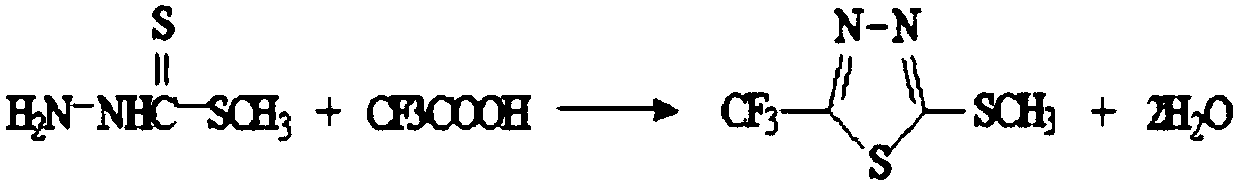
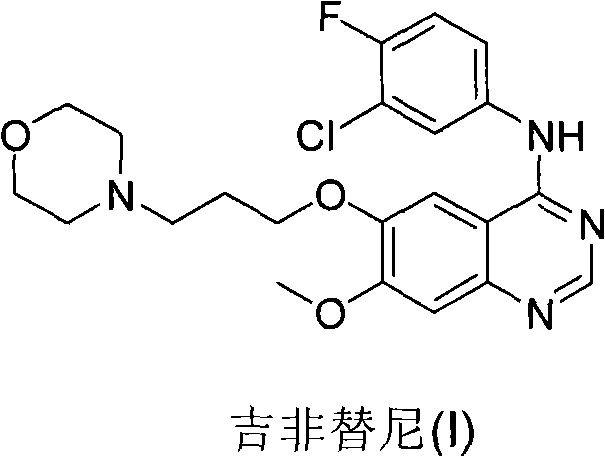
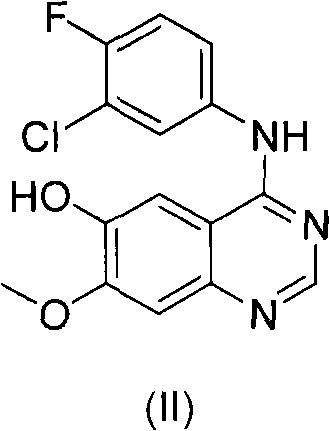
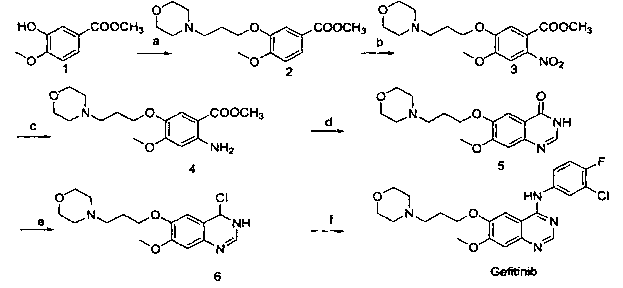
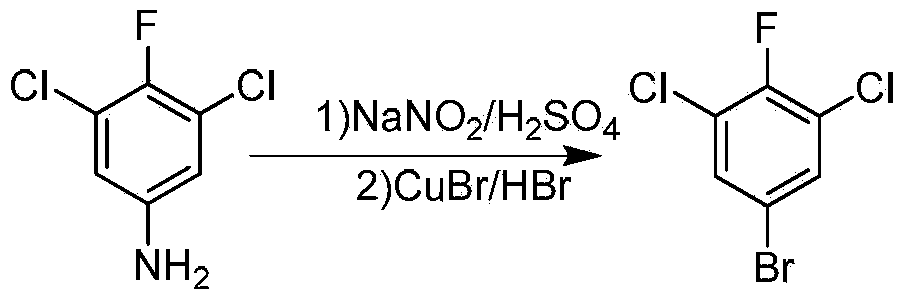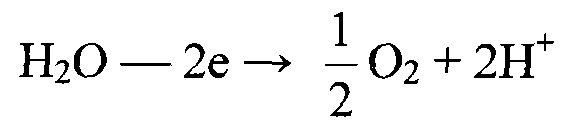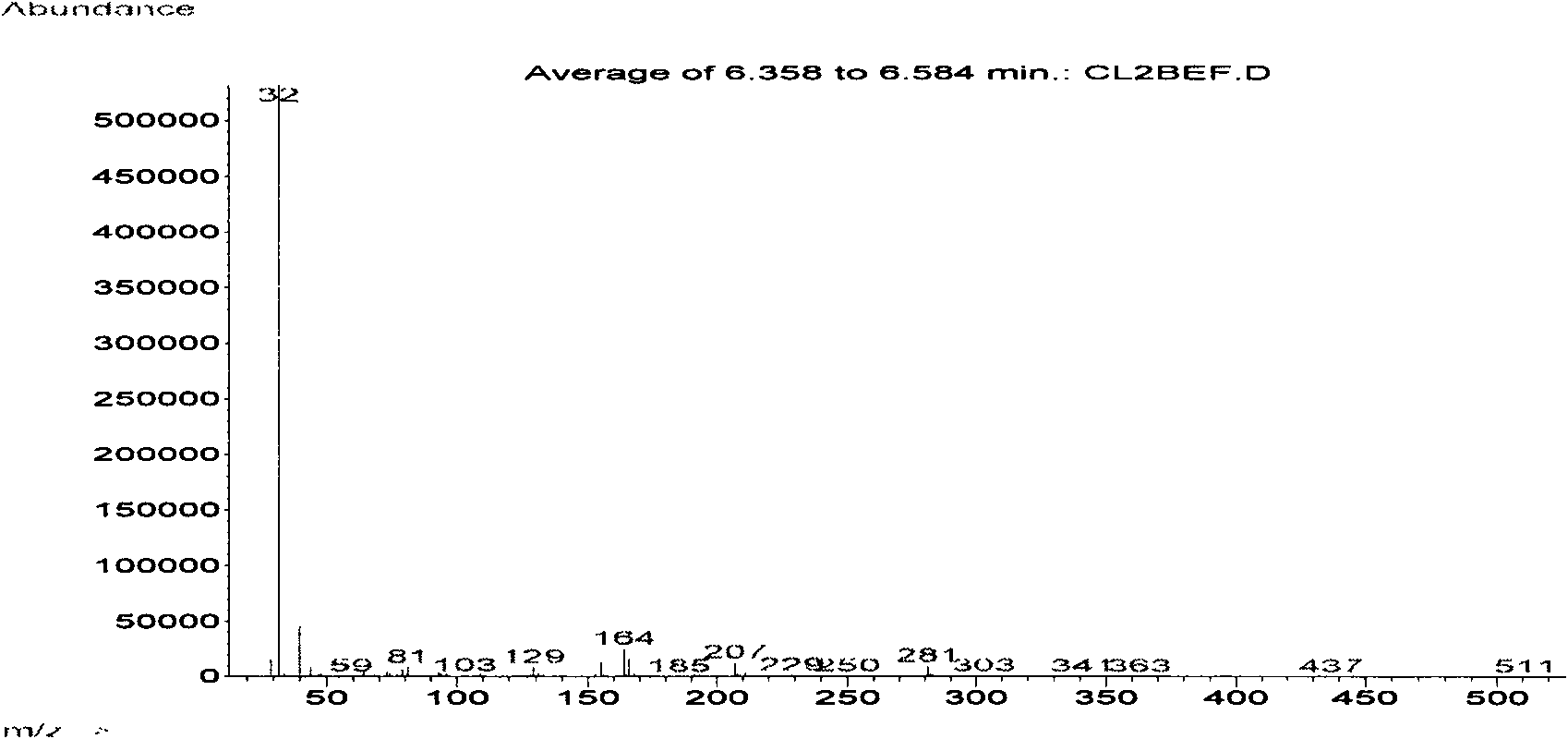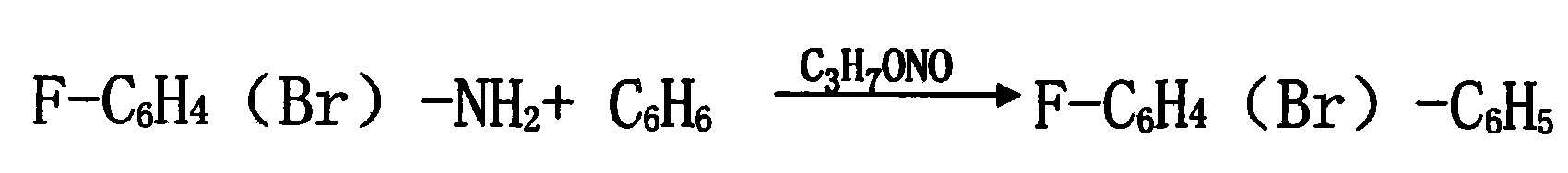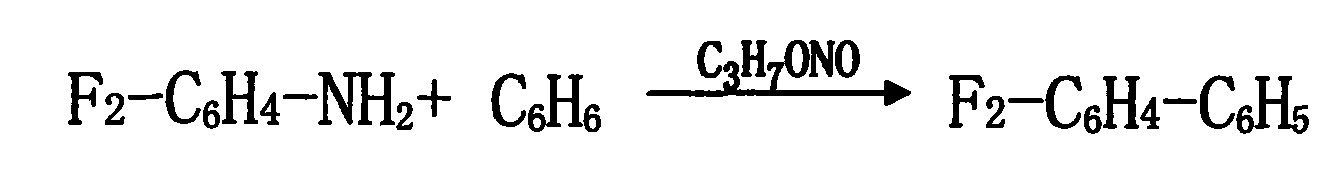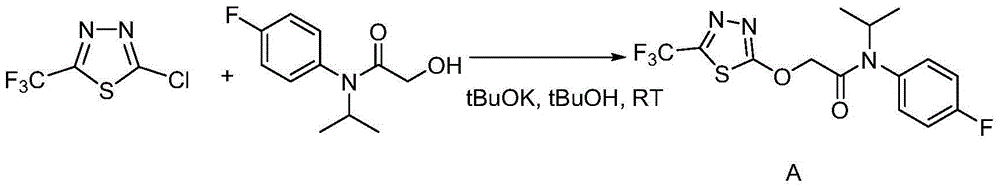Patents
Literature
78 results about "4-fluoroaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
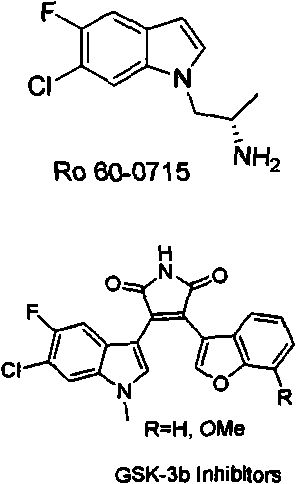
4-Fluoroaniline 99% Synonym: 1-Amino-4-fluorobenzene CAS Number 371-40-4. Linear Formula FC 6 H 4 NH 2. Molecular Weight 111.12 . Beilstein Registry Number 742030 . EC Number 206-735-5. MDL number MFCD00007829. PubChem Substance ID 24894858
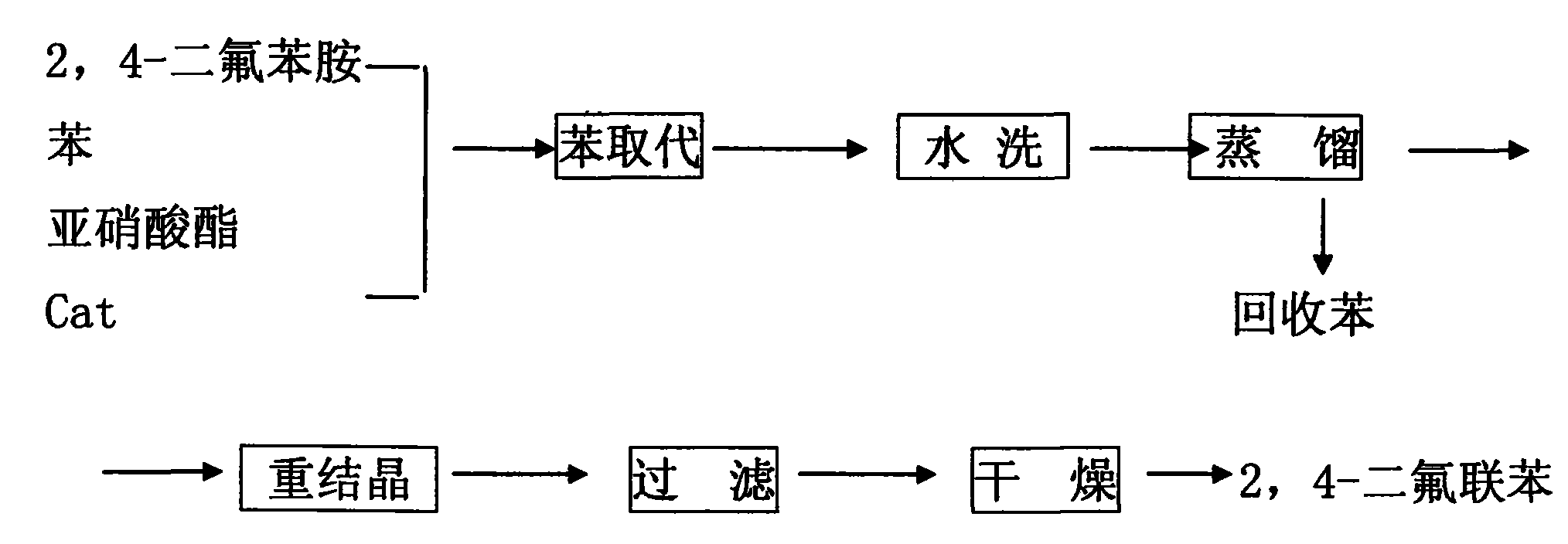
Preparation method of 5-bromo-1,3-dichloro-2-fluorobenzene
ActiveCN103664511AEasy to operateHigh purityHalogenated hydrocarbon preparationDecompositionSide reaction
The invention discloses a preparation method of 5-bromo-1,3-dichloro-2-fluorobenzene, which comprises the following steps: (1) simultaneously feeding ammonium salt and a sodium nitrite aqueous solution into a tubular reactor for tubular diazotization reaction so as to obtain a diazonium salt intermediate, wherein the ammonium salt is prepared through dissolving 3,5-dichloro-4-fluoroaniline in sulfuric acid; (2) dissolving cuprous bromide in hydrobromic acid, heating to 100-130 DEG C, dropwise adding the diazonium salt intermediate prepared in the step (1) for reaction, and after the complete reaction, performing after-treatment to obtain the 5-bromo-1,3-dichloro-2-fluorobenzene. According to the preparation method, the tubular diazotization reaction technology is adopted to prepare diazonium salt, so that the side reaction including diazonium salt coupling and decomposition and the like can be reduced, the diazotization reaction is enabled to be more stable, and the final yield is improved; in addition, the tubular diazotization reaction technology has a series of advantages of continuity in production, safety, short reaction time, energy saving and the like, and is relatively suitable for industrial production in the future.
