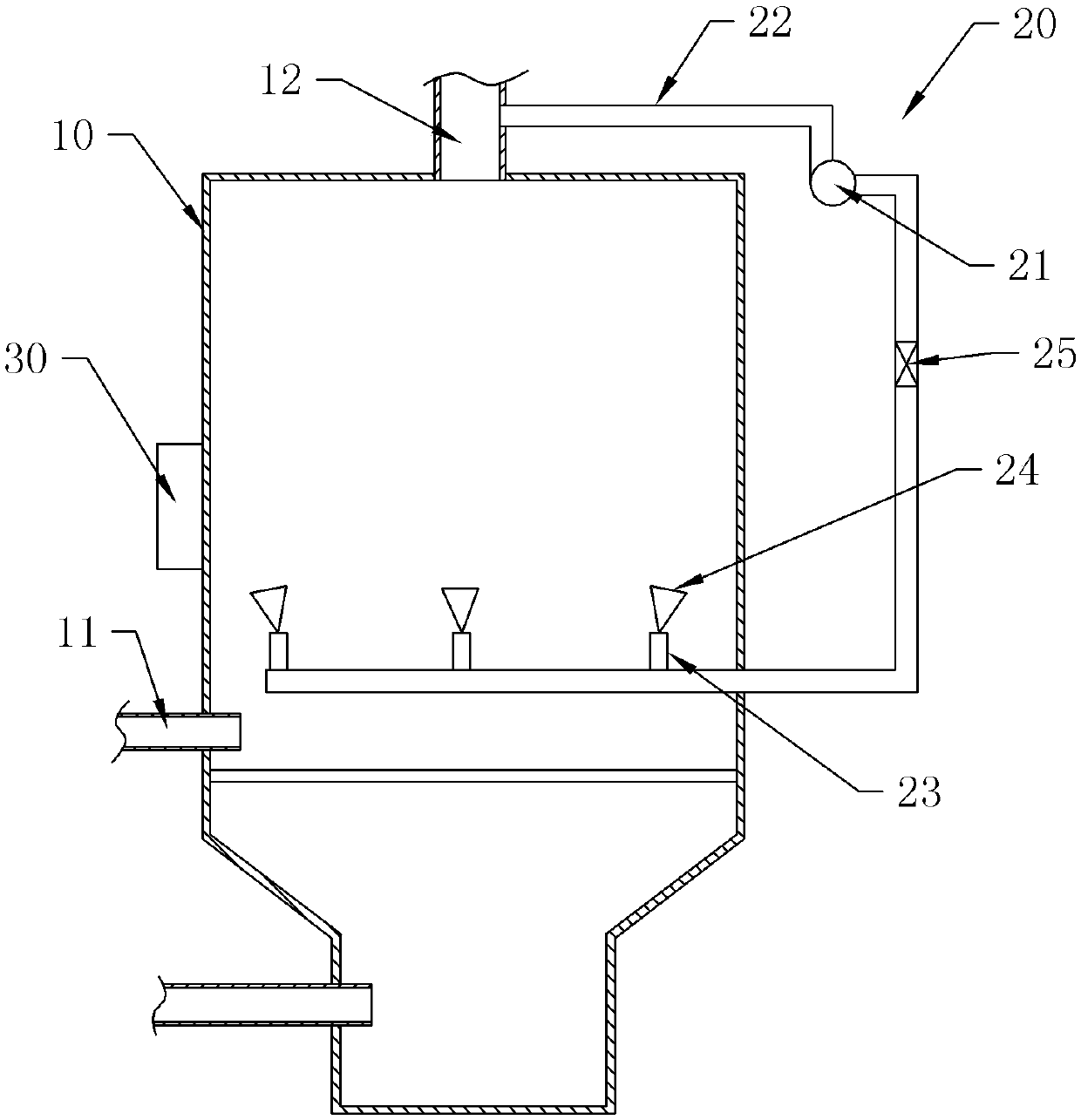
Fluidized bed dryer for isovanillin production
InactiveCN105371596AImprove discharge efficiencyWon't hinder blowingDrying solid materials with heatHearth type furnacesFluidized bed dryingSpray nozzle
The invention discloses a fluidized bed dryer for isovanillin production. The fluidized bed dryer for isovanillin production comprises a dryer shell; a material inlet and a material outlet are formed in the dryer shell; a cleaning structure is further arranged. The cleaning structure comprises an air blower, a pressure pipeline and spray nozzles, wherein one end of the pressure pipeline is communicated with the material outlet; multiple branch pipes are connected to the pressure pipeline; each branch pipe is connected with the corresponding spray nozzle; the multiple spray nozzles are all located in the dryer shell; the multiple spray nozzles are distributed in the dryer shell in the circumferential direction of the dryer shell; an outlet of each spray nozzle faces the inner wall of the dryer shell. By means of the fluidized bed dryer for isovanillin production, isovanillin particles stuck to the inner wall of the dryer can be automatically cleaned, manual cleaning is not needed, and the fluidized bed dryer for isovanillin production is quite convenient to use.
