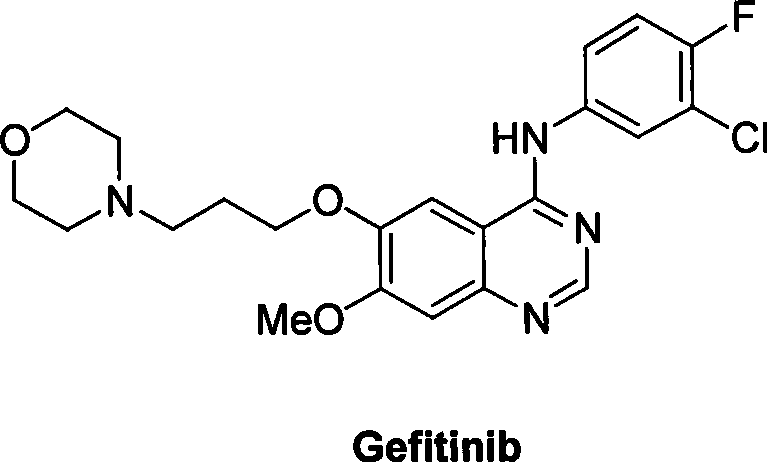
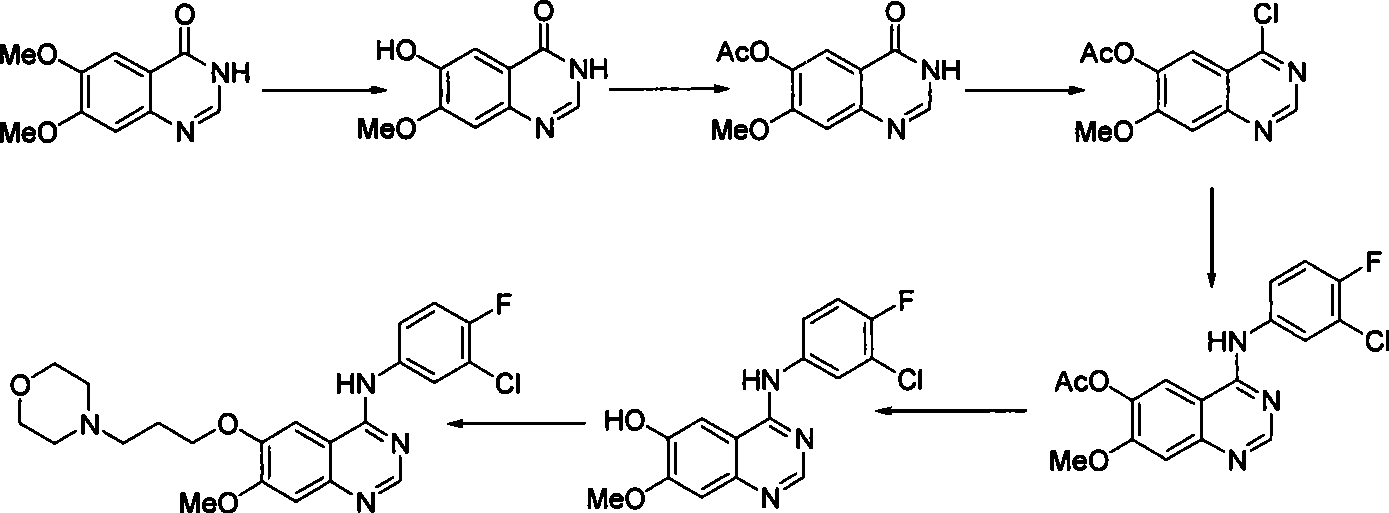
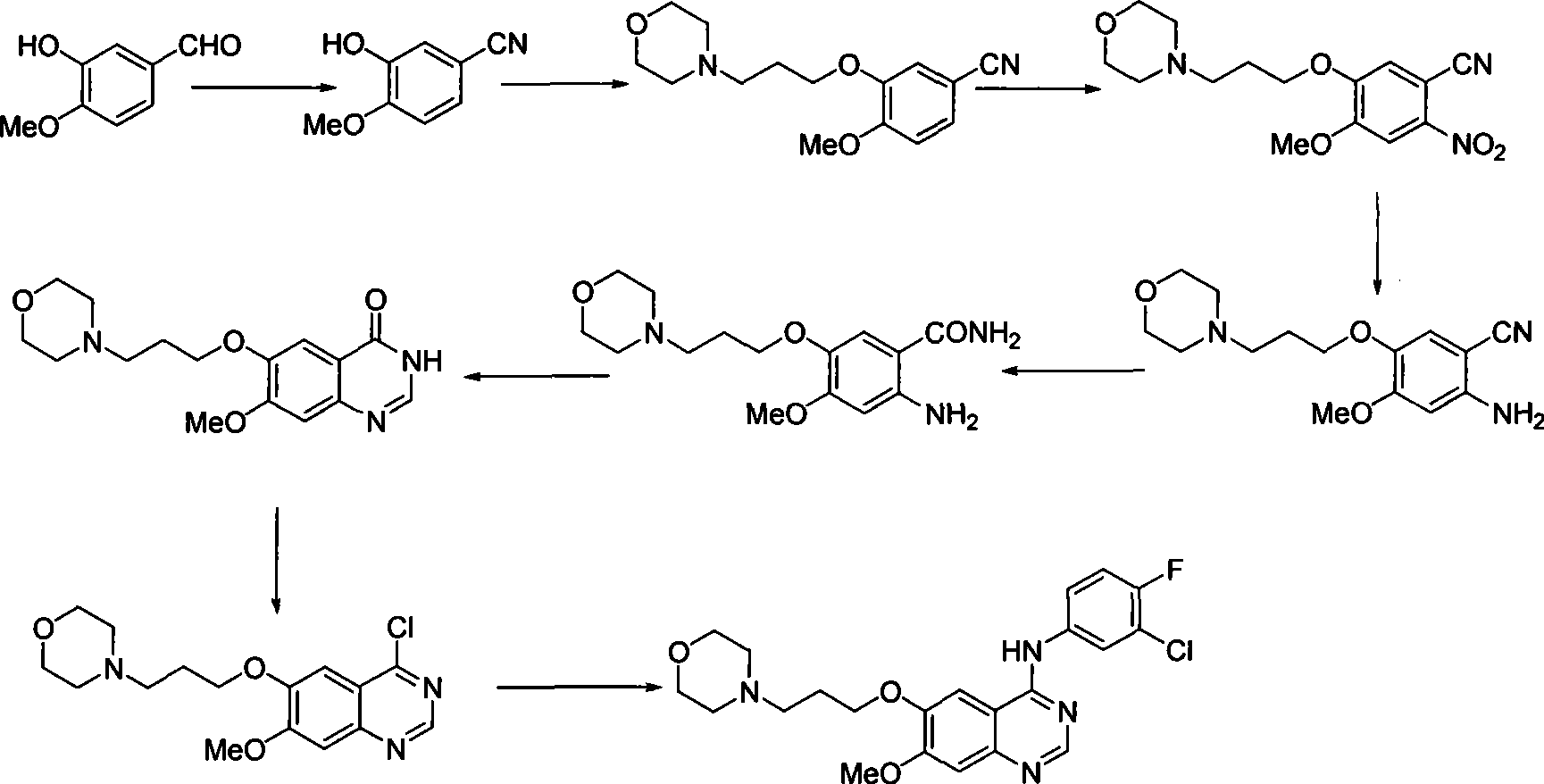
Preparation of gefitinib
A technology of gefitinib and compounds, applied in the field of preparation of 4--7-methoxy-6-quinazoline, which can solve the problems of many reaction by-products, difficult control of cyano hydrolysis, unavoidable by-products, etc. , to avoid excessive hydrolysis, shorten the synthesis route, and achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] ①Synthesis of Compound 1
[0042] Add 5L double-distilled toluene to a 10L four-neck bottle, add 1kg of isovanillin, 500g of hydroxylamine hydrochloride, 160g of p-toluenesulfonic acid and 5kg of anhydrous magnesium sulfate under stirring, heat and reflux for 6 hours, cool to room temperature, filter, and use as filter cake The hot ethyl acetate was extracted to TLC to detect no product, combined, washed with water, washed with saturated brine, dried, concentrated until a large amount of product precipitated, added 2.5L petroleum ether, suction filtered, washed with a small amount of petroleum ether, and dried to obtain about 750g of the product.
[0043] ② Synthesis of Compound 2
[0044] Add compound 1 (1kg), 2kg of 3-morpholine-1-chloropropane, 3kg of potassium carbonate, 500g of tetrabutylammonium iodide and 5 liters of DMSO into a 10-liter four-neck flask, heat to reflux, and after TLC detects that the reaction is complete, cool , poured into 10 liters of ice wate...
Embodiment 2
[0056] ①Synthesis of Compound 1