Owner:ZHEJIANG LINJIANG CHEM
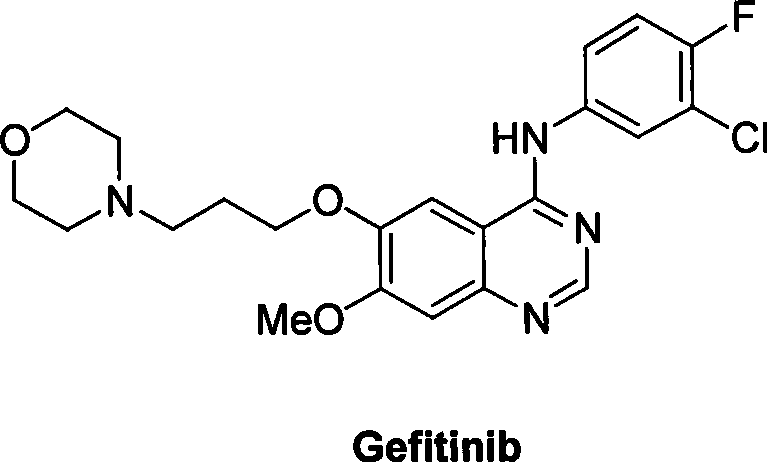
Preparation method of gefitinib
ActiveCN103570633AReduce usageFor the purpose of purificationOrganic chemistryPurification methodsMorpholine
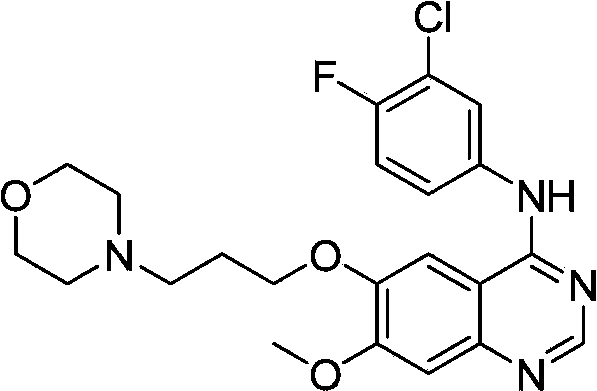
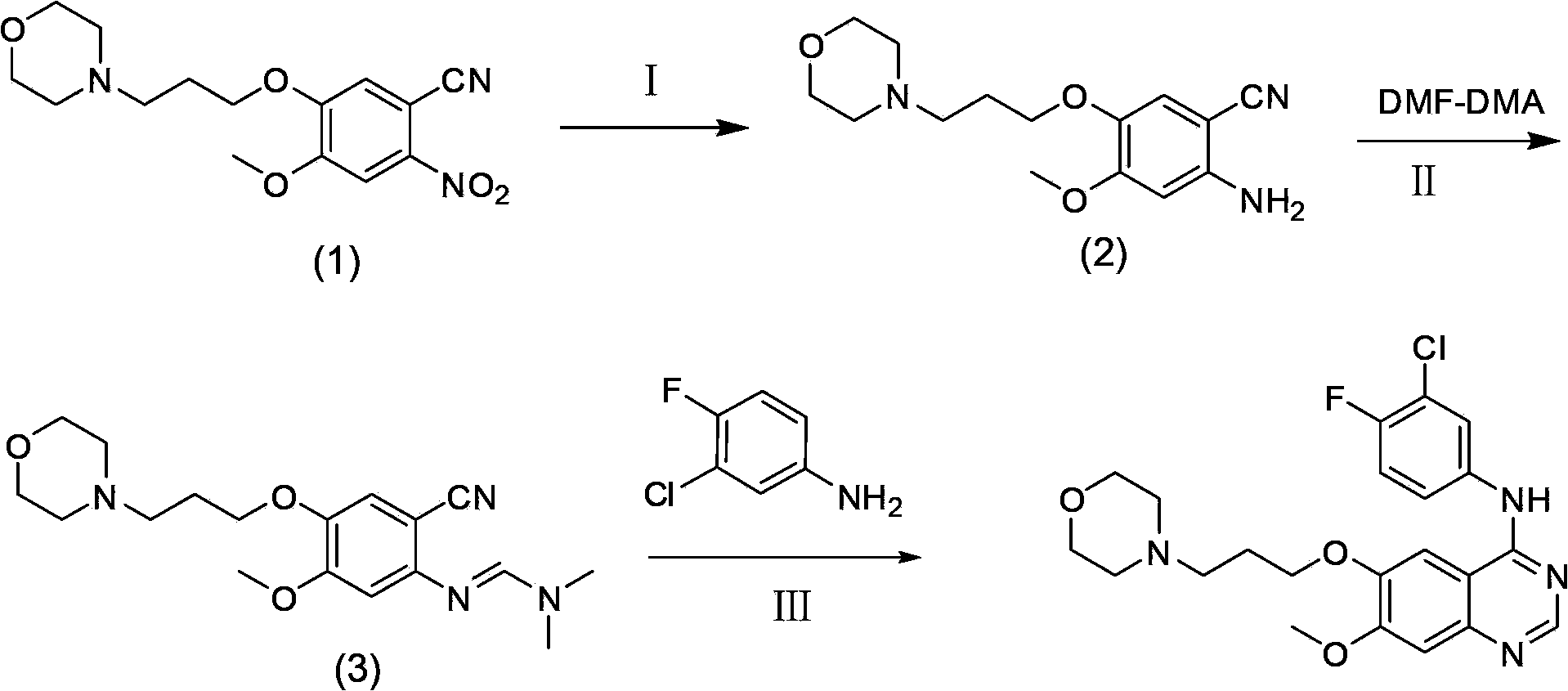
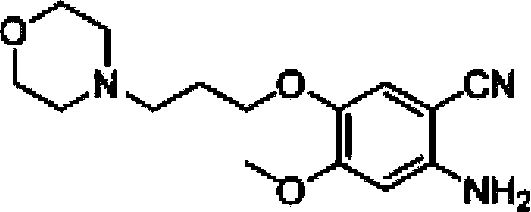
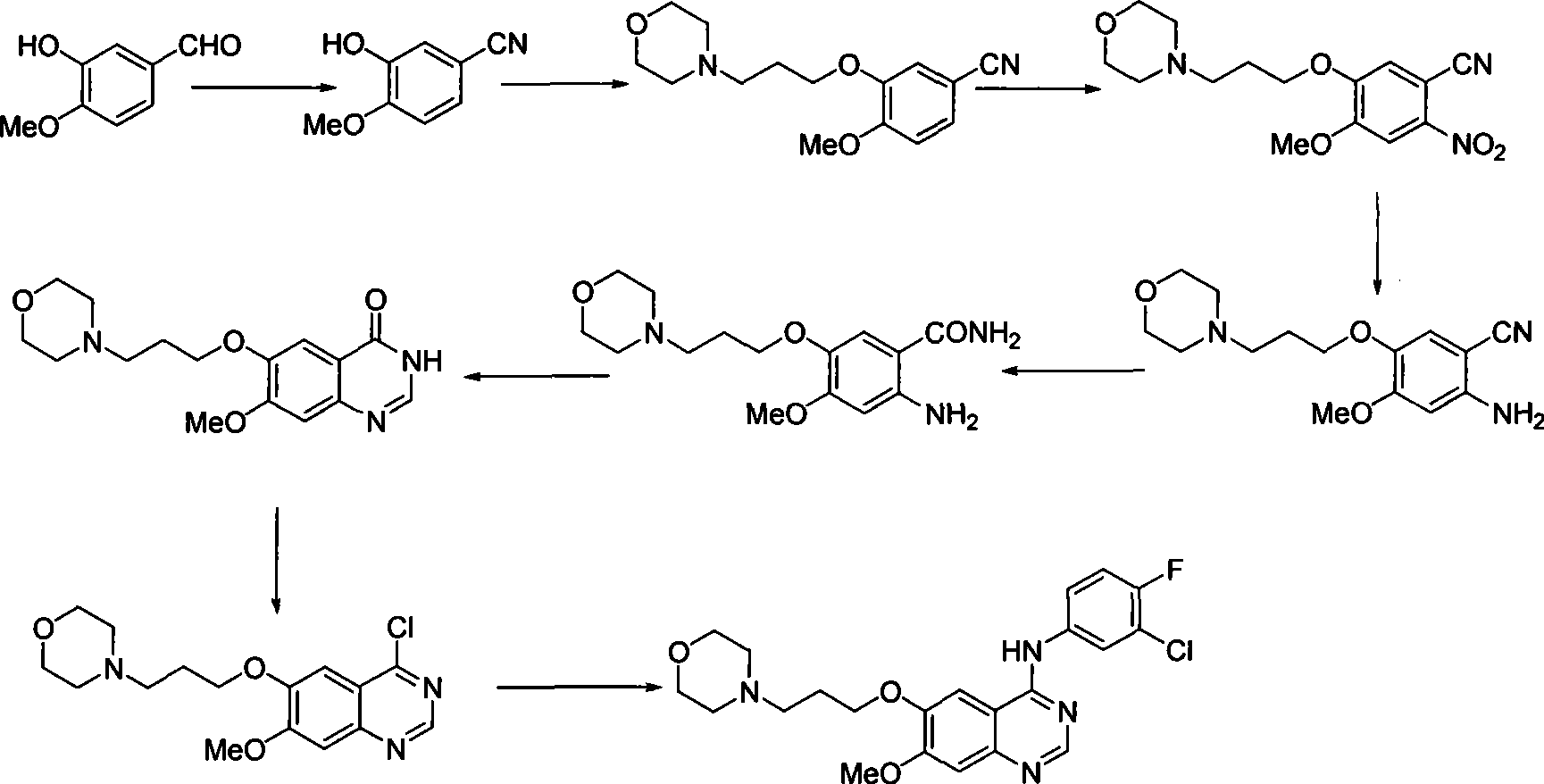
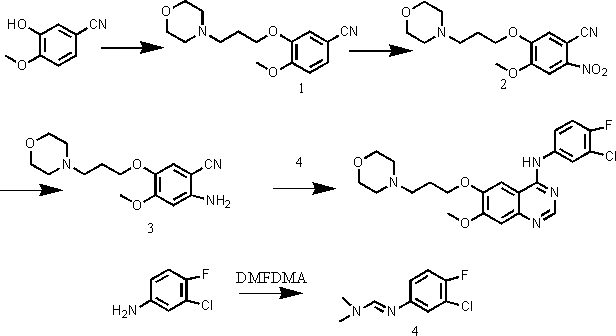
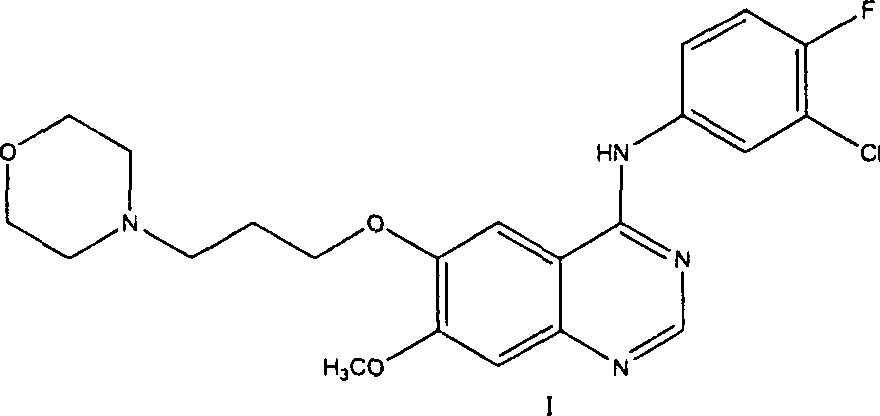
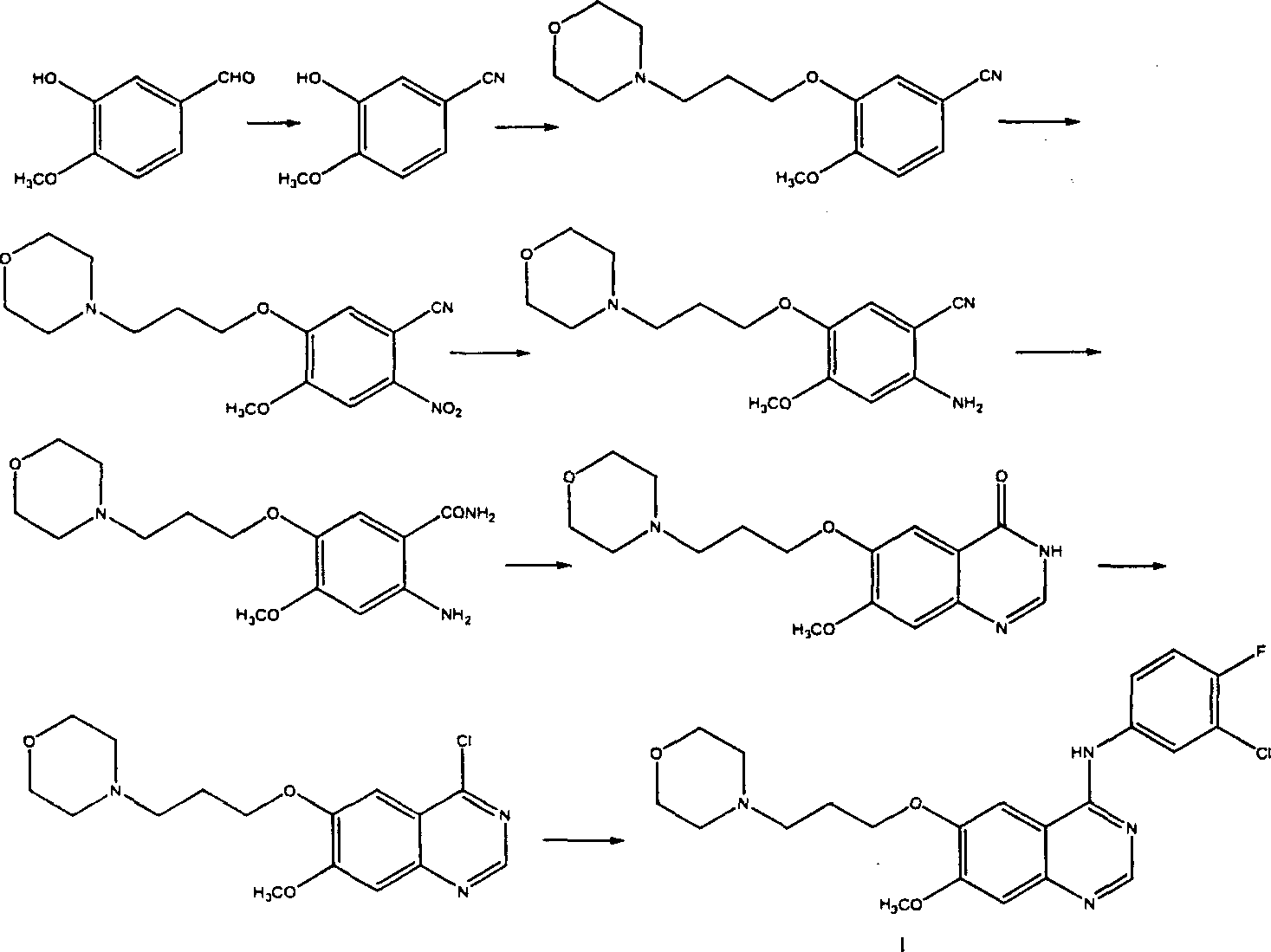
The invention discloses a preparation method of gefitinib. The preparation method takes 4-methoxyl-5-(3-morpholine propoxyl)-2-nitrobenzonitrile as a raw material, then subjecting the raw material to treatments of reduction and salt forming reactions so as to obtain an intermediate 2-amino-4-methoxyl-5-(3-morpholine propoxyl) benzonitrile hydrochloride, then directly subjecting the intermediate to react with N,N-dimethyl formamide dimethyl acetal so as to obtain N'-(2-cyano-5-methoxyl-4-(3-morpholinyl propoxyl)benzyl)-N,N-dimethyl formamidine, and finally subjecting the formamidine intermediate to carry out rearrangement reactions with 3-chloro-4-fluoroaniline so as to obtain the gefitinib. The preparation method has the advantages of mild reaction conditions, convenient intermediate purification method, and suitability for industrial production.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
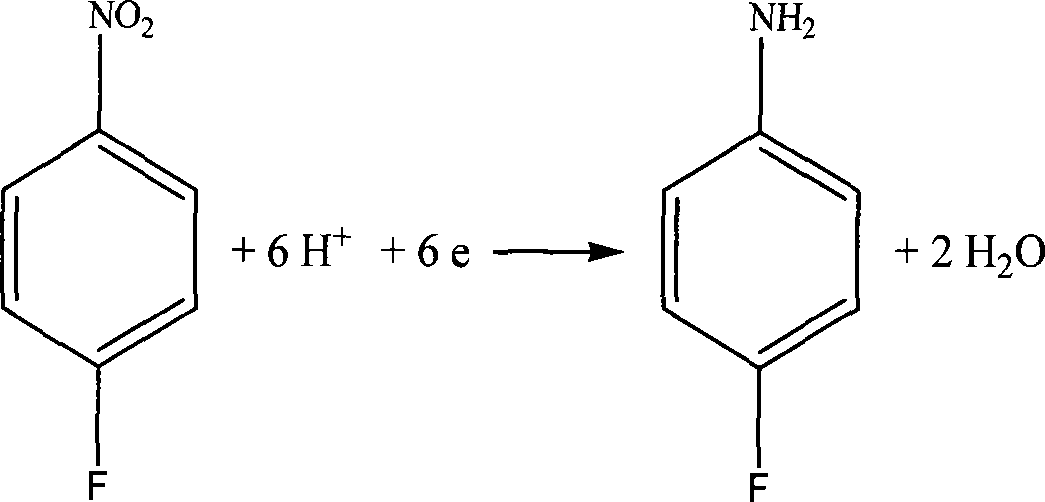
Technical method for synthesizing 4-fluoroaniline by electrochemistry method
ActiveCN101457368ARealize cleaner productionLow costOrganic chemistryElectrolysis componentsElectrochemical responseNitrobenzene
The invention relates to a technical method for electrochemically synthesizing para-fluoroaniline. An electrochemical reaction of the invention occurs in a double-chamber diaphragm electrolytic cell, and an anode chamber and a cathode chamber of the electrolytic cell are separated by a cation exchange membrane. An anode is made of such anode materials as graphite, DSA or lead and the like; and a cathode is made of such materials as platinum, silver, copper, lead, nickel, a copper nickel alloy, a copper amalgam alloy, carbon steel, stainless steel, the graphite, glassy carbon and the like. Catholyte consists of 1-20% of nitrobenzene, 1-30% of water and ionic liquid; and anolyte is 5-50% of sulfuric acid or other acidic solution, alkali solutions and salt solutions. An electrolysis product of the cathode is the para-fluoroaniline. The technical method for synthesizing the para-fluoroaniline in the ionic liquid has the advantages of simple process, high yield, little pollution and low cost.
