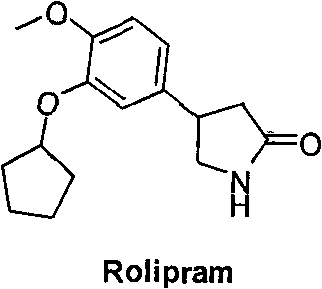
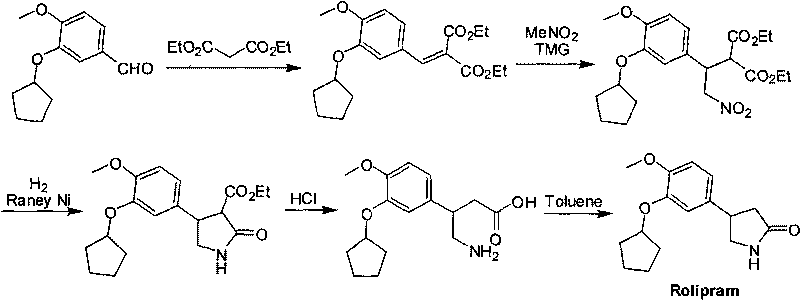
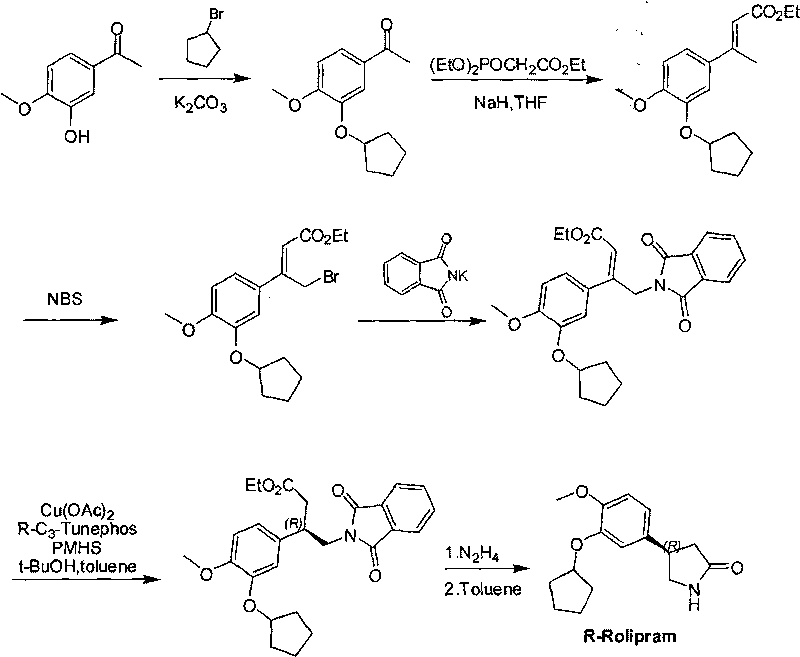
Synthetic method of R-structured Rolipram
A synthetic method and compound technology, applied in the direction of organic chemistry, can solve problems such as difficult industrialization, and achieve the effect of simple post-processing, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Step: in the 100ml there-necked flask, add dichloromethane (60ml), then (E)-2-(cyclopentyloxy)-1-methoxyl group-4-(2-nitrovinyl)benzene ( 10 g, 0.043 mol) and diethyl malonate (8.3 g, 0.052 mol) were added. Under nitrogen atmosphere, add chiral catalyst nickel(II) bromide-bis[(S,S)-N,N'-dibenzylcyclohexyl-1,2-diamine] complex (0.19mg, 0.2mmol) , Reacted for 6 hours at 0 degrees. The solvent was removed under reduced pressure to obtain 14.5 g of white solid (yield: 85.29%). Melting point 94-96 ℃; NMR spectrum (500MHz, CDCl3) δH 6.78 (d, J=8.0, 1H, Ar-H), 6.74-6.70 (m, 2H, Ar-H), 4.87 (dd, J= 13.0, 5.2, 1H, CHAHB-NO2), 4.82 (dd, J=13.0, 9.0, 1H, CHAHB-NO2), 4.73 (app.qd, J=9.3, 3.0, 1H, OCH(CH2)2), 4.15 (app.dt, J=9.0, 5.2, 1H, Ar-*CH), 3.84 (d, J=9.0, 1H, CH-*C), 3.80(s, 3H, OCH3), 3.75(s, 3H, OCH3), 3.57(s, 3H, OCH3), 1.97-1.88(m, 2H, cyclopentyl-H), 1.87-1.76(m, 4H, cyclopentyl-H), 1.65-1.56(m, 2H, cyclopentyl-H ); mass spectrum m / z (CI+) 413 (100%, MNH4+). Optic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com