Owner:CHONGQING THRIVE CHEM
Synthetic method for methyl caulophine
ActiveCN105001107AImprove stabilityMild reaction conditionsOrganic chemistryOrganic compound preparationHydroxybenzoate EthersBenzaldehyde
The invention provides a synthetic method for methyl caulophine. The method comprises the following steps: with isovanillin and p-hydroxyanisole as raw materials, successively subjecting isovanillin to bromination and methylation so as to obtain 2-bromo-3,4- dimethoxy benzaldehyde; successively subjecting p-hydroxyanisole to acetylation, bromination, hydrolyzation, allyl etherification, Claisen rearrangement and methylation so as to obtain 1-allyl-4-bromo-2,5-dimethoxybenzene; successively subjecting the obtained 1-allyl-4-bromo-2,5-dimethoxybenzene and 2-bromo-3,4- dimethoxy benzaldehyde to Grignard reaction, ruthenium chloride / sodium periodate double-bond oxidation, reductive amination, pyridine chlorodichromate oxidation and intramolecular coupling so as to obtain 3-(2-(N,N-dimethylamino)methyl)-1,4,5,6-tetramethoxyl-9-H-fluorene-9-ketone, i.e. the method for synthesizing methyl caulophine is completed. The method in the invention has the advantages of mild reaction conditions, simple operation, easily-available raw materials and reagents, etc., and is suitable for large-scale production of pharmaceutical enterprises
Owner:XI AN JIAOTONG UNIV
Therapeutic compositions containing harmine and isovanillin components, and methods of use thereof
Human therapeutic treatment compositions comprise at least two of a curcumin component, a harmine component, and an isovanillin component, and preferably all three in combination. The agents are effective for the treatment of human conditions, especially human cancers.
Owner:ANKH LIFE SCI LTD
Method for separating vanillin and isovanillin by three-zone asynchronous switching simulated moving bed
InactiveCN109785908AReduce consumptionThe purpose of continuous and large-scale preparation and separationChemoinformaticsInstrumentsSeparation technologySimulated moving bed
The invention discloses a method for separating vanillin and isovanillin by a three-zone asynchronous switching simulated moving bed, and relates to vanillin and isovanillin. The method comprises thesteps of preparing an eluent; preparing a vanillin and isovanillin sample solution; separating the obtained vanillin from the isovanillin sample solution by adopting an asynchronous switching simulated moving bed chromatography system to obtain the vanillin and the isovanillin. According to the invention, the asynchronous switching simulated moving bed