Owner:LAVIANA TAIZHOU PHARMACHEM
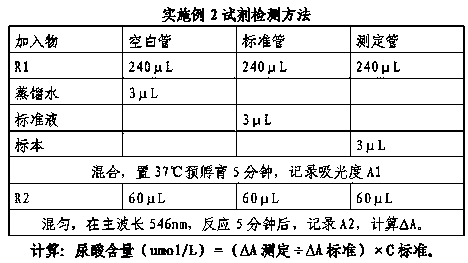
Enzymic uric acid detection agent with high interference preventing capacity
ActiveCN108287233AGuaranteed cushioning effectGuaranteed not to interfere with the responseBiological testingAmpyroneBetaine
The invention relates to an enzymic uric acid detection agent with high interference preventing capacity. The agent is characterized in that an agent R1 includes a buffering solution, 4-ampyrone, sodium nitrite, an ion balancing agent, ascorbic acid oxidase, a heavy metal ion chelator, bovine serum albumin, dodecyl trimethyl betaine, triton-305 and a preservative; an agent R2 includes a bufferingsolution, F-DAOS (N-ethyl-N-(2-hydroxyl-3-sulfopropyl)-3, 5-dimethoxy-4-fluoroaniline), uricase, bovine serum albumin, peroxidase, a preservative, and other components. The agent is high in interference preventing capacity and particularly capable of accurately detecting a sample of a newborn.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
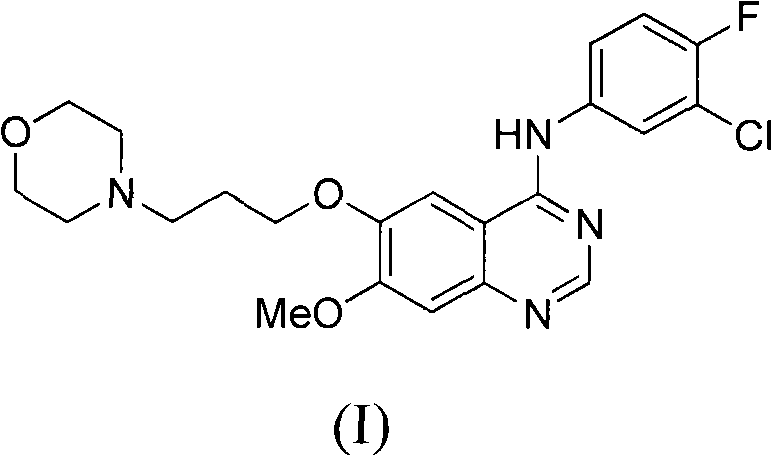
Novel method for preparing 4-(3-chlorine-4-fluorophenylamino)-7-methoxyl-6-(3-morpholinepropoxy)quinazoline
The invention discloses a novel method for preparing 4-(3-chlorine-4-fluorophenylamino)-7-methoxyl-6-(3-morpholinepropoxy)quinazoline (gefitinib, I). The method comprises the following steps of: preparing an intermediate (VI), performing functional group transformation twice under a hydrogenation condition to generate 2-(N,N-dimethylformylimido)-4-methoxyl-5-(3-morpholinepropoxy)cyanophenyl (VIII), and performing ring closure rearrangement on the obtained (VIII) and 3-chlorine-4-fluoroaniline to generate the gefitinib (I). The method is short in steps and high in yield in each step, the intermediate is convenient to purify, a target product has high purity, and the method is suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
Catalyst for continuously preparing N-isopropyl-4-fluoroanilines, and preparation method and application of catalyst
ActiveCN104971740AHigh catalytic activityImprove catalytic stabilityOrganic compound preparationPreparation by reductive alkylationActivated carbonDehydrogenation
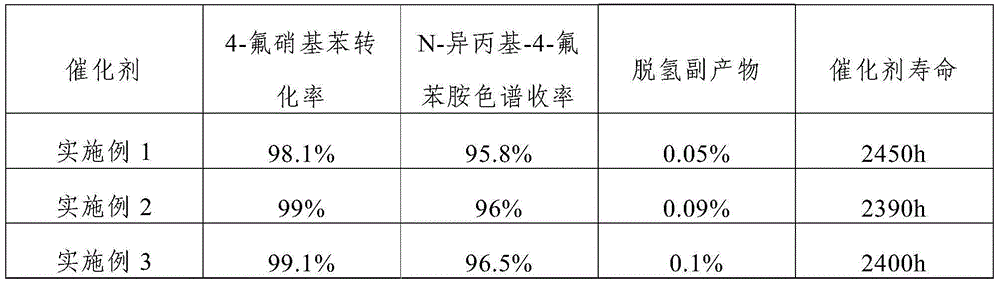
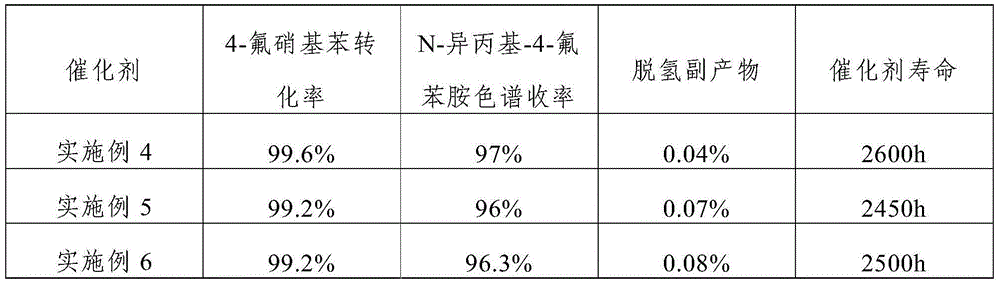
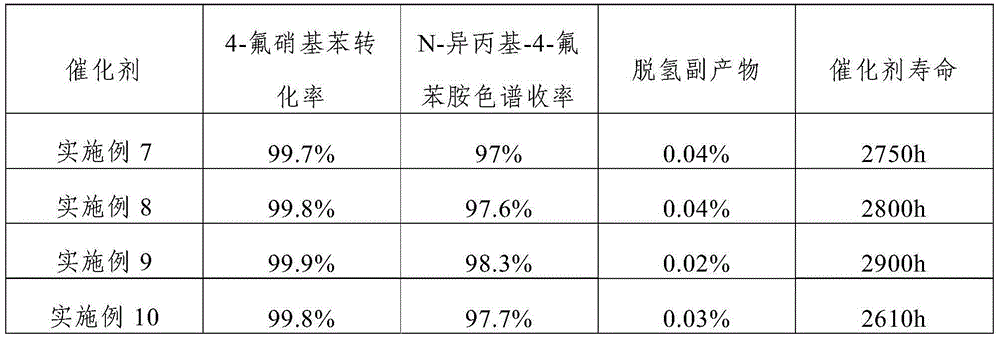
The invention discloses a catalyst for continuously preparing N-isopropyl-4-fluoroanilines. The catalyst comprises an activated carbon carrier, Pt, metal M1 and metal M2, wherein the Pt, metal M1 and metal M2 are loaded on the activated carbon carrier; the Pt accounts for 0.3-1.0wt% of the catalyst; the metal M1 accounts for 0.05-0.5wt% of the catalyst; the metal M2 accounts for 0.05-0.5wt% of the catalyst; the metal M1 is selected from Fe, Cu or Sn; the metal M2 is selected from K, Ce, or Ag. In addition, the invention also discloses a preparation method and application of the catalyst. The catalyst disclosed by the invention has the characteristics of being high in activity, high in selectivity and high in stability and can be used for preparing N-isopropyl-4-fluoroanilines in a high-efficiency catalyzing manner; the conversion ratio of a raw material, namely 4-fluoronitrobenzene, is 98% or more, the yield of a product, namely N-isopropyl-4-fluoroanilines, is 95% or more, and the yield of dehydrogenation by-products is not more than 0.1%.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
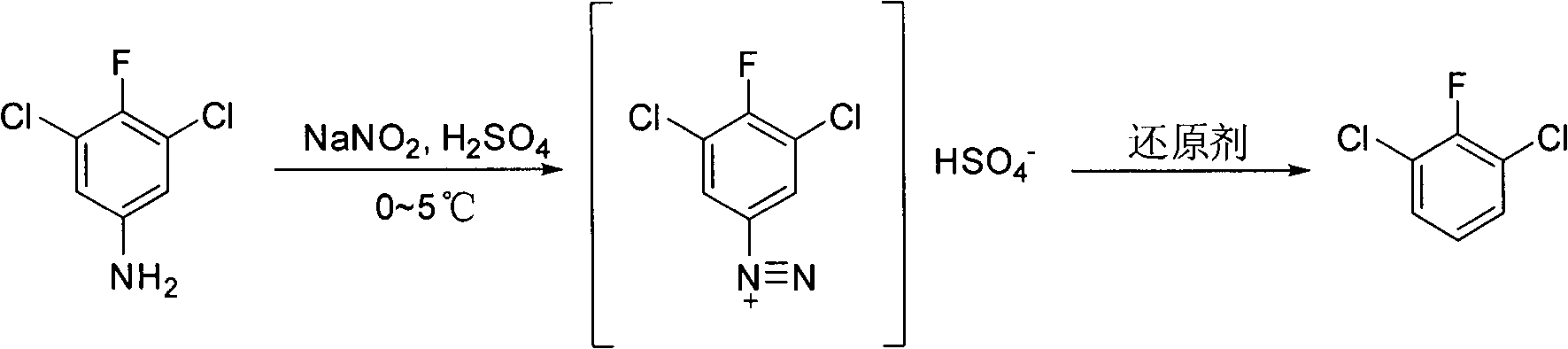
Preparation method of 2, 6-dichlor fluorbenzene
InactiveCN101643386ASolve the use problemWide variety of sourcesHalogenated hydrocarbon preparationWater vaporPotassium iodine
The invention discloses a preparation method of 2, 6-dichlor fluorbenzene, including that diazonium salt is made by taking 3, 5-dichlor-4-fluoroanilines as raw material and the 2, 6-dichlor fluorbenzene is obtained through diazo hydrogen replacement in the presence of reducing agent. The method concretely includes the following steps: (1) 3, 5-dichlor-4-fluorbenzene is taken as raw material and reacts with inorganic acid, so as to obtain solution containing ammonium salt; (2) the solution containing ammonium salt reacts with nitrite aqueous solution, so as to obtain solution containing diazonium salt; (3) reducing agent is dropwise added into the solution containing diazonium salt until reaction product causes starch potassium iodide paper to turn blue; (4) the reaction solution obtained by the step (3) is subject to dispensing, washing and steam distillation, so as to obtain the product 2, 6-dichlor fluorbenzene. The preparation method has the characteristics of simple technology, high yield and low cost.