[0057] Add 5L double-distilled toluene to a 10L four-neck bottle, add 1kg of isovanillin, 500g of hydroxylamine hydrochloride, 160g of p-toluenesulfonic acid and 4kg of anhydrous sodium sulfate under stirring, heat and reflux for 16 hours, cool to room temperature, filter, and use as filter cake The hot ethyl acetate was extracted to TLC to detect no product, combined, washed with water, washed with saturated brine, dried, concentrated until a large amount of product precipitated, added 2.5L petroleum ether, suction filtered, washed with a small amount of petroleum ether, and dried to obtain about 800g of product.
[0058] ② Synthesis of compound 2
[0059] Add compound 1 (1kg), 2kg of 3-morpholine-1-chloropropane, 3kg of potassium carbonate, 100g of tetrabutylammonium bromide and 5 liters of DMF into a 10-liter four-neck flask, heat to reflux, and after TLC detects that the reaction is complete, cool , poured into 10 liters of ice water, extra...
Embodiment 3
[0071] ①Synthesis of Compound 1
[0072] Add 5L double-distilled benzene into a 10L four-necked bottle, add 1kg of isovanillin, 500g of hydroxylamine hydrochloride, 160g of p-toluenesulfonic acid and 5kg of anhydrous magnesium sulfate under stirring, heat and reflux for 10 hours, cool to room temperature, filter, and use as filter cake The hot ethyl acetate was extracted to TLC to detect no product, combined, washed with water, washed with saturated brine, dried, concentrated until a large amount of product precipitated, added 2.5L petroleum ether, suction filtered, washed with a small amount of petroleum ether, and dried to obtain about 780g of the product.
[0073] ② Synthesis of compound 2
[0074] Add compound 1 (1kg), 3kg of 3-morpholine-1-chloropropane, 2kg of potassium carbonate, 250g of tetrabutylammonium bromide and 5 liters of DMSO into a 10-liter four-neck flask, heat to reflux, and after TLC detects that the reaction is complete, cool , poured into 10 liters of ic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com