chromatography system is adopted, so that vanillin and isovanillin are successfully separated. The purpose of continuous and large-scale preparation and resolution is achieved. Under the condition that equipment investment and solvent consumption are not increased, the feeding flow is increased. Vanillin and isovanillin with the recovery rate and purity higher than 99.0% are obtained. The method has the advantages of high yield, low solventconsumption, automatic continuous production and the like. A product with stable quality can be prepared, and the method is a separation technology with a good commercial application prospect.
Owner:XIAMEN UNIV
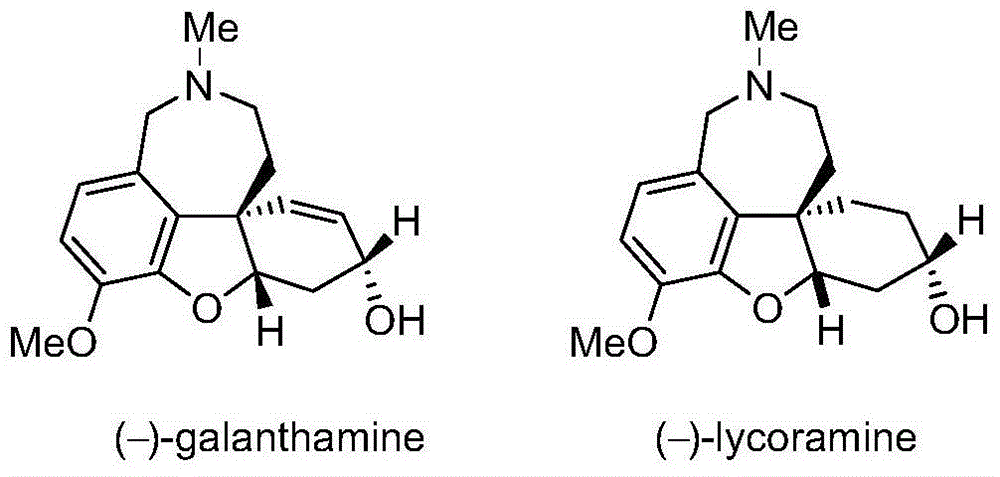
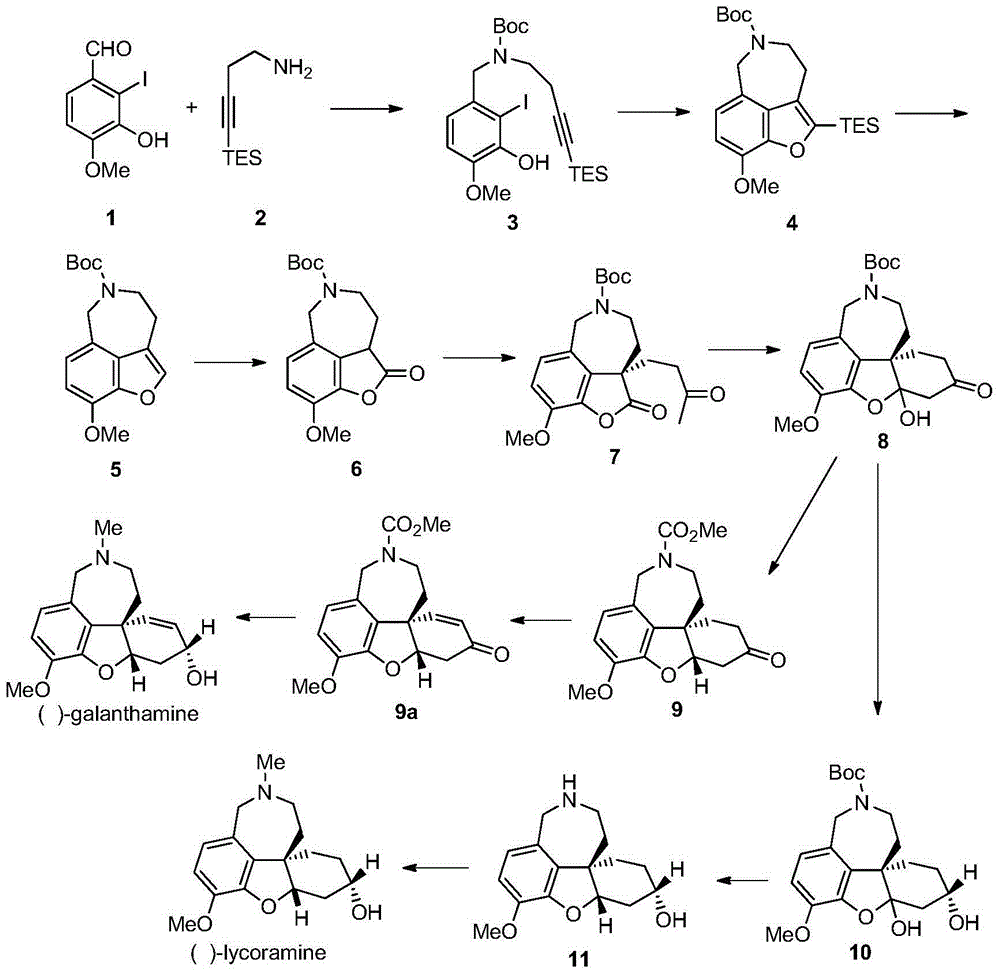
Asymmetric synthesis method of galanthamine and lycoramine
InactiveCN104592243AImprove noveltyShort synthetic stepsBulk chemical productionAsymmetric synthesesMethylvinyl ketoneSynthesis methods
The invention discloses an asymmetric synthesis method of galanthamine and lycoramine. According to the asymmetric synthesis method, the iodide of cheap and easily available raw material isovanillin and easily prepared 4-triethylsilyl-3-butyne-1-amidogen are taken as raw materials, a coumarone compound can be produced by virtue of a palladium catalytic Larock cyclization reaction after the nitrogen atom is protected by virtue of reductive amination, next, an optical pure key intermediate can be obtained at the ee value of 94% by virtue of the asymmetric michael addition of p-methyl vinyl ketone under the catalysis of critical metal Sc(OTf)3 and oxynitride ligands after the coumarone compound is transformed into a coumaranone structure, and the intermediate is subjected to the asymmetric adol reaction induced chirally by a substrate and subsequent selective reduction of lithium tri-sec-butylborohydride so that optically pure galanthamine and lycoramine can be obtained. The asymmetric synthesis method has the advantages of shorter synthesis route and higher efficiency.
Owner:PEKING UNIV
Methods and dosage forms for the treatment of human cancers
Methods for the treatment of human cancers, daily dosage forms for cancer patients, and methods of formulating the dosage forms are provided wherein the daily dosage form contains from about 10-6,000 mg of each of β-sitosterol, isovanillin, and linolenic acid. Preferably, the dosage forms are formulated by first creating an aqueous decoction of Arum palaestinum Boiss, followed by fortification of the decoction with additional quantities of β-sitosterol, isovanillin, and linolenic acid.
Owner:LIFE PLUS MICHIGAN