Owner:ZHEJIANG UNIV
Preparation of gefitinib
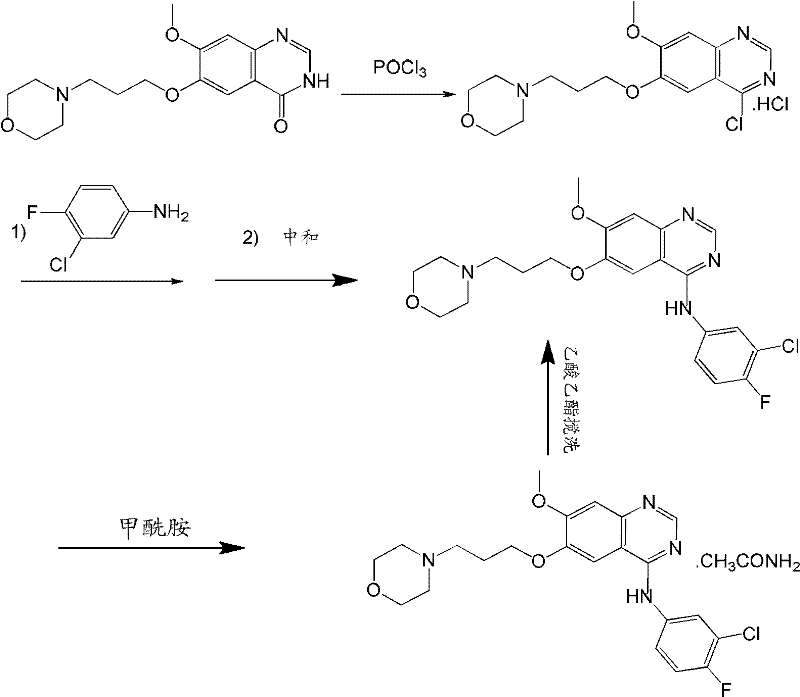
ActiveCN101463012AAvoid the risk of overhydrolysisMild reaction conditionsOrganic chemistryMorpholineQuinazoline
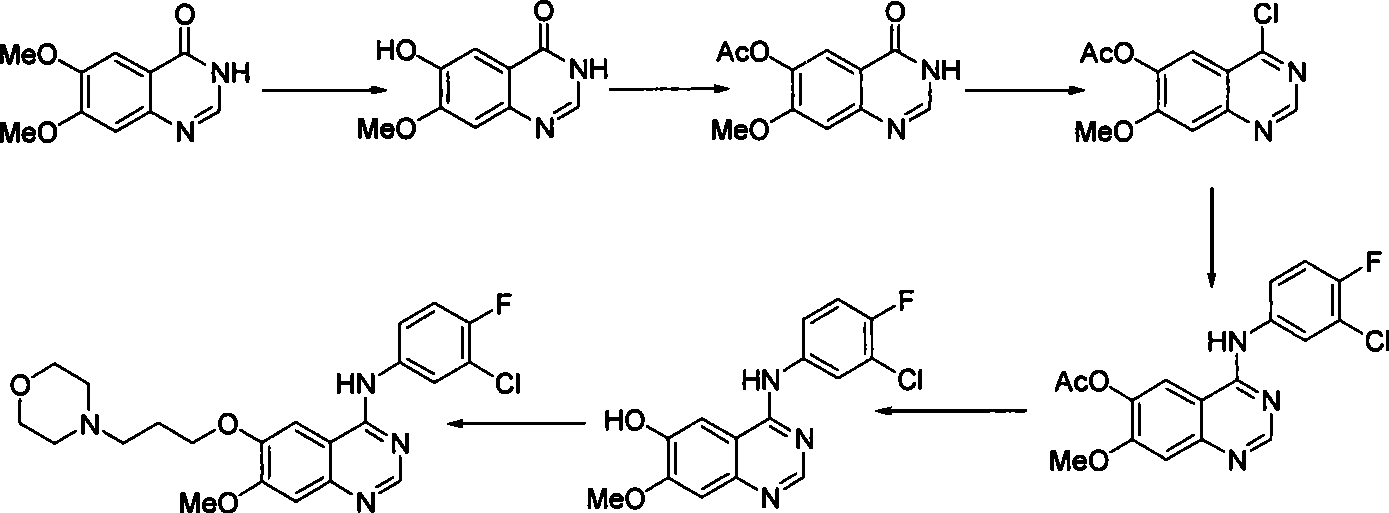
The invention discloses a preparation method of gefitinib. In the method, isovanillin is taken as a raw material and synthesized to obtain 7-methoxy-6-(3-morpholine-propoxy)quinazoline-4-one which is directly chloridized to obtain a product, the product is allowed to react with 3-chlorine-4-fluoroaniline to obtain gefitinib hydrochloride which neutralized off hydrochloric acid to obtain the gefitinib. The method has mild reaction condition and is applicable to industrialized production.
Owner:FUJIAN SOUTH PHARMA CO LTD
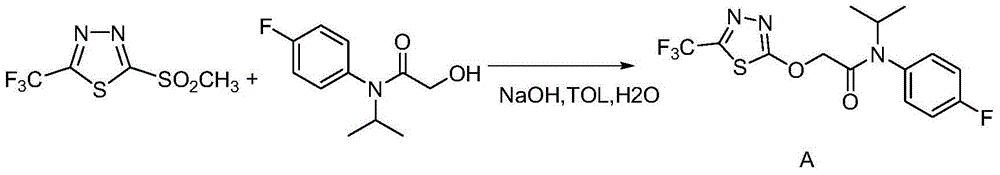
Flufenacet preparation method
InactiveCN107721948AAchieve recyclingReasonable process designOrganic chemistryReaction rateReaction temperature
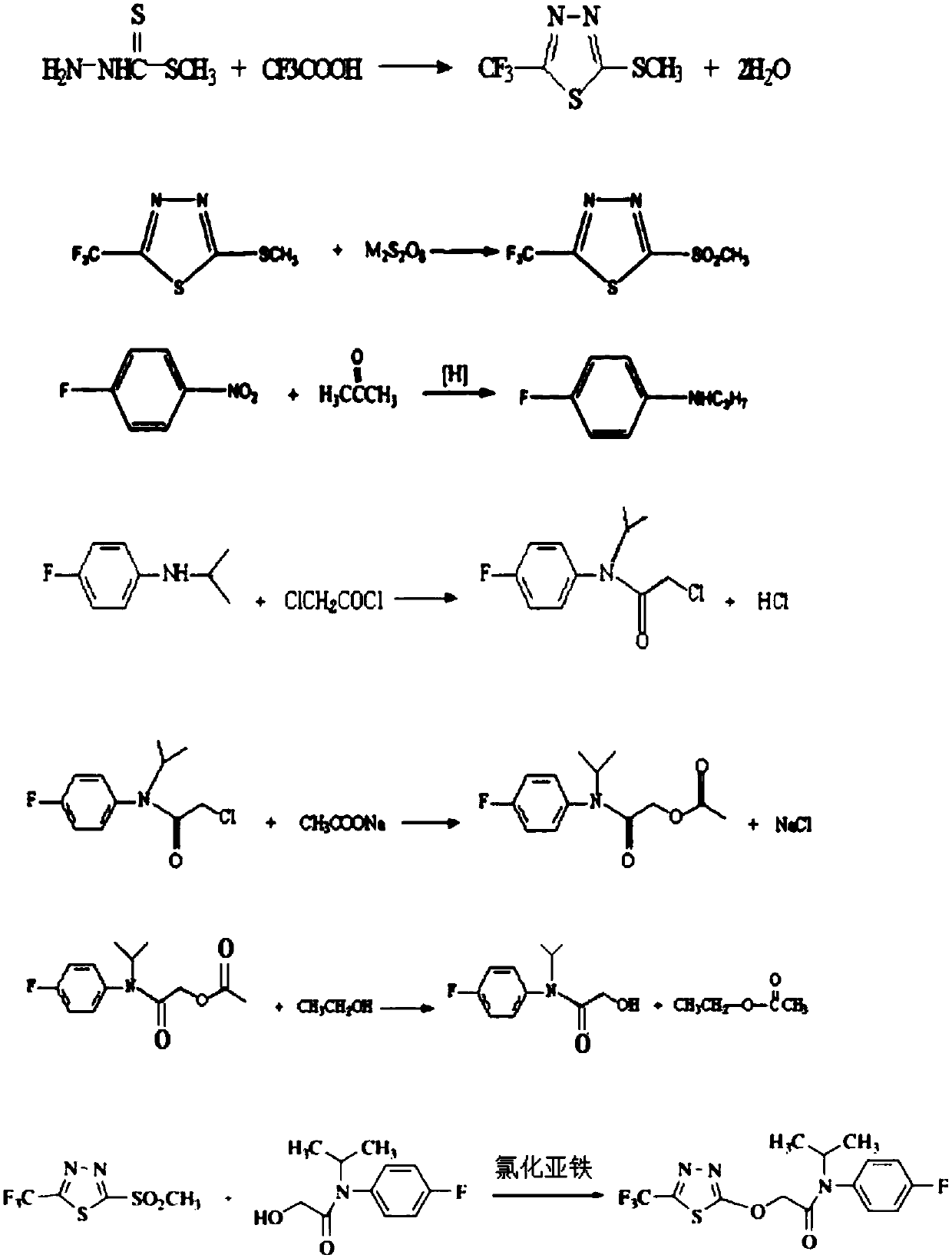
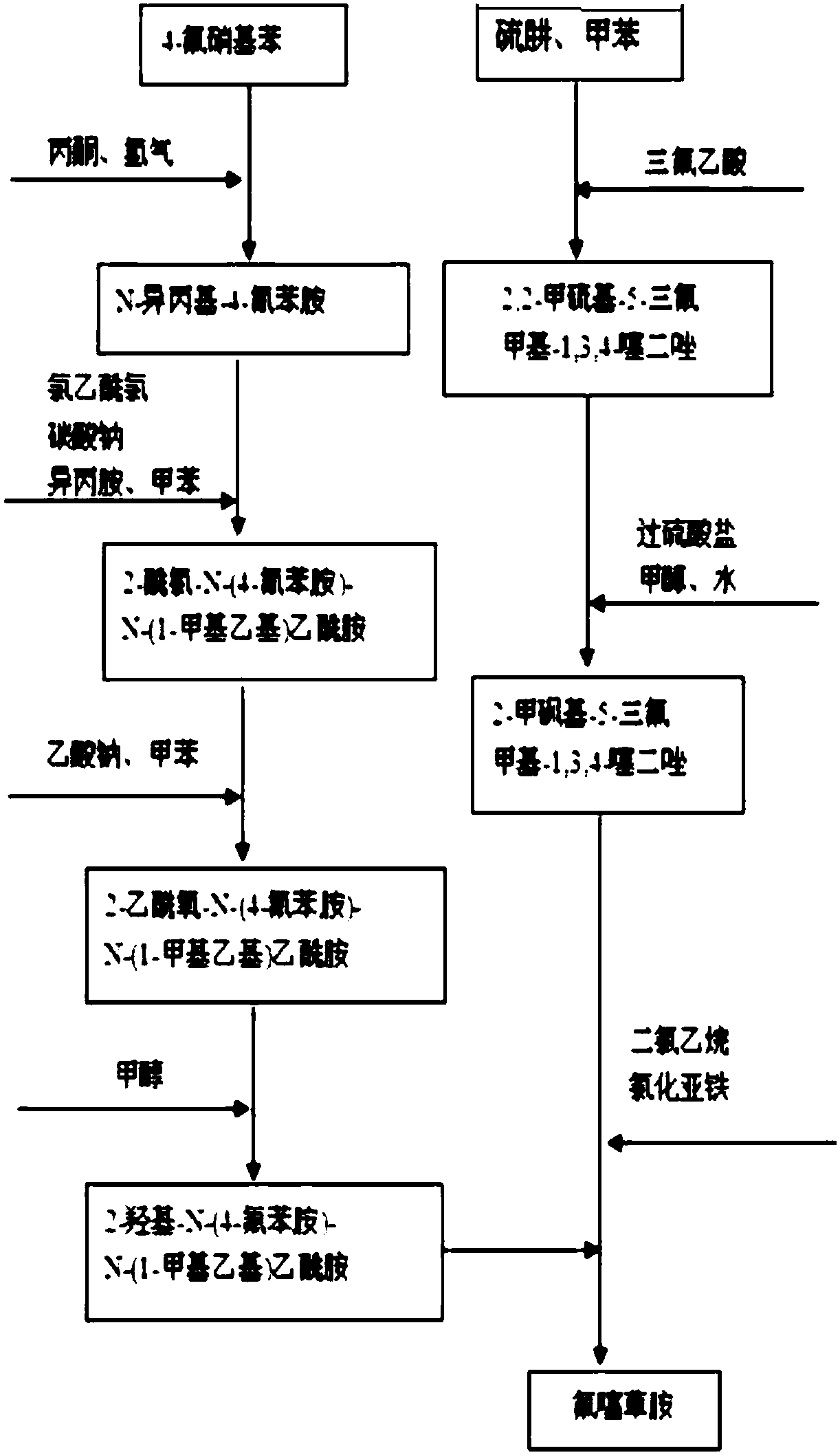
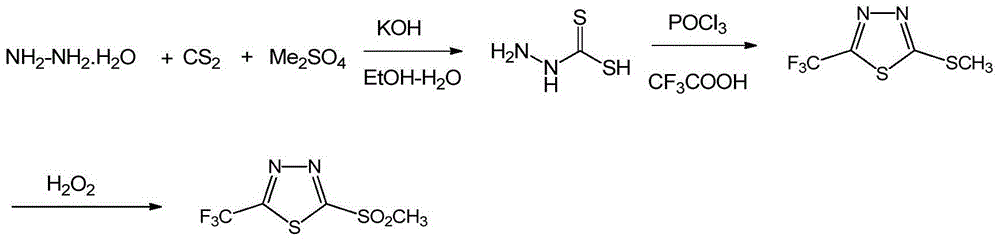
The invention discloses a flufenacet preparation method, which comprises synthesis of 2-methylsulfonyl-5-trifluoromethyl-1,3,4-thiadiazole, synthesis of 2-hydroxy-N-(4-fluoroaniline)-N-(1-methylethyl)acetamide and synthesis of 4'-fluoro-N-isopropyl-2-[5-(trifluridine)-1,3,4-thiadiazole-2-imide]acetamide. The optimal flufenacet preparation method is screened by a large number of experiments, the whole process is reasonable in design, particularly the steps of screening optimal reaction conditions and the optimal amount ratio, reaction temperature, reaction time and the like of reaction raw materials, the reaction yield (capable of reaching 90 percent or more) can be greatly increased, side reaction can be reduced, the reaction rate can be increased, the reaction raw materials can be recycled, the production cost is greatly reduced, and a broad application prospect is achieved.
Owner:江苏绿叶农化有限公司
Preparation method of Gefitinib
The invention relates to a preparation method of 4-(3-chloro-4-fluorophenylamido)-7-methoxy-6-(3-morpholinylpropoxy)quinazoline (Gefitinib, I). The preparation method of Gefitinib is characterized in that etherification reaction is carried out on a Gefitinib intermediate 4-(3-chloro-4-fluorophenylamido)-6-hydroxy-7-methoxy-quinazoline and 4-(3-hydroxypropyl)-morpholine under proper conditions to obtain the target product Gefitinib. Gefitinib (I).
