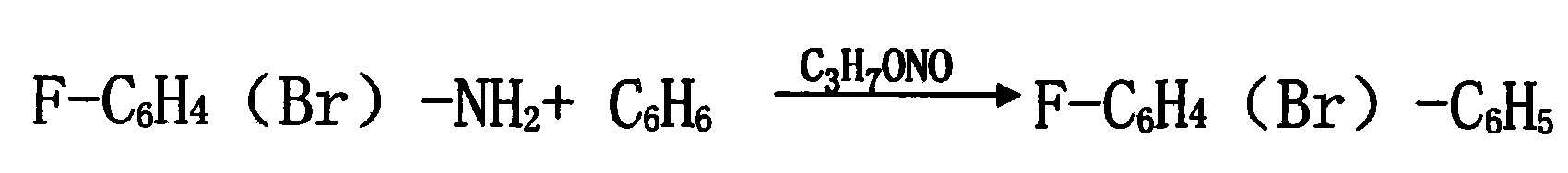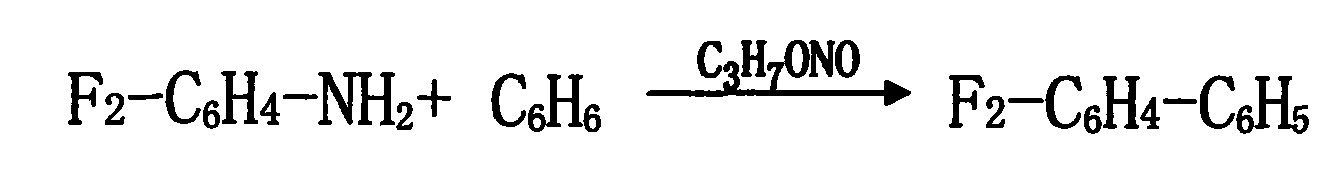Method for preparing halogenated biphenyl
A technology of halogenated aniline and biphenyl, which is applied in the field of preparation of halogenated biphenyl, can solve problems such as low yield of target product, difficulty in separation and purification, and easy disproportionation reaction, so as to improve equipment production capacity and shorten production cycle , The effect of increasing product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
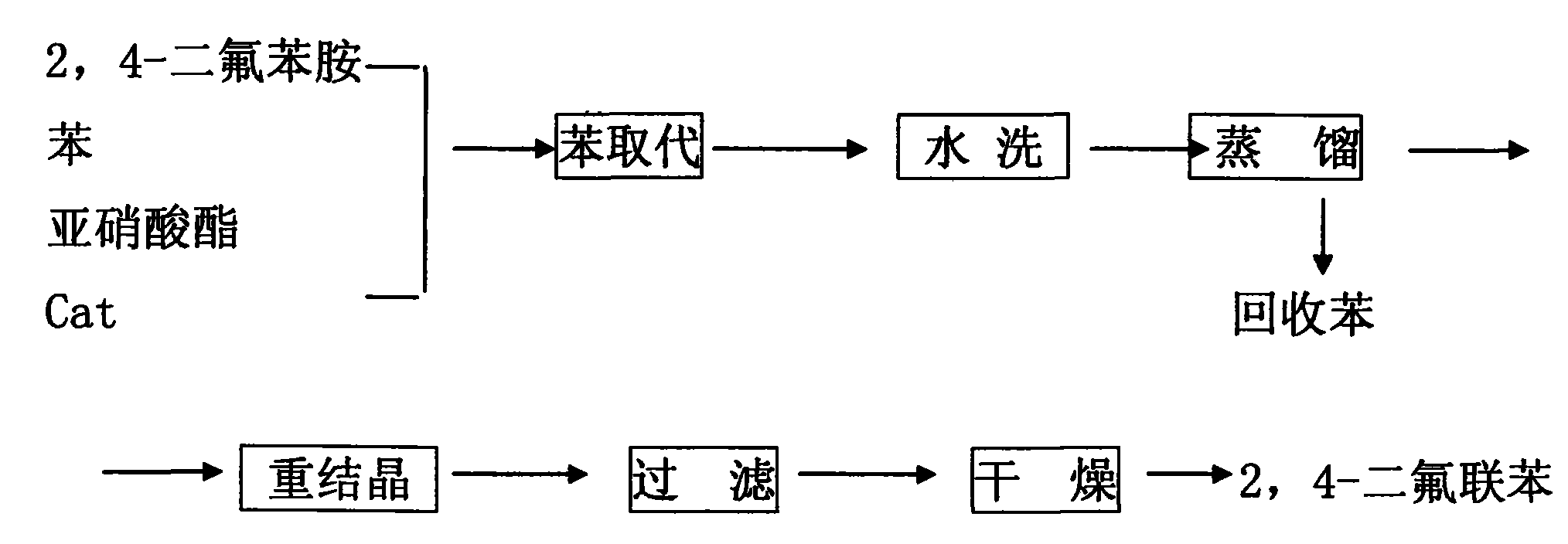
[0023] 1. Make a self-made copper-containing composite catalyst, add dimethylformamide into the reactor, fill with nitrogen for protection, then add 0.1 equivalent of ammonium formate and 0.5 equivalent of N, N, N', N'-tetraphenylethylenediamine and 0.1 equivalent of CuCl 2 and 0.01 equivalent of PdCl 2 , and then the mixture was heated to 30 ° C, fully stirred and maintained for 15 hours, cooled, filtered, and vacuum-dried.
[0024] 2. Add 100KG trichloroacetic acid, 5KG anhydrous magnesium sulfate, 1KG self-made copper-containing composite catalyst, 1000L benzene, and 50KG 2,4-difluoroaniline into the synthesis kettle, start stirring, open the jacket to cool the brine, slowly Slowly cool down to 10-20°C. Add isopropyl nitrite dropwise, and control the reaction temperature at 10-20°C. After the reaction is completed, use compressed air to hydraulically press the synthesis kettle to wash the kettle, add 200L of water each time, stir for 15-30 minutes, and wash for 3 times. ...
Embodiment 2
[0026] 1. Make a self-made copper-containing composite catalyst, add dimethylformamide into the reactor, fill with nitrogen for protection, then add 0.5 equivalent of ammonium formate and 0.8 equivalent of N, N, N', N'-tetraphenylethylenediamine and 0.2 equivalents of CuCl 2 and 0.02 equivalent of PdCl 2 , and then the mixture was heated to 45 ° C, fully stirred and maintained for 20 hours, cooled, filtered, and vacuum-dried.