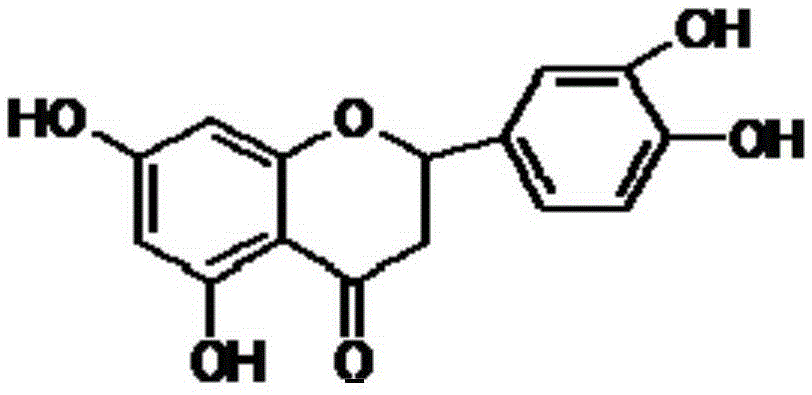
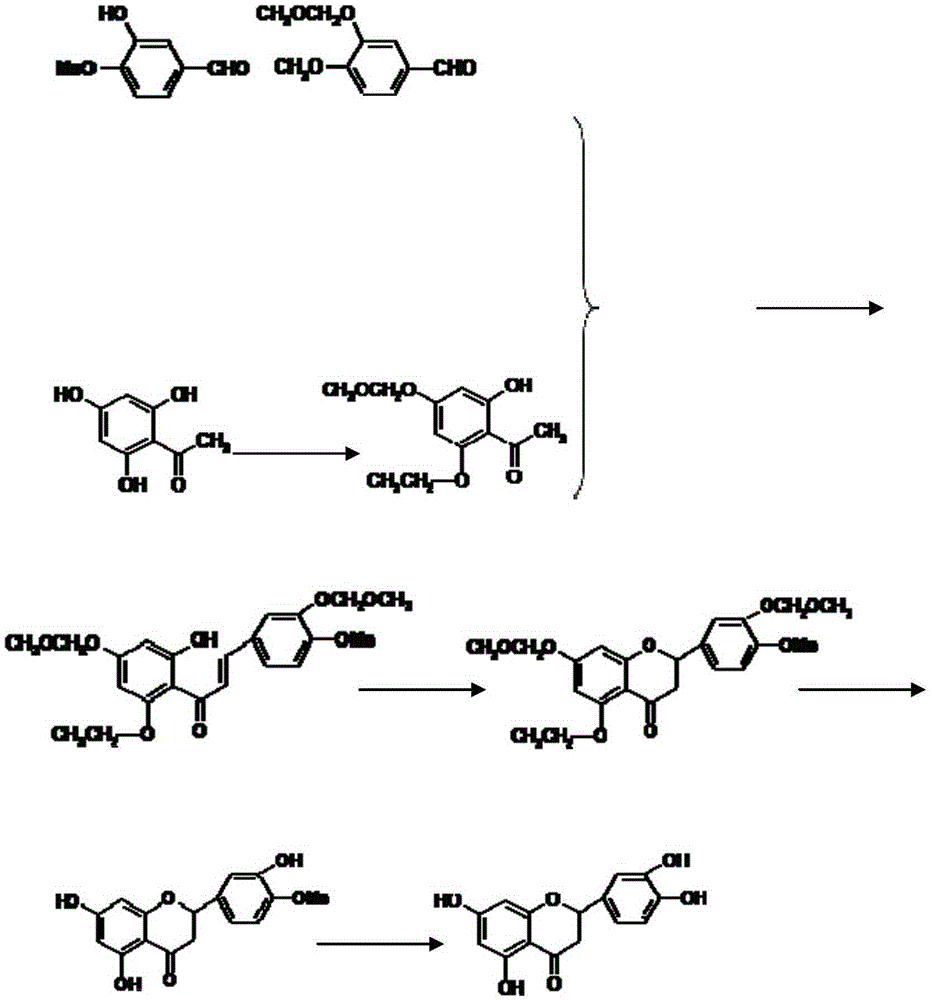
Eriodictyol synthesis method
InactiveCN105348245AHigh yieldSuitable for industrial productionOrganic chemistrySynthesis methodsCombinatorial chemistry
The invention provides an eriodictyol synthesis method. According to the eriodictyol synthesis method, isovanillin and 2,4,6-trihydroxyacetophenone are selected as starting materials, and eriodictyol is obtained through the following five steps: firstly, performing MOMCl protection on a hydroxyl of isovanillin; secondly, performing MOMCl protection on a hydroxyl of 2,4,6-trihydroxyacetophenone; thirdly, performing an aldol condensation reaction to generate chalcone; fourthly, performing Michael addition and cyclization; finally, carrying out MOM deprotection to obtain eriodictyol of which the total yield is 50-65%. As isovanillin and 2,4,6-trihydroxyacetophenone are selected as the starting materials, the eriodictyol synthesis method is high in yield and suitable for industrialized production.
Owner:SHAANXI JIAHE PHYTOCHEM
Method for synthesizing neohesperidin by taking naringin as raw material
ActiveCN106432386AHigh purityLow impurity contentSugar derivativesSugar derivatives preparationNaringinHistidine
The invention provides a method for synthesizing neohesperidin by taking naringin as a raw material. The method comprises the following steps: (1) hydrolyzing: dissolving the naringin into an aqueous alkali; heating, pressurizing and hydrolyzing; cooling to room temperature and adjusting the pH (Potential of Hydrogen) value with an acid; cooling and crystallizing; filtering and drying to obtain a hydrolyzed product-PN; (2) carrying out condensation: dissolving the PN into an organic solvent; adding isovanillin and histidine; carrying out a refluxing reaction; freezing and crystallizing; filtering and drying to obtain a neohesperidin crude product; (3) refining: heating and dissolving the neohesperidin crude product with a low-carbon alcohol water solution; cooling and crystallizing; filtering and drying a filter cake to obtain a neohesperidin fine product. According to the method provided by the invention, the purity of the neohesperidin in the product can reach 99.5 percent, the content of impurities is less and the yield of the neohesperidin is high; the dosage of an alkali and a catalyst in the method is less, the reaction time is short and the cost is low; in a technological process, protection of inert gas is not needed and anhydrous reaction conditions are not needed, so that the method is suitable for industrial production.