Owner:JIANGSU FARMTEC RES
Preparation method of gefitinib
InactiveCN103304491AReduce pollutionShort process routeOrganic chemistryN dimethylformamideMorpholine
The invention relates to a preparation method of gefitinib. The preparation method comprises the following steps of: reacting under the action of an appropriate solvent and alkali by taking 3-hydroxy-4-methoxybenzonitrile and 4-(3-chloropropyl) morpholine as raw materials to prepare 4-(3-chlorine-4-fluorophenyl amido)-7-methoxyl-6-[3-(4-morpholinyl)propoxy] quinazoline; nitrifying the 4-(3-chlorine-4-fluorophenyl amido)-7-methoxyl-6-[3-(4-morpholinyl)propoxy] quinazoline to obtain 2-nitryl-4-methoxyl-5-[3-(4-morpholinyl)propoxy] methyl benzoate, and then reducing the 2-nitryl-4-methoxyl-5-[3-(4-morpholinyl)propoxy] methyl benzoate to obtain 2-amido-4-methoxyl-5-[3-(4-morpholinyl)propoxy] methyl benzoate; reacting 3-chlorine-4-fluoroaniline with N,N-dimethylformamide dimethylacetal to obtain (3-chlorine-4-fluorophenyl)-N,N-dimethyl dimethyleneimine; and carrying out loop closing on the 2-amido-4-methoxyl-5-[3-(4-morpholinyl)propoxy] methyl benzoate and the (3-chlorine-4-fluorophenyl)-N,N-dimethyl dimethyleneimine to obtain the gefitinib. The preparation method disclosed by the invention has the advantages of short process route, safety and easiness for operation, easiness and convenience for post processing, high reduction transformation ratio and yield, less environment pollution, and safety, reliability and stability in product quality.
Owner:LIANYUNGANG SHENGHE BIOTECH
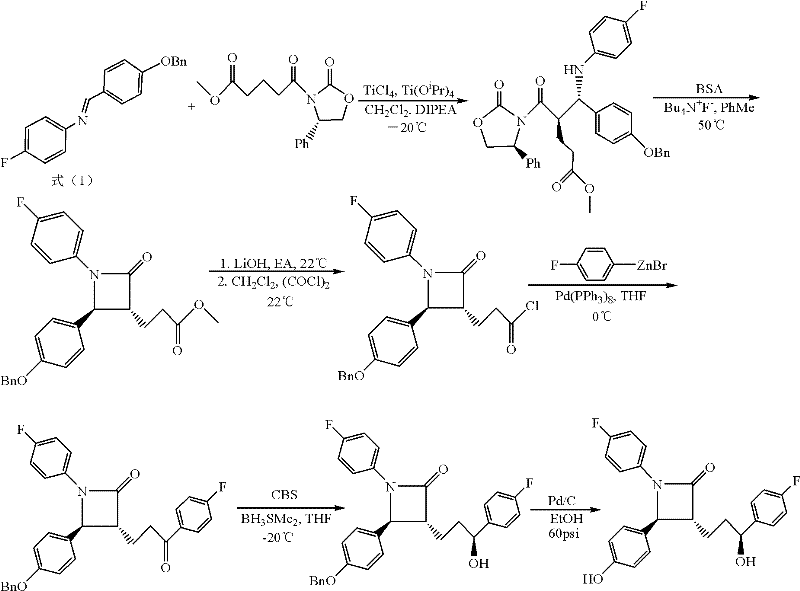
Method for synthesizing ezetimibe
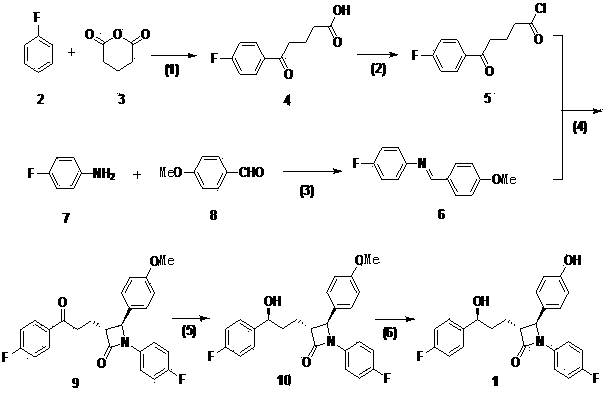
The invention discloses a novel technical method for synthesizing ezetimibe (a compound 1), and belongs to the field of organic chemical medical synthesis. The method comprises the following steps: synthesizing a (3R, 4S)beta-lactam ring of a key intermediate (a compound 9) by taking p-anisaldehyde, 4-fluoroaniline and glutaric anhydride as main raw materials; and reducing and hydrolyzing the compound to obtain ezetimibe. The method disclosed by the invention adopts simply and easily-available materials, and is less in synthesis steps, and stable in process; a chiral four-membered ring of the key intermediate is directly constructed by virtue of one-step reaction to obtain an important intermediate (3R, 4S)-1-(4- fluorophenyl)-3-[(3S)-3-]4-fluorophenyl)-3-carbonyl propyl]-4-(4-methoxypheyl)-2-azetidinone (a compound 9); a target enantiomer proportion in the product is high, post-treatment operation is simple, and industrial large-scale production is easy to realize.
Owner:HENAN ACADEMY OF SCI CHEM RES INST CO LTD
Method for preparing halogenated biphenyl
InactiveCN101550059AReduce labor intensityImproved Coupling ReactionHalogenated hydrocarbon preparationMetal/metal-oxides/metal-hydroxide catalystsEquivalent weightNitrogen gas
The present invention relates to a method for synthesizing halogenated biphenyl, wherein one halogenated aniline of 4-bromium-2-fluoroaniline or 2, 4-difluoro aniline or 2-fluoroaniline or 4-fluoroaniline or the like, and the benzene are used as the principal raw material under a acidic condition, the trichloroacetic acid, the anhydrous magnesium sulphate and homemade composite catalyst containing copper or zinc or iron are added into a same reactor, and a diazotization reaction and a coupling reaction are completed synchronously. The invention also discloses a method for preparing a homemade composite catalyst containing a metal, including the steps: adding the dimethylformamide into a reactor, filling the nitrogen to protect, adding 0.1-1.0 equivalent weight ammonium formate, 0.5-1.0 equivalent weight N, N, N', N'-tetraphenyl ethylene diamine and 0.1-0.3 equivalent weight CuCl2 or ZnCl2 or FeCl3 and 0.01-0.03 equivalent weight PdCl2, then mixing and heating until 30-60 DEG C, agitating thoroughly and holding for 15-24 hours, cooling, filtrating and vacuum drying, the composite catalyst containing a metal will be obtained. By means of using a same reactor, the halogenated biphenyl may be prepared by the diazotization and the coupling reaction completed in one same step, the yield is more than 85% by the halogenated aniline. The product content achieves 99.5%, the production period is shortened greatly, the equipment production capacity is improved, the energy consumption and material consumption are all reduced, and the work intensity of workers is alleviated greatly, thus the method of the invention is very suitable for an industrialization great production.
Owner:江西省化学工业研究所 +1
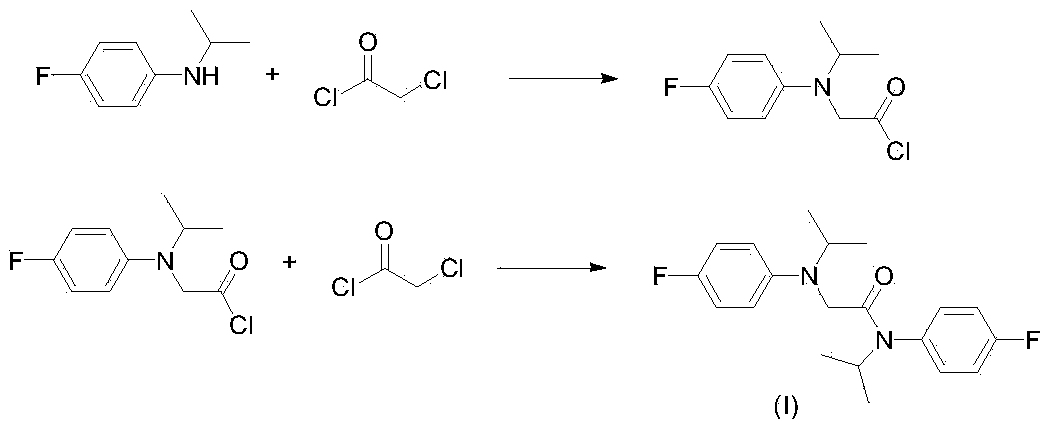
Method for preparing 2-chloro-N-(4-fluorophenyl)-N-isopropylacetamide
ActiveCN103664675ASimple process recyclingSpeed up the amidation reactionOrganic compound preparationCarboxylic acid amides preparationChloroacetyl chlorideImpurity
The invention discloses a method for preparing 2-chloro-N-(4-fluorophenyl)-N-isopropylacetamide. The method comprises the steps as follows: N-isopropyl-4-fluoroaniline and chloroacetyl chloride are taken as raw materials, triethylamine is taken as an acid-binding agent, a reaction is performed under the mild condition, and 2-chloro-N-(4-fluorophenyl)-N-isopropylacetamide is obtained. Triethylamine is recovered with a follow-up simple technology, so that N-alkylate impurities are effectively avoided; and the technology mainly has numerous advantages as follows: the reaction is mild, the operation is simple and convenient, the equipment corrosiveness is small, the yield and the purity are high, and the method is suitable for industrial production.
Owner:ORIENTAL LUZHOU AGROCHEM
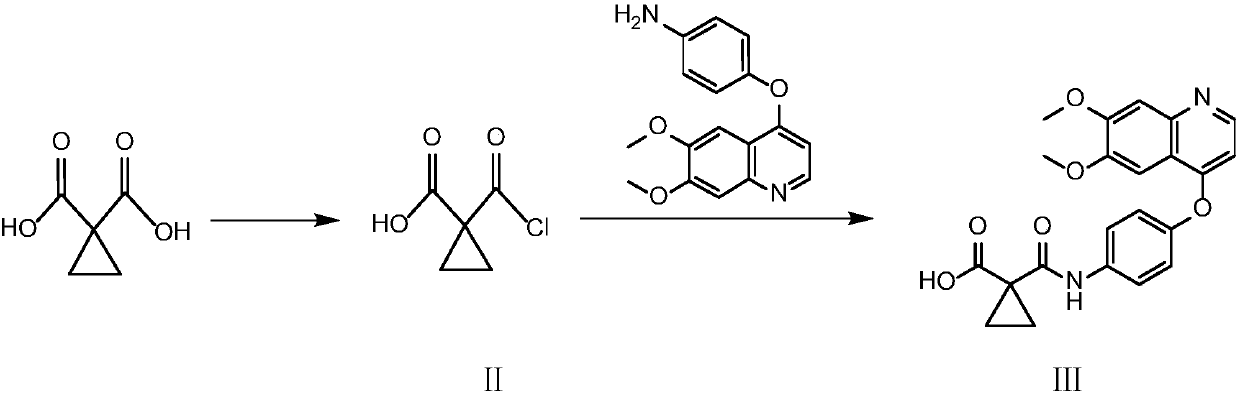
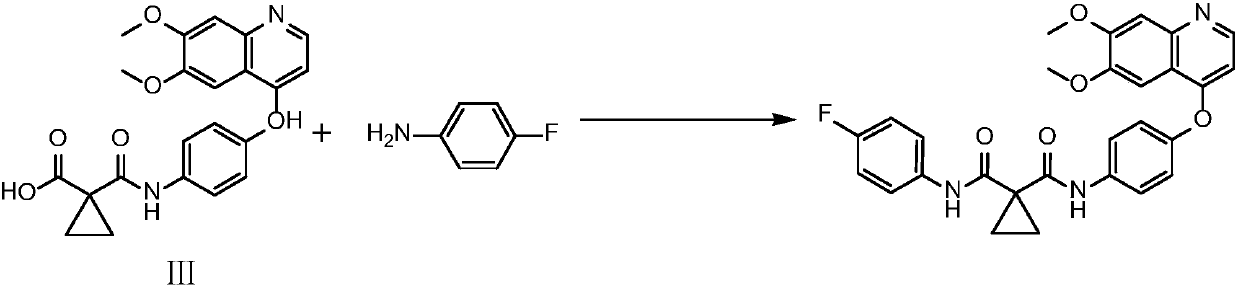
Synthetic method for cabozantinib
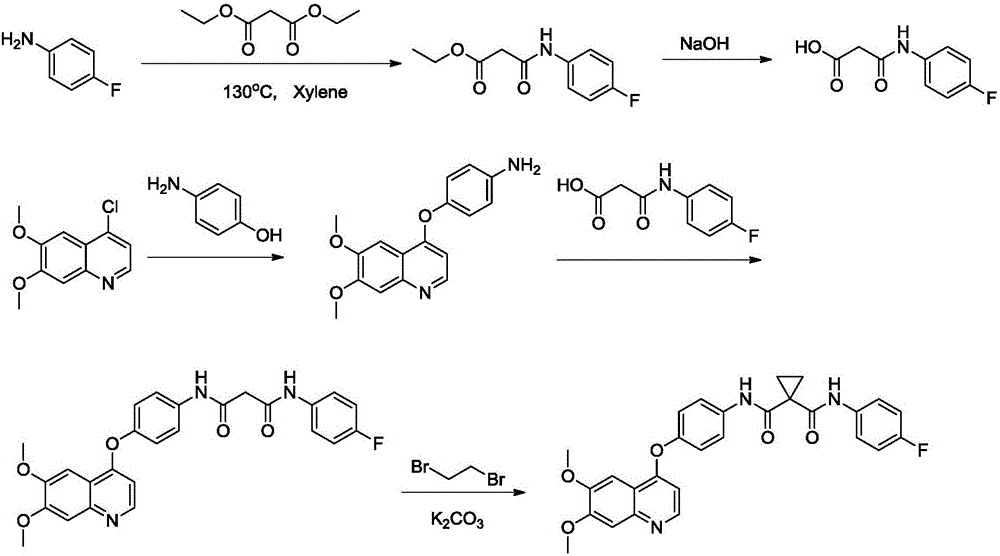
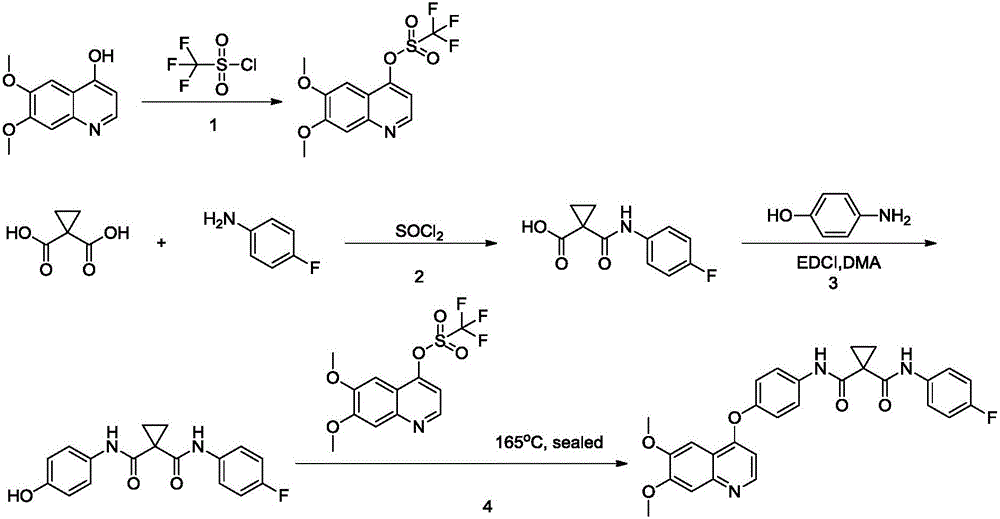
The invention provides a synthetic method for cabozantinib. The method comprises the following steps: 1,1-cyclopropanedicarboxylic acid is used a starting raw material, acylation is performed, condensation is performed on an acylation product and 4-[6,7-dimethoxy-4-quinolinyl]oxy]aniline, condensation is further performed on an obtained product and 4-fluoroaniline under effects of a polypeptide condensing agent, and therefore the cabozantinib is obtained. According to the method, the reaction conditions are mild, a chlorinating reagent is used just in one step, and the method is suitable for industrialized production.
