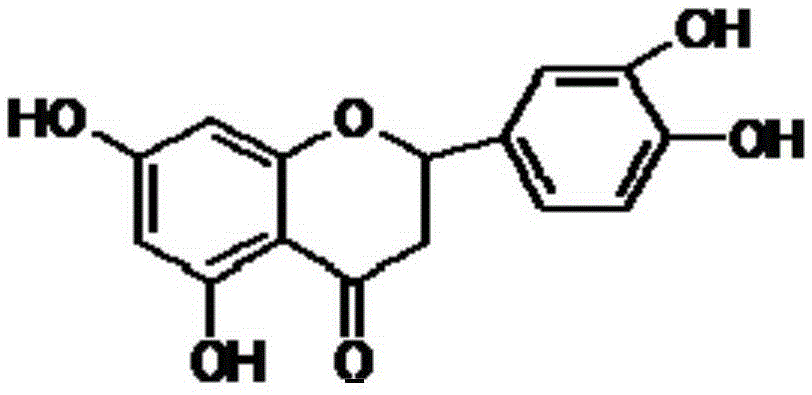
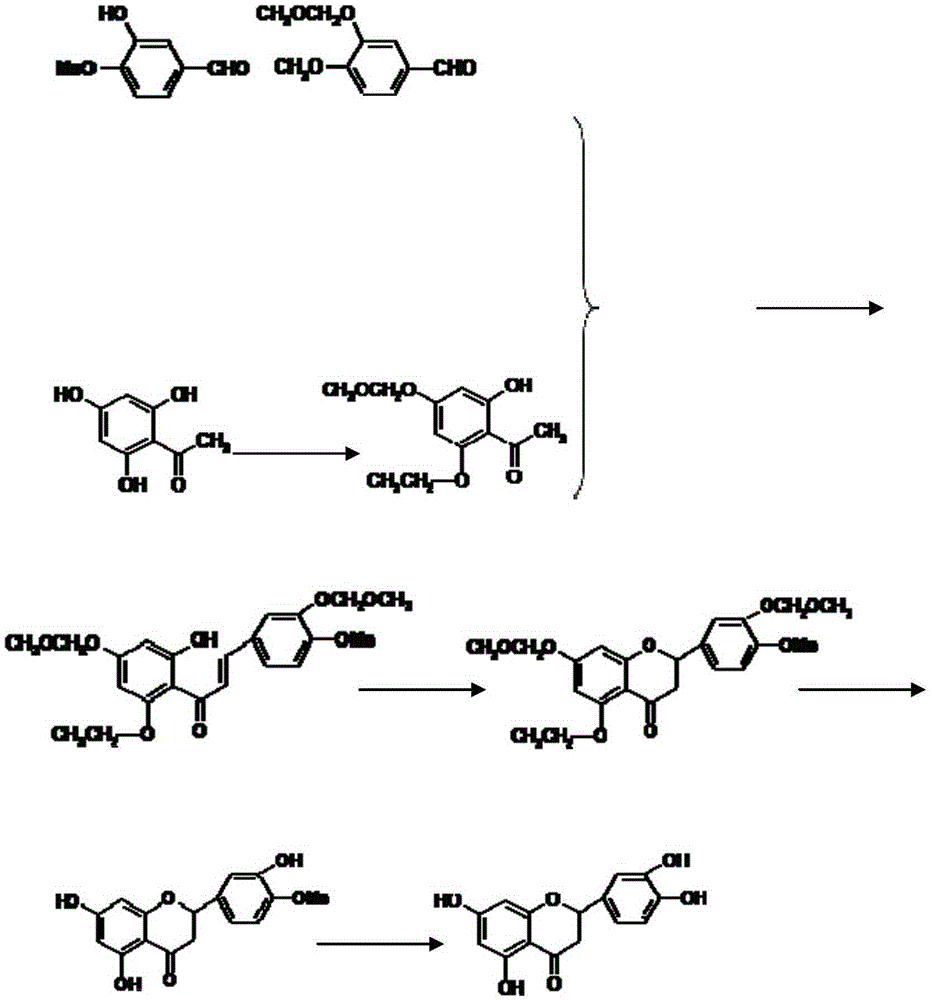
Eriodictyol synthesis method
A synthesis method and technology for eriodictyol, applied in the biological field, can solve the problems of complex process, no technical documents for the total synthesis of eriodictyol, and high cost, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Step 1 Add 15.2 g of isovanillin, 0.54 g of sodium methoxide, and 100 mL of acetone into a 1000 mL round bottom flask, stir at room temperature for 1 h, then add 18 g of dimethyl carbonate, and continue stirring for 4 h. Add 100 mL of water, extract three times with 300 mL of ethyl acetate, combine the organic layers, anhydrous Na 2 SO 4 After drying for 30 minutes, the ethyl acetate was distilled off under reduced pressure to obtain crude 3-methoxymethoxy-4-methoxybenzaldehyde as a yellow-green solid with a yield of 86.4%.