Owner:HUNAN HUACHENG BIOTECH
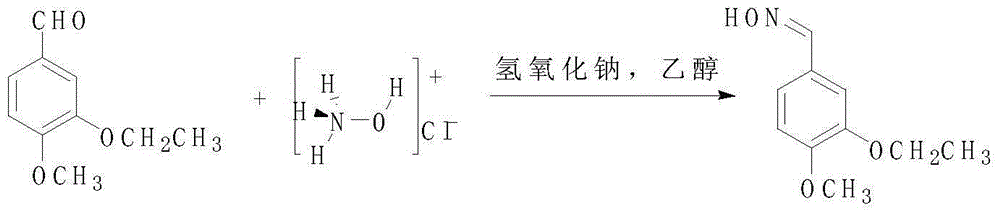
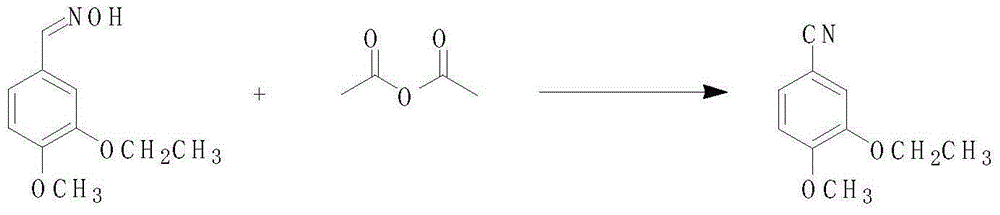
3-ethoxy-4-methoxy benzonitrile preparing method
InactiveCN105175283AReduce pollutionRaw materials are easy to getCarboxylic acid nitrile preparationOrganic compound preparationAcetic anhydrideOrganic synthesis
The invention discloses a 3-ethoxy-4-methoxy benzonitrile preparing method and relates to the technical field of organic synthesis. Isovanillin, bromoethane and hydroxylamine hydrochloride serve as raw materials for reaction, acetic anhydride serves as a dehydrating agent, activated carbon serves as a decolorizing agent, and 3-ethoxy-4-methoxy benzonitrile is obtained through ethylation, aldehyde oximation, acetic anhydride dehydration and absolute ethyl alcohol decoloration treatment. The raw materials adopted by the 3-ethoxy-4-methoxy benzonitrile preparing method are easy to obtain, reaction conditions are moderate, post-treatment is simple and convenient, the acetic anhydride can be recycled and reused, the production cost is low, environmental pollution is low, and the product yield and purity are high.
Owner:BENGBU CHINA SYNCHEM TECH CO LTD
Sharing synthesis method for vanillin and isovanillin
InactiveCN102617313AReasonable designHigh yieldOrganic compound preparationCarbonyl compound preparationAlkyl transferSynthesis methods
The invention relates to a sharing synthesis method for vanillin and isovanillin. The method comprises the following steps of: performing alkylation reaction on guaiacol and halogenated hydrocarbon to prepare a 1-methoxy-2-alkoxylbenzene compound 1 in a high-yield way; performing Vilsmeier-Haack reaction on the 1-methoxy-2-alkoxylbenzene compound 1 and N,N-methylformanilide under the action of phosphorus oxychloride to generate a mixture of 3-methoxy-4-alkyloxybenzaldehyde 2 and 3-methoxy-4-methoxybenzaldehyde 3, separating the mixture out, selectively removing alkyl groups by directly using lewis acid to obtain a mixture of vanillin and isovanillin, and separating to prepare two compounds, namely vanillin and isovanillin. The method has the advantages of simplicity and high yield.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method of 3-hydroxyl-4-methoxycinnamaldehyde
InactiveCN107556182AHigh activityReduce dosageOrganic compound preparationCarbonyl compound separation/purificationPotassium hydroxidePotassium ions
The invention provides a preparation method of 3-hydroxyl-4-methoxycinnamaldehyde and relates to the 3-hydroxyl-4-methoxycinnamaldehyde. The 3-hydroxyl-4-methoxycinnamaldehyde is prepared by taking potassium hydroxide as a catalyst and polytetrahydrofuran as a cocatalyst and carrying out condensation reaction on isovanillin and acetaldehyde. The adopted cocatalyst polytetrahydrofuran is polyetherdiol and can be complexed with potassium ions, so that the activity of the catalyst potassium hydroxide is improved. Compared with an existing preparation method of the 3-hydroxyl-4-methoxycinnamaldehyde, the dosage of alkali is greatly reduced, so that the amount of generated wastewater is also greatly reduced, the reaction time is greatly shortened and the yield of the product is also improved.
Owner:XIAMEN UNIV
Process for producing cinnamaldehyde derivatives, use thereof and the like
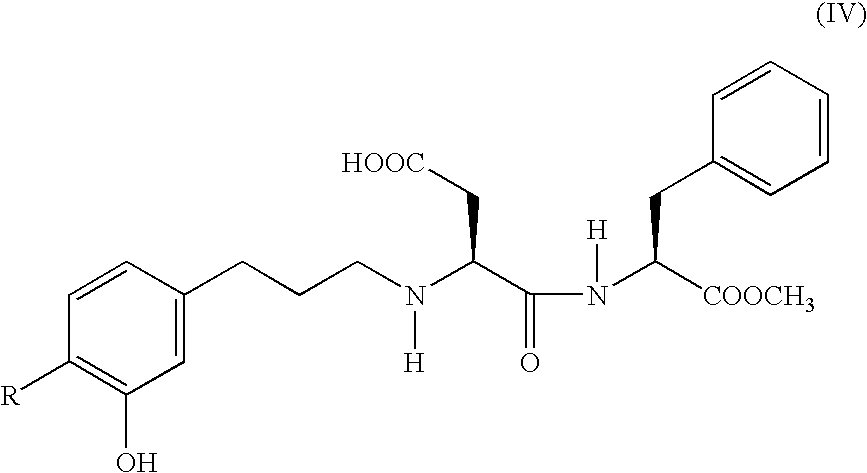
InactiveUS20050245759A1Secondary reaction can be minimally suppressedHigh yieldOrganic compound preparationPeptidesAlkyl transferBenzaldehyde
A process for conveniently and efficiently producing a highly pure cinnamaldehyde derivative, for example, (2E)-(3-hydroxy-4-methoxy)cinnamaldehyde, which comprises reacting a benzaldehyde derivative, such as isovanillin and the like, with acetaldehyde in the presence of an alkali, in particular preferably, dropping acetaldehyde little by little thereto in an aqueous solution at a low temperature for the reaction thereof. The cinnamaldehyde derivative thus obtained is selectively reduced to produce 3-(3-hydroxy-4-substituted (with methoxy group or the like)phenyl)propionaldehyde. Further, through a reductive alkylation reaction of the compound thus produced with an aspartame, N-[N-[3-(3-hydroxy-4-substituted (with methoxy group or the like)phenyl)propyl]-L-aspartyl]-L-phenylalanine 1-methyl ester, which is useful as a sweetener having a high sweetening potency, can be industrially and efficiently produced. The processes therefor are also provided.