Owner:深圳万乐药业有限公司
Preparation method of 2-bromo-4-fluoroacetanilide
InactiveCN104447382AReduce concentrationEasy to handleOrganic compound preparationCarboxylic acid amides preparation4-fluoroacetanilideReaction temperature
The invention discloses a preparation method of 2-bromo-4-fluoroacetanilide. The preparation method comprises steps as follows: 4-fluoroaniline is taken as a raw material and subjected to acetylation and bromination to obtain 2-bromo-4-fluoroacetanilide; the bromination reaction is that acetylate is bromized by hydrobromic acid to produce 2-bromo-4-fluoroacetanilide under the presence of an oxidizing agent; a mole ratio of 4-fluoroaniline, hydrobromic acid and the oxidizing agent is 1: (1.0-2.0): (1.0-3.5); the oxidizing agent is one of hydrogen peroxide, sodium hypochlorite, sodium hypobromite and peracetic acid; and the bromination reaction temperature is in a range from 30 DEG C to 60 DEG C. The bromination reaction adopts hydrobromic acid to replace bromine to serve as a bromination reagent, so that very little 2, 6-dibromo-4-fluoroacetanilide is generated in the bromination reaction, and the yield of 2-bromo-4-fluoroacetanilide is increased.
Owner:常州化工研究所有限公司
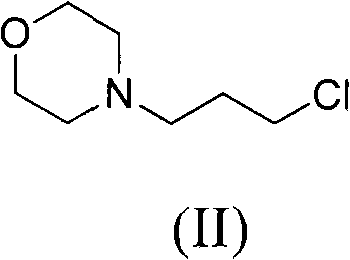
Gefitinib synthesis intermediate, and its preparing method and use
ActiveCN100420676CQuality improvementReduced two-step reactionOrganic chemistryCombinatorial chemistryQuinazoline
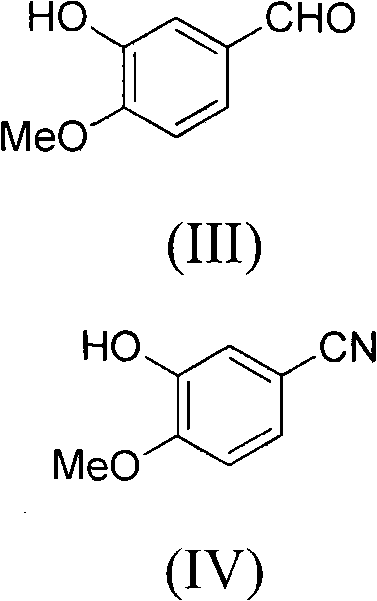
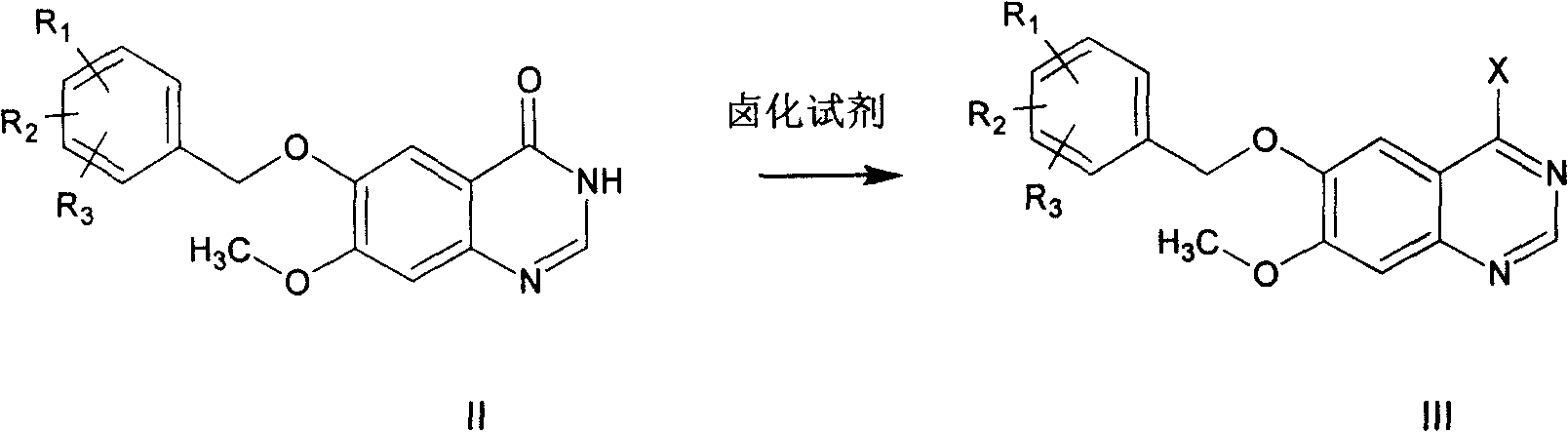
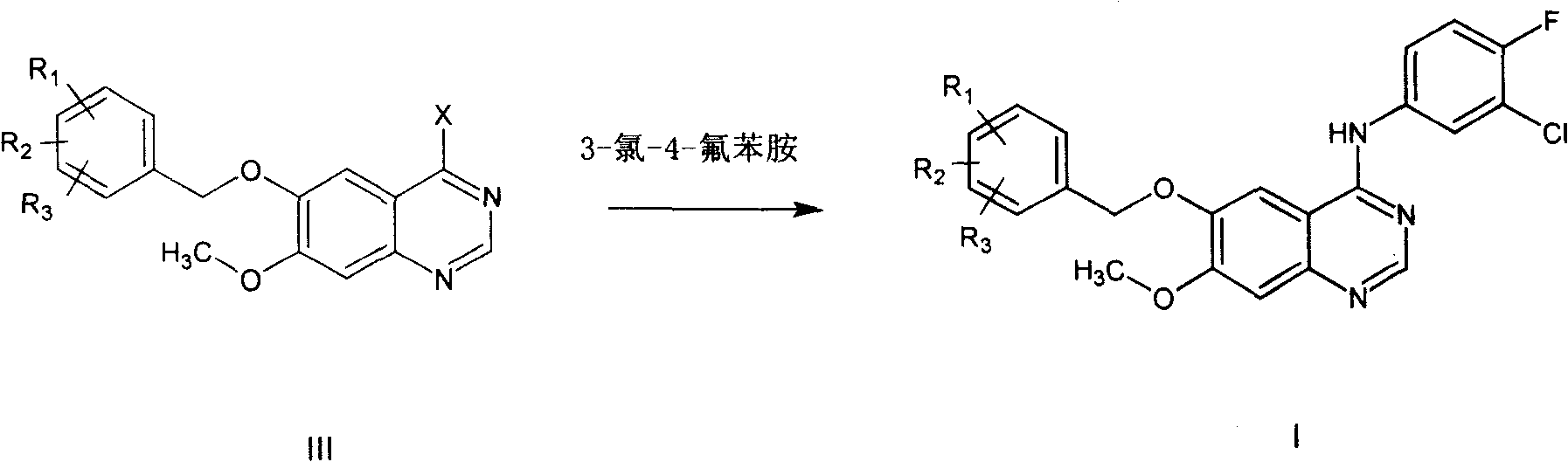
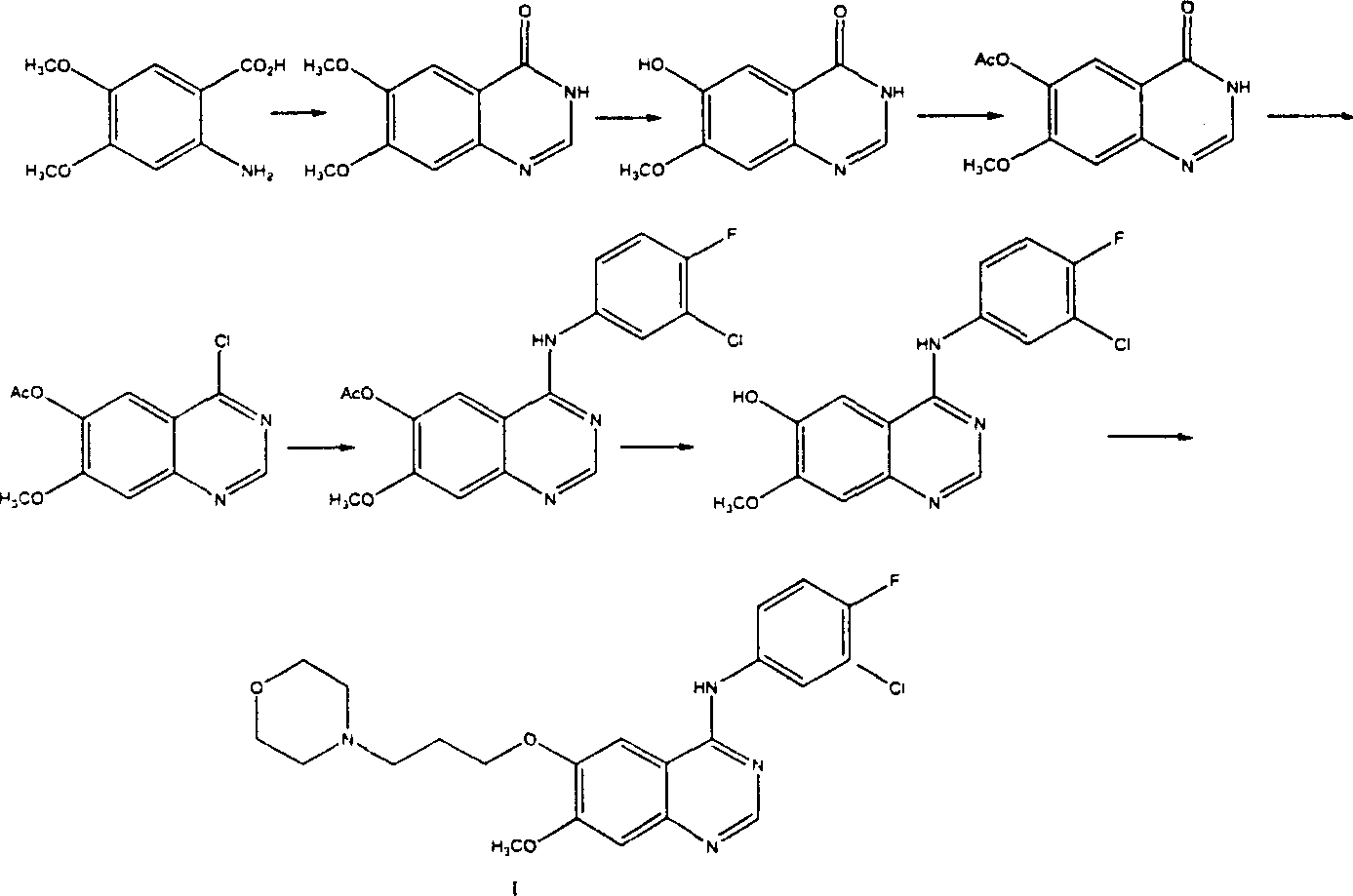
This invention relates to gefetinib synthesis intermediate and its preparation method and application. Compound of formula (II) is halogenated to make formula (III) compound, then condensed with 3-chlorine-4-fluoroaniline to make formula (I) compound. The formula (I) compound is new intermediate compound that used to synthesize the gefetinib, another important intermediate compound 6-hydroxy group- 7-methoxyl group-4-(3'-chlorine-4'-fluoroaniline) quinazoline (2) that used to synthesize gefetinib can be easily made by (I) compound.