[0027] Step 2 Add 2,4,6-trihydroxyacetophenone 18.2g in a 1000ml round bottom flask, K 2 CO 3 82.8g, acetone 100mL, stirred at room temperature for 1h, then added MOMCl22.8ml, continued stirring for 4h, added water 100mL, extracted with ethyl acetate 300ml, combined organic layers, anhydrous Na 2 SO 4 After drying for 30 min, ethyl acetate was distilled off under reduced pressure to obtain crude 2-hydroxy-4-methoxymethoxy-6-ethoxyacetophenone a...
Embodiment 2
[0032] Step 1 Add 30.4g of isovanillin, 1.08g of sodium methoxide and 200mL of acetone into a 1000mL round bottom flask, stir at room temperature for 1h, then add 36g of dimethyl carbonate, and continue stirring for 4h. Add 200mL of water, extract three times with 600ml of ethyl acetate, and combine Organic layer, anhydrous Na 2 SO 4 After drying for 30 min, the ethyl acetate was distilled off under reduced pressure to obtain the crude product 3-methoxymethoxy-4-methoxybenzaldehyde as a yellow-green solid with a yield of 87%.
[0033]Step 2 Add 2,4,6-trihydroxyacetophenone 36.4g in a 1000ml round bottom flask, K 2 CO 3 165g, acetone 100mL, stirred at room temperature for 1h, then added MOMCl46ml, continued to stir for 4h, added water 200mL, extracted with ethyl acetate 500ml, combined organic layers, anhydrous Na 2 SO 4 After drying for 30 minutes, ethyl acetate was distilled off under reduced pressure to obtain 28 g of crude 2-hydroxy-4-methoxymethoxy-6-ethoxyacetophenone...
Embodiment 3
[0038] Step 1: Add 45g of isovanillin, 1.5g of sodium methoxide and 300mL of acetone into a 2000mL round bottom flask, stir at room temperature for 1h, then add 54g of dimethyl carbonate, and continue stirring for 4h. Add 300mL of water, extract three times with 900ml of ethyl acetate, combine organic layer, anhydrous Na 2 SO 4 Dry for 30 minutes, distill off the ethyl acetate under reduced pressure to obtain 40 g of the crude product 3-methoxymethoxy-4-methoxybenzaldehyde as a yellow-green solid, with a yield of 85%.
[0039] Step 2 Add 2,4,6-trihydroxyacetophenone 54g in a 2000ml round bottom flask, K 2 CO 3 250g, acetone 300mL, stirred at room temperature for 1h, then added MOMCl70ml, continued to stir for 4h, added water 300mL, extracted with ethyl acetate 900ml, combined organic layers, anhydrous Na 2 SO 4 After drying for 30 minutes, the ethyl acetate was distilled off under reduced pressure to obtain 38.34 g of crude 2-hydroxy-4-methoxymethoxy-6-ethoxyacetophenone a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com