Owner:AJINOMOTO CO INC
Method for preparing novolac epoxy resin by utilizing vanillin production waste liquid
The invention relates to a method for preparing novolac epoxy resin by utilizing vanillin production waste liquid. A polymerization reaction is performed on the waste liquid containing 20% to 30% of o-vanillin, 10% to 15% of isovanillin, 10% to 15% of vanillin, 10% to 15% of dimerized vanillin, 15% to 20% of vanillic acid, 5% to 10% of mandelic acid and 5% to 10% of dimerized mandelic acid and formaldehyde solution to generate a linear phenolic polymer. Under the condition of existence of sodium hydroxide and a surfactant, a condensation reaction is further performed on the linear phenolic polymer and epichlorohydrin to obtain the novolac epoxy resin of which an epoxy value is 0.1 to 0.27mol / 100g and a viscosity is 1.5 to 3.0mPa.s. According to the invention, the novolac epoxy resin is prepared by the vanillin production waste liquid, so that the difficult problem of treating the vanillin production waste liquid is solved, comprehensive utilization of waste is implemented, production cost of the novolac epoxy resin is greatly reduced, and product performance of the novolac epoxy resin is improved.
Owner:TIANJIN VOCATIONAL INST
Therapeutic compositions containing harmine and isovanillin components, and methods of use thereof
InactiveUS20170105975A1Useful in treatmentRaise countInorganic non-active ingredientsKetone active ingredientsDiseaseHuman cancer
Human therapeutic treatment compositions comprise at least two of a curcumin component, a harmine component, and an isovanillin component, and preferably all three in combination. The agents are effective for the treatment of human conditions, especially human cancers.
Owner:ANKH LIFE SCI LTD
Synergistic cancer therapy drug combinations
InactiveUS20150140125A1Good treatment effectTherapy is simpleBiocideHeavy metal active ingredientsChemotherapeutic drugsTherapeutic effect