[0027] 2. Add 150KG trichloroacetic acid, 8KG anhydrous magnesium sulfate, 1.5KG self-made copper-containing composite catalyst, 1500L benzene, and 80KG 2,4-difluoroaniline into the synthesis kettle, start stirring, open the jacket to cool the brine, Slowly cool down to 10-20°C. Add isopropyl nitrite dropwise, and control the reaction temperature at 10-20°C. After the reaction is completed, use compressed air to hydraulically press the synthesis kettle to wash the kettle, add 350L of water each time, stir for 15-30 minutes, wash 3 times, discharge th...
Embodiment 3
[0029] 1. Make a self-made copper-containing composite catalyst, add dimethylformamide into the reactor, fill with nitrogen for protection, then add 1.0 equivalent of ammonium formate and 1.0 equivalent of N, N, N', N'-tetraphenylethylenediamine and 0.3 equivalents of CuCl 2 and 0.03 equivalent of PdCl 2 , and then heat the mixture to 60°C, keep it fully stirred for 24 hours, cool, filter, and vacuum dry to obtain the final product;
[0030] 2. Add 200KG trichloroacetic acid, 10KG anhydrous magnesium sulfate, 2KG self-made copper-containing composite catalyst, 1500L benzene, and 100KG 2,4-difluoroaniline into the synthesis kettle, start stirring, open the jacket to cool the brine, and slowly Cool down to 10-20°C. Add isopropyl nitrite dropwise, and control the reaction temperature at 10-20°C. After the reaction is completed, use compressed air to hydraulically press the synthesis kettle to wash the kettle, add 500L of water each time, stir for 15-30 minutes, and wash 3 time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com