Owner:ZHEJIANG HISUN PHARMA CO LTD
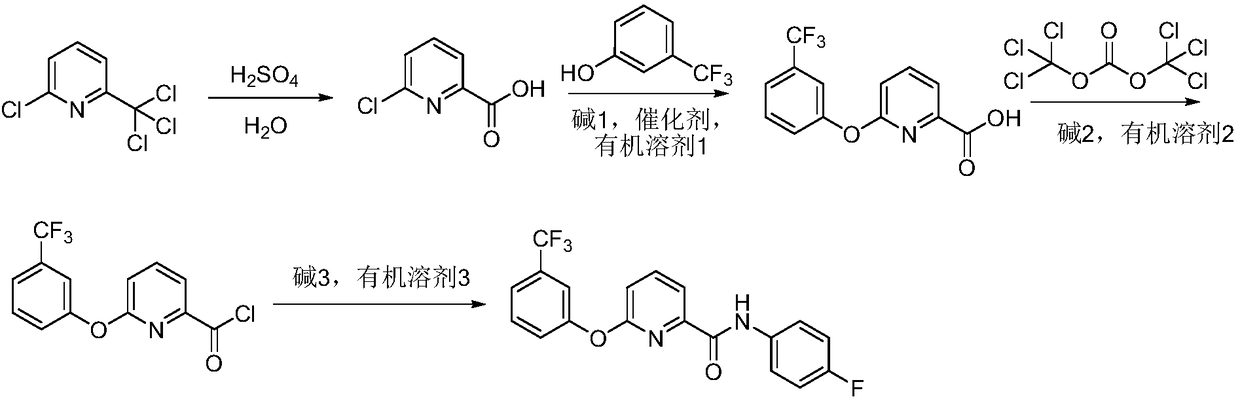
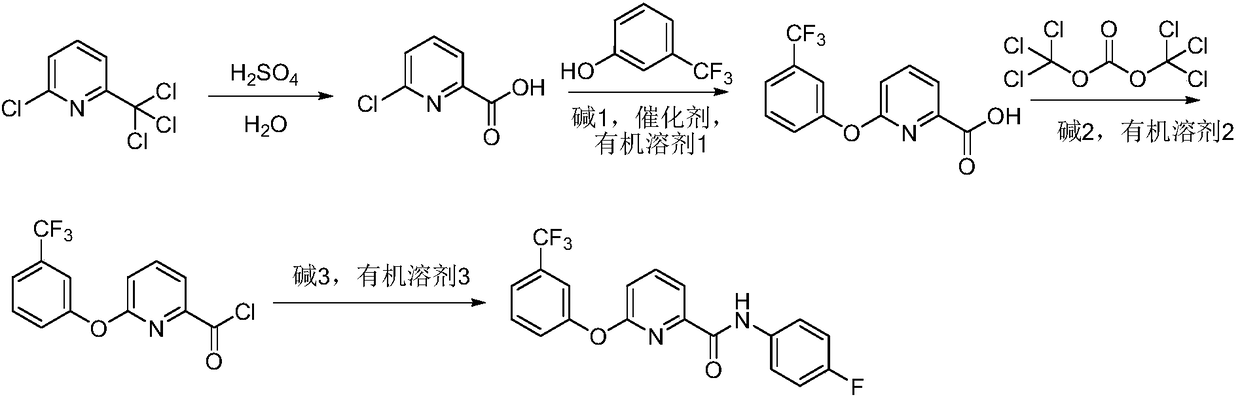
Method for preparing picolinafen
InactiveCN108530351AEmission reductionLow equipment requirementsOrganic chemistryCarboxylic acidHydrolysis
The invention discloses a method for preparing picolinafen, and belongs to the field of pesticide original medicine technology. The method comprises the following steps that (1) 2-chlorine-6-nitrapyrin serves as a raw material, and acid catalysis hydrolysis is carried out to synthesize 2-chlorine-6-pyridine carboxylic acid; (2) under conditions of alkali 1, catalyst and organic solvent 1, etherification reaction is carried out on 2-chlorine-6-pyridine carboxylic acid and m-trifluoromethylphenol to obtain 2-(3-trifluoromethylphenoxy pendant)-6-picolinic acid; (3) the 2-(3-trifluoromethylphenoxypendant)-6-picolinic acid and di(trichloromethyl) dimethyl carboxylate are reacted in alkali 2 and organic solvent 2 to synthesize intermediate 2-(3-trifluoromethylphenoxy pendant)-6-pyridinecarbonylchloride; (4) the intermediate 2-(3-trifluoromethylphenoxy pendant)-6-pyridinecarbonyl chloride and 4-fluoroaniline are reacted in alkali 3 and organic solvent 2 to prepare the picolinafen. The method for preparing the picolinafen has the advantages that the content of the prepared picolinafen product is 98.1%, the yield of the prepared picolinafen product is 83%, synthesis conditions are mild and safe, the operation process is controlled easily, aftertreatment is simple and convenient, the wastewater quantity is less, and the method is a green synthetic method for preparing the picolinafen.
Owner:陈磊
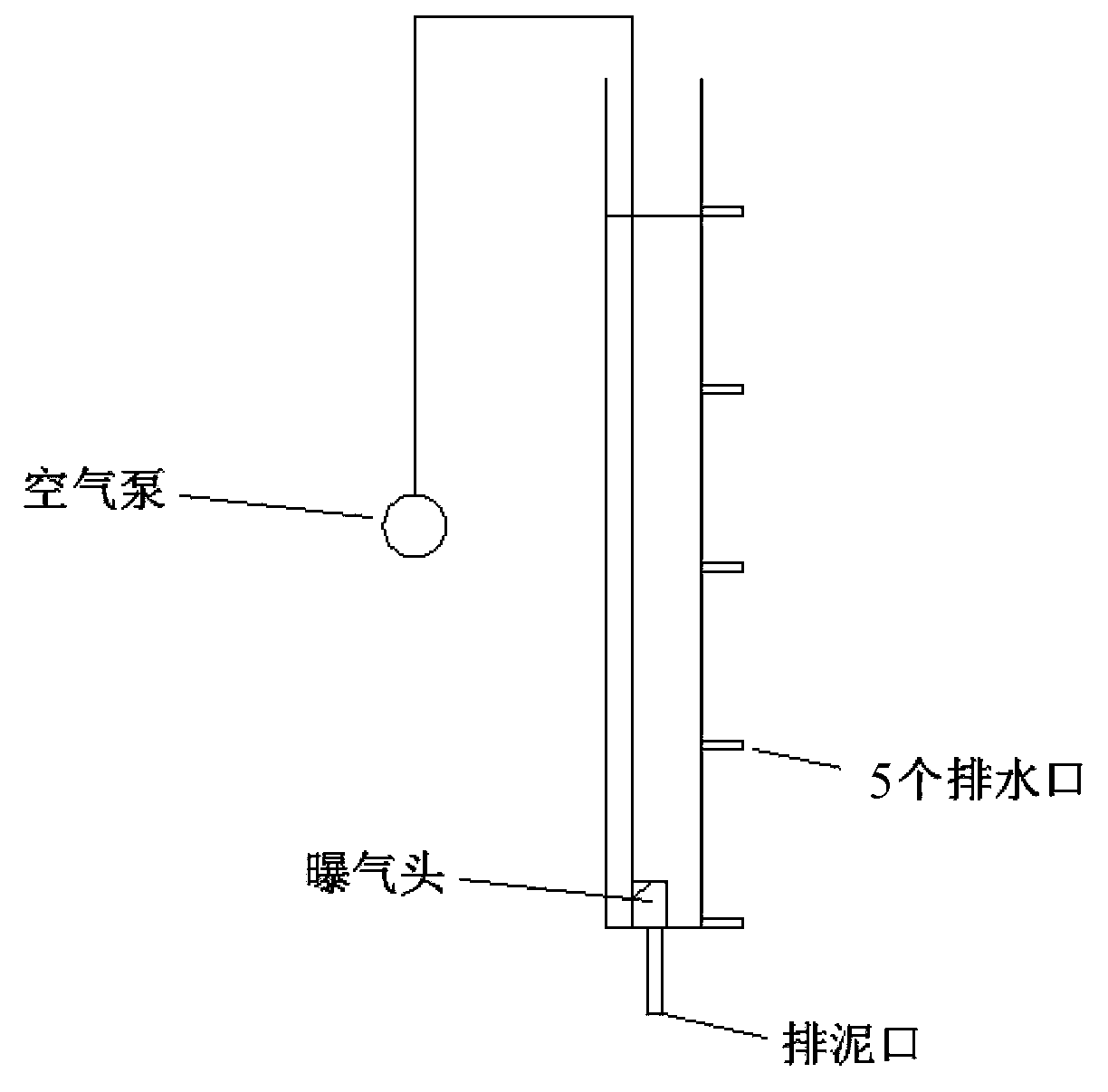
Method for processing tobacco waste or organic fluorine wastewater
ActiveCN103381418AImprove naturePreferential degradationSolid waste disposalWater contaminantsActivated sludgeSignalling molecules
The invention discloses a method for processing tobacco waste or organic fluorine wastewater. The method for processing tobacco waste comprises the following steps: adding tobacco waste into water and uniformly mixing to obtain a mixed liquor, adding activated sludge, an Arthrobacte TW seed liquid and a signal molecule preparation, and carrying out aeration treatment for 18-48h. The method for processing organic fluorine wastewater comprises the following steps: adding activated sludge, an Arthrobacte TW seed liquid and a signal molecule preparation into organic fluorine wastewater, and carrying out aeration treatment for 24-72h, wherein the collection number of the Arthrobacte TW is CGMCC No.7.47; and the signal molecule preparation is prepared by mixing an N-3oxo-caproyl-homoserinelactone solution and an N-caproyl-homoserinelactone solution. According to the method, adding frequency is low. The method is economical and environmentally friendly, can be adopted to preferentially, rapidly and efficiently degrade nicotine in tobacco or 4-fluoroaniline in organic fluorine wastewater so as to reduce the harm, and is of great significance for environmental protection.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Preparation method of flufenacet
The invention discloses a preparation method of flufenacet, and belongs to the technical field of pesticide preparation. The method comprises the steps: adding 2-hydroxy-N-(4-fluoroaniline)-N-(1-methylethyl)acetamide into an alkali-containing organic solvent A, dropping 2-chloro-5-trifluoromethyl-1,3,4-thiadiazole into the reaction liquid, carrying out a reaction at room temperature, adding an organic solvent B, extracting, washing with water, drying, and concentrating to obtain a crude product flufenacet; and refining the crude product to obtain the pure product flufenacet. The preparation method has the advantages that the required raw materials are easy to obtain, less pollution to the environment exists, the synthesis is simple and convenient, the process conditions are not harsh, the yield can reach more than or equal to 85%, the purity can reach more than or equal to 98%, and the preparation method is suitable for industrialized production.
Owner:ZHEJIANG YONGTAI TECH CO LTD
Preparation technology o high-purity gefitinib
The invention relates to the preparation technology of high-purity gefitinib and belongs to the technical field of preparation of medical compounds. The technology is characterized in that 4-chlorine-7-methoxyl -6-(3-morpholin-4-ylpropoxy) quinazoline hydrochloride and 3-chlorine-4-fluoroaniline are in direct reaction, so as to obtain gefitinib hydrochloride, then gefitinib crude products can be obtained through neutralization, and finally, the high-purity gefitinib is obtained by post processing. In the invention, the the 4-chlorine-7-methoxyl-6-(3-morpholin-4-ylpropoxy) quinazoline hydrochloride and the 3-chlorine-4- fluoroaniline are in direct reaction, the production process is simplified, the production period is shortened, energy consumption is lowered, the types of all solvents are decreased at the same time, the use level of solution medium is reduced greatly, the production cost is obviously lowered, and pollutions caused by the solvents to human body and the environment are reduced; and in the invention, by preparing formylamine solvate, the purity of prepared gefitinib is high.
Owner:REYOUNG PHARMA
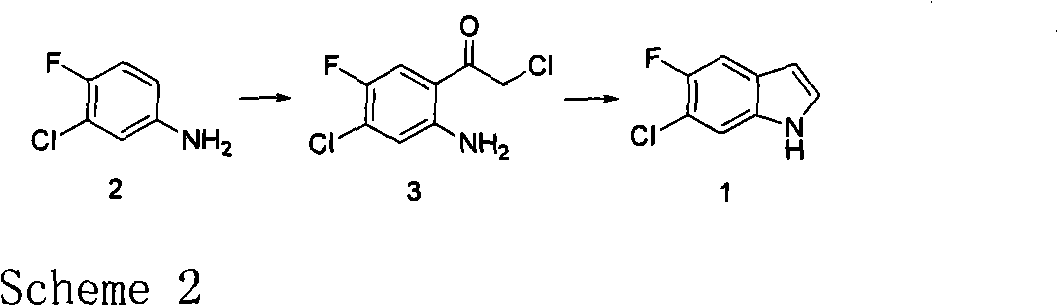
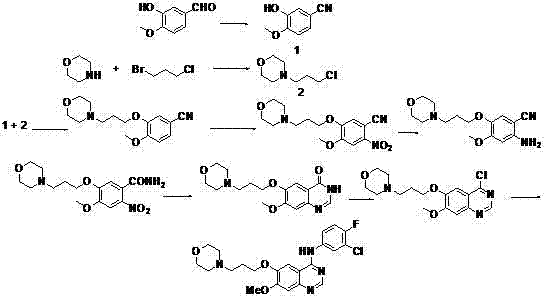
Preparation method of 3-chloro-4-fluoroaniline
InactiveCN104292113AHigh reaction conversion rateHigh yieldOrganic compound preparationAmino compound preparationOrganic solventHydrogen atmosphere
The invention discloses a preparation method of 3-chloro-4-fluoroaniline, and belongs to the technical field of organic chemical industry. The preparation method comprises the following steps of weighing 3-chloro-4-fluoronitrobenzene and 1% Pt / C catalyst and reacting for 1-10 hours at a temperature of 50-100 DEG C and in a hydrogen atmosphere of 0.1-5 MPa, wherein a mass ratio of 3-chloro-4-fluoronitrobenzene to 1% Pt / C catalyst is (200-400):1. The preparation method takes 3-chloro-4-fluoronitrobenzene as a raw material and prepares 3-chloro-4-fluoroaniline by a hydrogenation substitution reaction under catalysis of 1% Pt / C catalyst, has high reaction conversion rate, high yield and high selectivity, adopts no organic solvent, is simple and reasonable in process, and is suitable for large-scale production. The purity of 3-chloro-4-fluoroaniline prepared by the method can reach over 99.5%; and the yield reaches over 94%.
Owner:DO FLUORIDE CHEM CO LTD
Preparation method of 4-(3-chlor-4-fluorobenzeneamidocyanogen)-7-methoxy-6-(3-morpholine oxypropyl)quinazoline
InactiveCN1300118CReduce pollutionReduce manufacturing costOrganic chemistryAntineoplastic agentsMorpholine3-chloro-4-fluoroaniline
Owner:江苏吴中苏药医药开发有限责任公司
Preparation method of 3-chloro-4-fluoroaniline hydrochloride
InactiveCN103709044AAvoid pollutionStable in natureOrganic compound preparationAmino compound preparationHydrogen3-chloro-4-fluoroaniline
The invention provides a preparation method of 3-chloro-4-fluoroaniline hydrochloride. The 3-chloro-4-fluoroaniline hydrochloride is prepared from a raw material 3,4-dichloronitrobenzene through a three-step reaction comprising the steps of fluorine displacement, hydrogenation reduction and salt formation. The preparation method has the advantages of few byproducts, stable product properties, low reaction apparatus requirements because of the implementation of the nitro group reduction reaction using hydrogen and Pd-C at normal temperature, recycling of a solvent and Pd-C, and avoiding of the environmental pollution by introducing an excess HCl gas into an alkaline solution.
Owner:JIANGSU JIXIAN GREEN CHEM TECH RESINST
Cabozantinib preparation method
ActiveCN106632028AReduce manufacturing costReduce usageOrganic chemistryEthylene DibromideCabozantinib
The invention discloses a cabozantinib preparation method. The target product cabozantinib is prepared by performing five-step reaction on diethyl malonate, 4-fluoroaniline, 4-chloro-6,7-dimethoxyquinoline, 4-aminophenol, 1,2-dibromoethane and the like used as raw materials. The preparation method is simple to operate and friendly to environment; the comprehensive yield is more than 50% and is obviously increased in comparison with the yield of 20% in the prior art; and the preparation method greatly lowers the existing medicine production cost, and is suitable for industrial large-scale production.