Synergistic cancer therapy drug combinations include therapeutically effective amounts of at least one chemotherapeutic drug or agent with a fortified decoction dosage form comprising from about 10 mg to about 6,000 mg each of β-sitosterol, isovanillin, and linolenic acid. The decoction dosage preferably includes plant extract(s) of the genus Arum fortified with effective amounts of β-sitosterol, isovanillin, and linolenic acid not derived from the plant. The combination may be in various forms including aqueous dispersions, gels, ampules, powders, capsules, pills, or tablets, and are normally administered orally to patients. The anticancer combinations have therapeutic effects on cancerous tissue which are greater than the sum of the individual therapeutic effects of the fortified decoction dosage form and the at least one chemotherapeutic agent on the cancerous tissue.
Owner:LIFE PLUS MICHIGAN
Application of 2-bromide-isovanillin for the manufacture of a medicament for anti-cancer or/and radiation/chemotherapy sensitization
ActiveUS20110098362A9Induce apoptosisHigh sensitivityBiocideAldehyde active ingredientsSide effectTherapeutic effect
Use of 2-bromo-isovanillin in the preparation of an anticancer medicament and / or radio- and chemotherapy sensitizing medicament is disclosed. The medicament for the treatment of cancers and / or for radio- and chemotherapy sensitization comprising 2-bromo-isovanillin as active ingredient provided herein has the following features: (1) low toxicity, without evident adverse effects; (2) significant therapeutic effect, with remarkable proliferation inhibiting and pro-apoptotic effects in tumor cells; (3) a broad-spectrum anticancer activity; (4) suitable to be used in combination with antimetabolites, to enhance the effects and meanwhile lower the toxicity, and also to reduce multi-drug resistance; (5) convenient and safe administration, the main route being oral.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
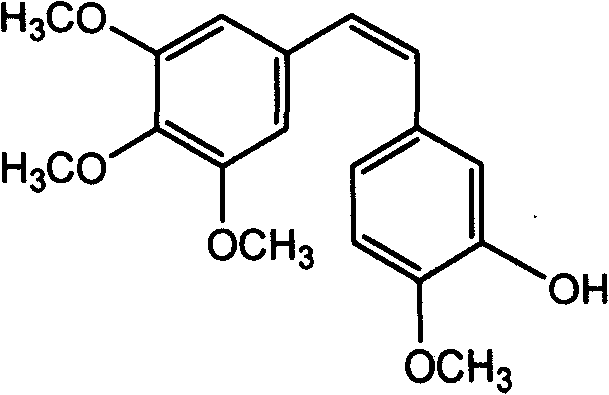
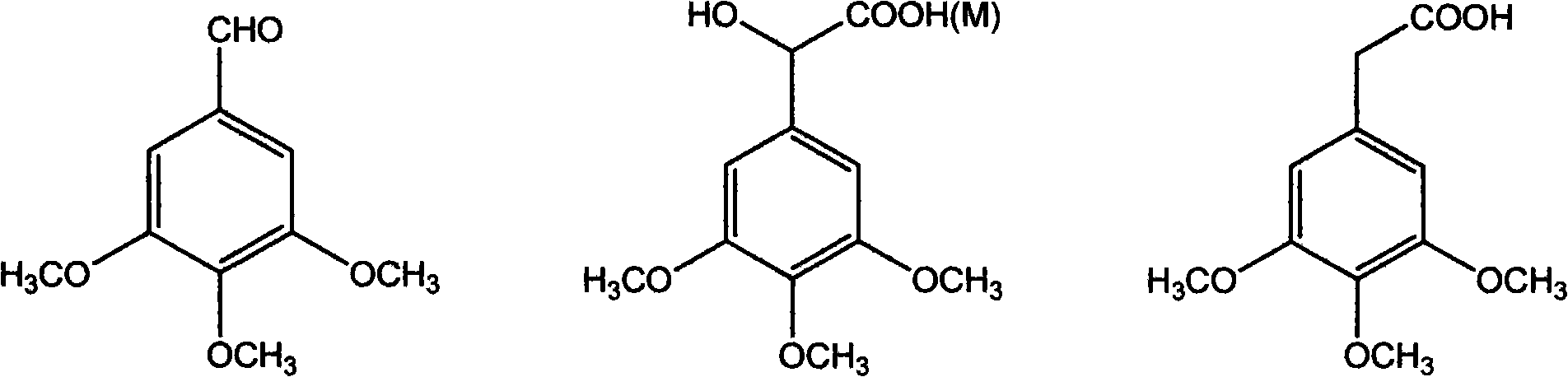
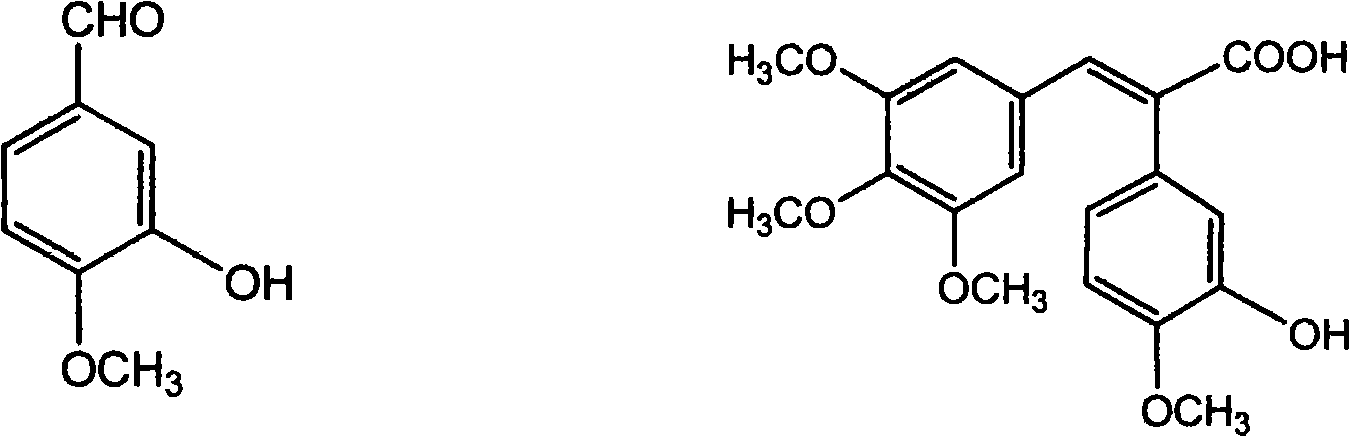