Owner:SHANGHAI ZAIQI BIO TECH
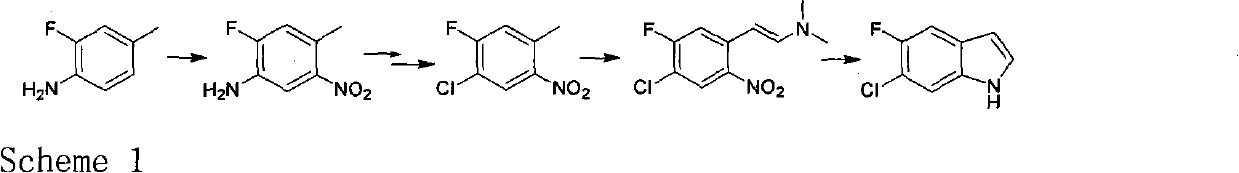
Novel method for preparing intermediate 6-chloro-5-fluoroindole used for synthesizing anticancer and weight-reducing medicine
InactiveCN102702066ARaw materials are easy to getShort reaction stepsOrganic chemistryDistillationBoron trichloride
The invention relates to a novel method for synthesizing an important intermediate 6-chloro-5-fluoroindole for anticancer and weight-reducing medicines. The method comprises the steps that: 3-chloro-4-fluoroaniline is reacted with boron trichloride in a toluene solution under the action of a Lewis acid aluminium trichloride to produce imine which is then hydrolyzed under the action of hydrochloric acid to get an intermediate, a target product is generated by the reduction of the intermediate by sodium borohydride in a dioxane / water system and then by reflux dewatering, and the 6-chloro-5-fluoroindole is produced by reduced pressure distillation of the initial product. The method has the advantages of readily available raw materials, short reaction steps and low costs, and is suitable for industrial production.
Owner:SUZHOU BAILINGWEI HYPERFINE MATERIAL
Water-based dispersive polyanion anticorrosive additive and preparation method thereof
InactiveCN108976870AGood water dispersibilityImprove corrosion resistanceAnti-corrosive paintsWater basedWater dispersible
The invention relates to the field of paintings and particularly relates to a water-based dispersive polyanion anticorrosive additive and a preparation method thereof. The preparation method comprisesthe following steps: step 1, selecting sodium lignosulfonates, phosphoric acid and de-ionized water, and performing ultrasonic agitation for dissolution; step 2, then adding aniline or 4-fluoroaniline or a mixture of aniline and 4-fluoroaniline, and performing ultrasonic agitation, thereby obtaining a mixture; step 3, reacting ammonium persulfate with the mixture obtained in the step 2, thereby obtaining a reactant liquor; and step 4, separating a solid precipitate out from the reactant liquor obtained in the step 3, and performing vacuum drying on the solid precipitate. The preparation method has the beneficial effects that natural reproduceable papermaking byproduct sodium lignosulfonates is adopted as a modifier and is blended with aniline and derivatives thereof for reaction, so as toobtain the modified water-based dispersive polyanion anticorrosive additive. The additive has favorable water dispersion property and excellent corrosion prevention effect. According to the scheme, adopted reaction conditions are mild, and the raw material cost is low. The preparation method is simple to operate and easy to realize.
Owner:FUJIAN NORMAL UNIV
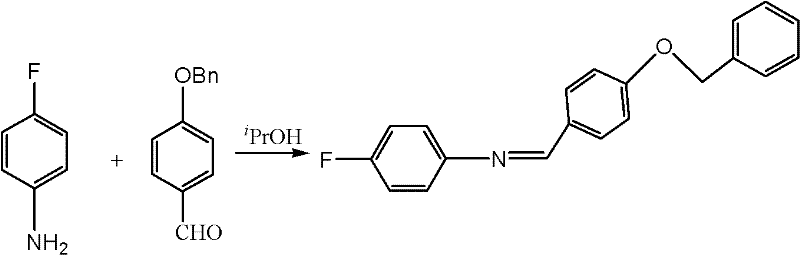
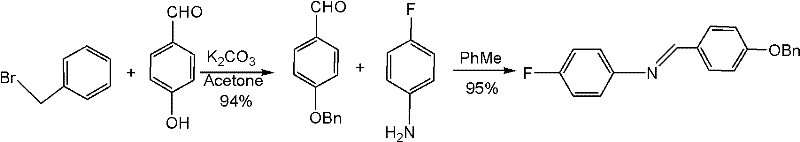
Preparation method of N-(4-fluorophenyl)-4-benzyloxy benzylidene amine
InactiveCN102358723ALow costLower reaction costBulk chemical productionImino compound preparationBenzoyl bromideBenzyl chloride
The invention discloses a preparation method of N-(4-fluorophenyl)-4-benzyloxy benzylidene amine. The method comprises steps of: adding 4-hydroxybenzaldehyde, alkali carbonate, catalyst and benzyl chloride into a reaction solvent; stirring and reacting completely; adding 4-fluoroaniline and reacting completely; and carrying out a post-treatment to obtain the N-(4-fluorophenyl)-4-benzyloxy benzylidene amine. Beneficial effect of the invention lays in that the method of the invention employs cheap benzyl bromide to substitute expensive benzyl bromide for benzyl protection in order to substantially reduce reaction costs; meanwhile introduction of the protective group and a condensation reaction are integrated into a ''one kettle way'' reaction, which simplifies operating steps of the reaction, further reduces reaction costs and is suitable for industrialization.
Owner:ZHEJIANG UNIV
New method for performing microwave synthesis on gefitinib and derivative thereof
The invention discloses a new method for performing microwave synthesis on gefitinib and a derivative thereof. The method comprises the following steps of: at first, synthesizing 6-methoxyl-7-hydroxyl quinazoline-4-one from 2-iodo (bromo)-4-methoxyl-5-hydroxyl cyanophenyl used as an initial raw material and formamidine hydrochloride in a microwave mode; then performing reaction with 4-(3-bromo propyl) morpholine to introduce an alkyl side chain so as to obtain 7-methoxyl-6-[3-(4-morpholinyl) propoxy] quinazoline-4-ketone; in the presence of a catalyst, performing reaction on the obtained product and a chlorination reagent to obtain 4-chloro-7-methoxyl-6-[3-(4-morpholinyl) propoxy] quinazoline; finally, performing reaction on 4-chloro-7-methoxyl-6-[3-(4-morpholinyl) propoxy] quinazoline and 3-chloro-4-fluoroaniline to obtain a final product 4-(3-chloro-4-fluoroanilino)-7-methoxyl-6-(3-morpholinyl propoxy) quinazoline (gefitinib). The whole route steps are simplified into four steps, the yield is high, the method is convenient to carry out, the dangerousness is low, and the application of high-pollution reagents is reduced; moreover, the whole route is high in economy and suitable for industrial production, and the product cost is reduced. The reaction formula is as shown in the specification.
Owner:FUJIAN MEDICAL UNIV
Preparation method for revaprazan hydrochloride
ActiveUS20170267646A1Easy to industrializeHigh yieldOrganic active ingredientsOrganic chemistryEthyl ChlorideMedicinal chemistry
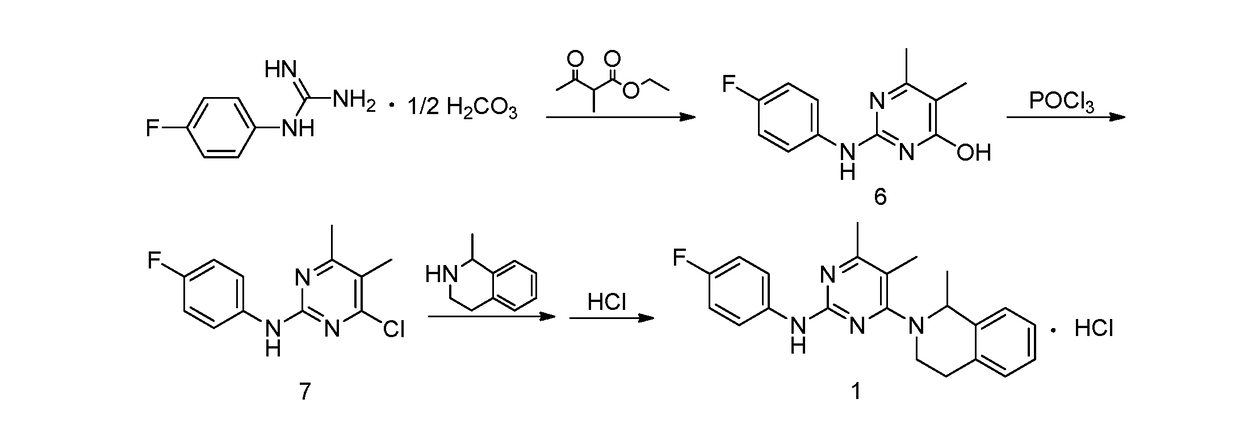
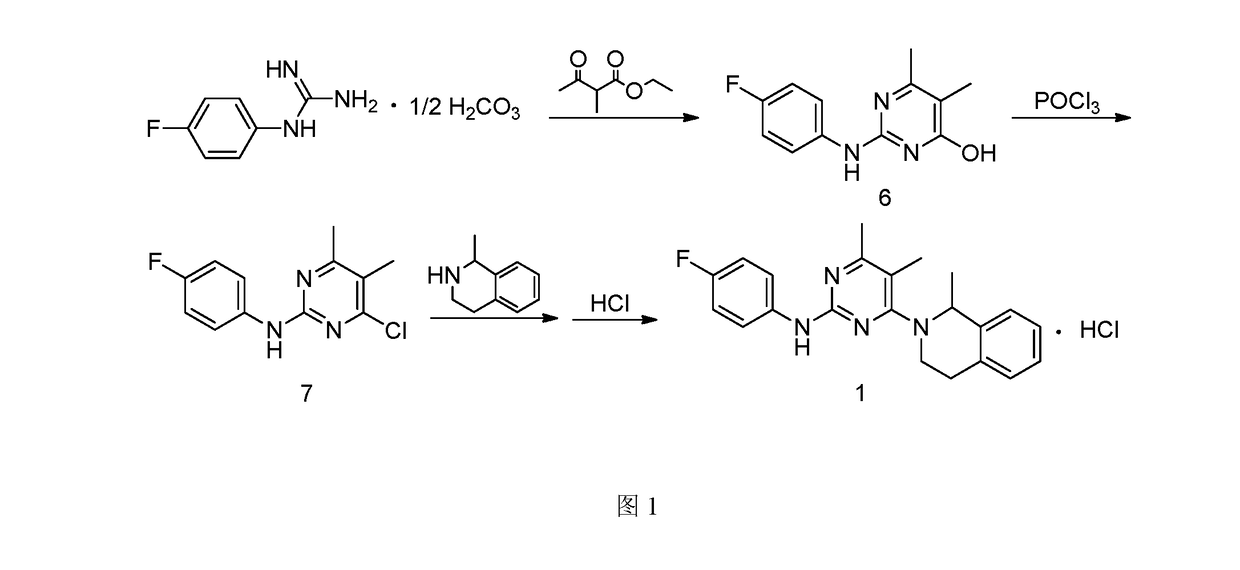
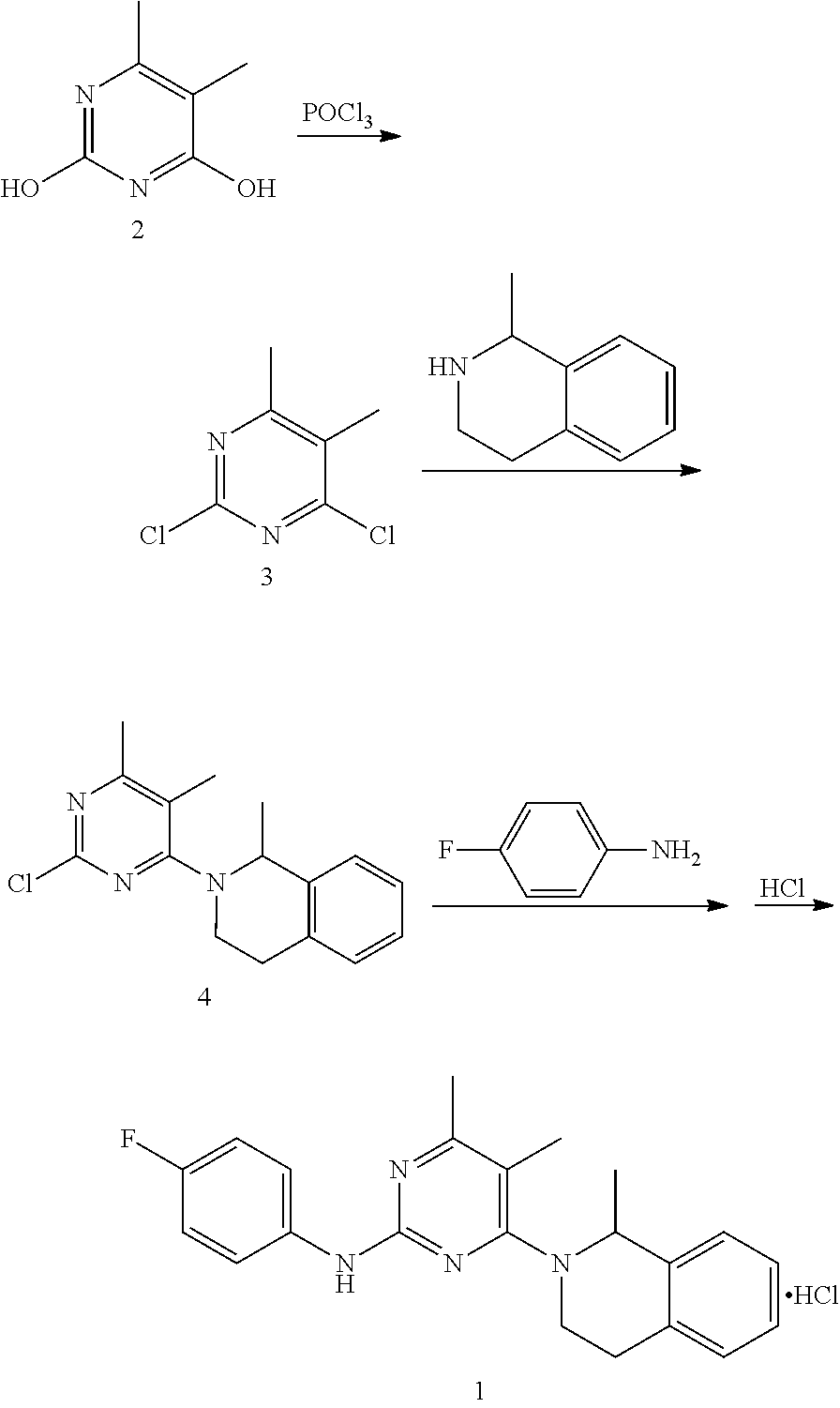
Provided is a preparation method for revaprazan hydrochloride, the method comprising: (1) the preparation of 4-hydroxyl-2-(4-fluoroaniline)-5,6-dimethylpyrimidine; (2) the preparation of 4-chloro-2-(4-fluoroaniline)-5,6-dimethylpyrimidine; (3) the preparation of revaprazan hydrochloride. The method has advantages such as simple operations, high product purity, good yield and suitability for industrial production.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com