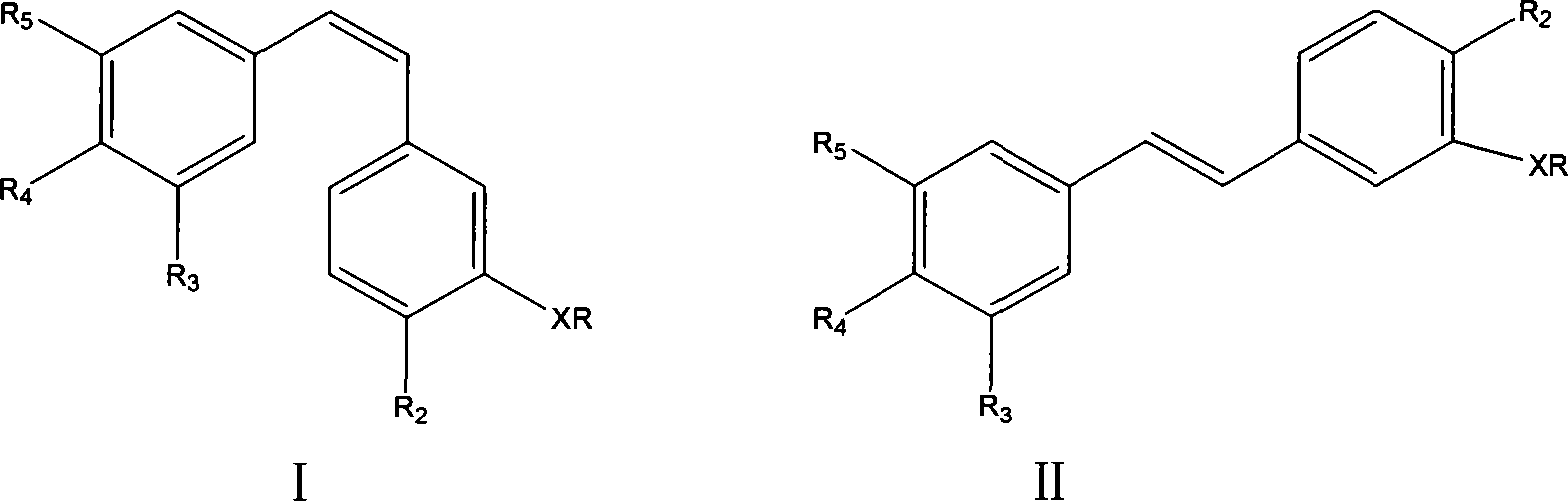
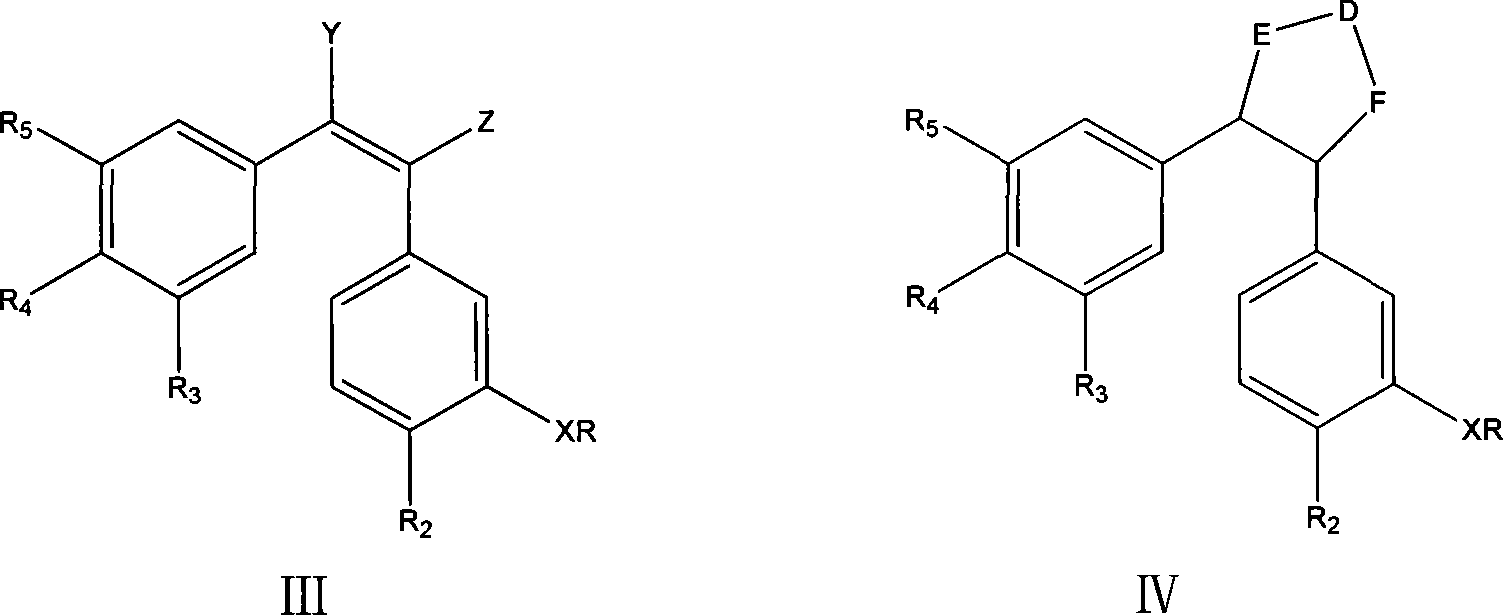
Antineoplastic drug Combretastatin water-soluble derivative and method for making same
InactiveCN101157600AGood water solubilityHigh selectivityEther/acetal active ingredientsPharmaceutical non-active ingredientsChemical industryLipid formation
The invention discloses water-solubility derivate of the antineoplastic medicine Combretastatin and the corresponding preparing method, and belongs to the technical field of medical and chemical industry. The compound of the invention takes substituted phenylacetic acid and isovanillin or the substitutional isovanillin as the initial material, and by condensation obtains (E)-substituted styrene acid; after that decorates a target group by amphotericity multi-polymer micromolecule with ether linkage, and finally, obtains water-solubility antineoplastic medicine Combretastatin derivate decorated by amphotericity multi-polymer micromolecule through decarboxylation or further decorating alkene linkage. The water-solubility is remarkably improved compared with the Combretastatin and the derivates before decoration, and basically maintains the intrinsic lipid solubility. Apart from easy material obtaining, the invention has the advantages of short synthesis line, simple operation and high yield as well as possessing strong selectivity to the cis-product with activity.
Owner:SHANGHAI JIAO TONG UNIV
Process for preparing erianin
ActiveUS7897820B2Easy to getOrganic compound preparationCarbonyl compound preparationPhosphonium saltBenzaldehyde
A process for preparing Erianin (Dihydro Combretastation A-4), wherein 3,4,5-trimethoxy benzaldehyde is converted to phosphonium salt or phosphonate ester or the likes thereof, then reacted with isovanillin (3-hydroxyl-4-methoxyl benzaldehyde) including a protected hydroxyl in the 3-position, followed by hydrogenation and deprotection.
Owner:ZHEJIANG TIANHUANG MEDICINAL PLANT PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com