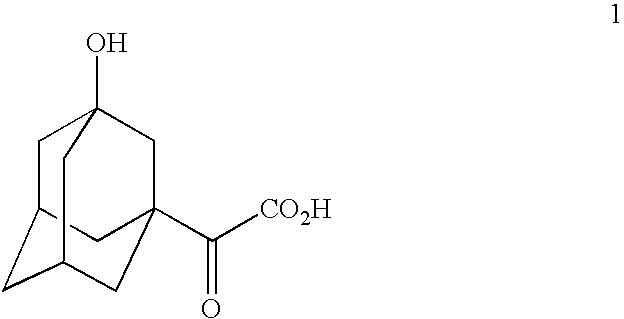
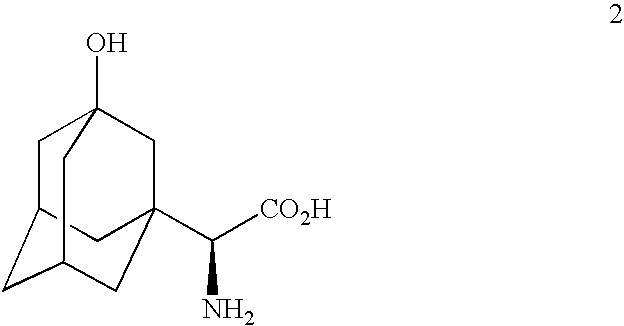
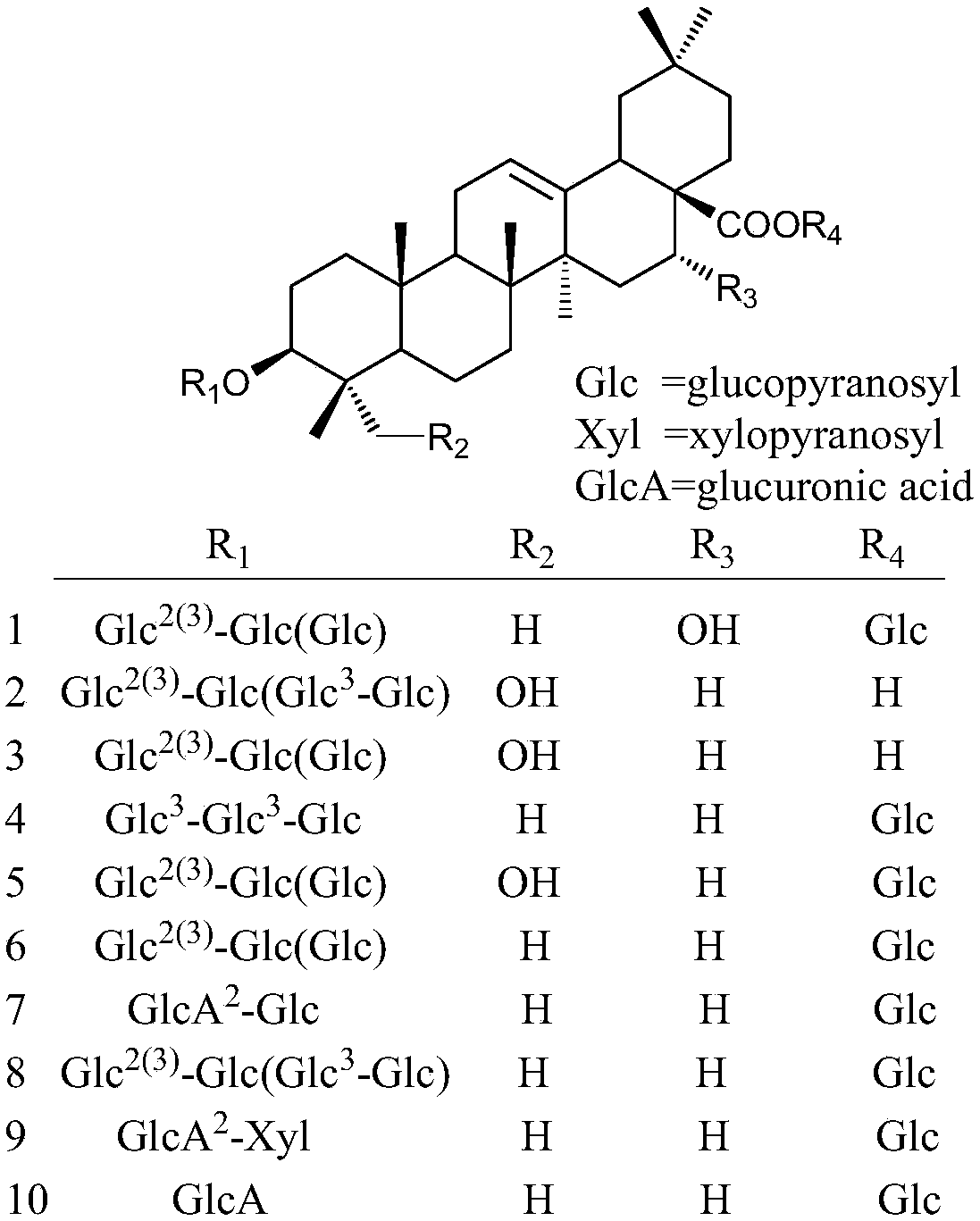
Patents
Literature
468 results about "Ammonium formate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ammonium formate, NH₄HCO₂, is the ammonium salt of formic acid. It is a colorless, hygroscopic, crystalline solid.
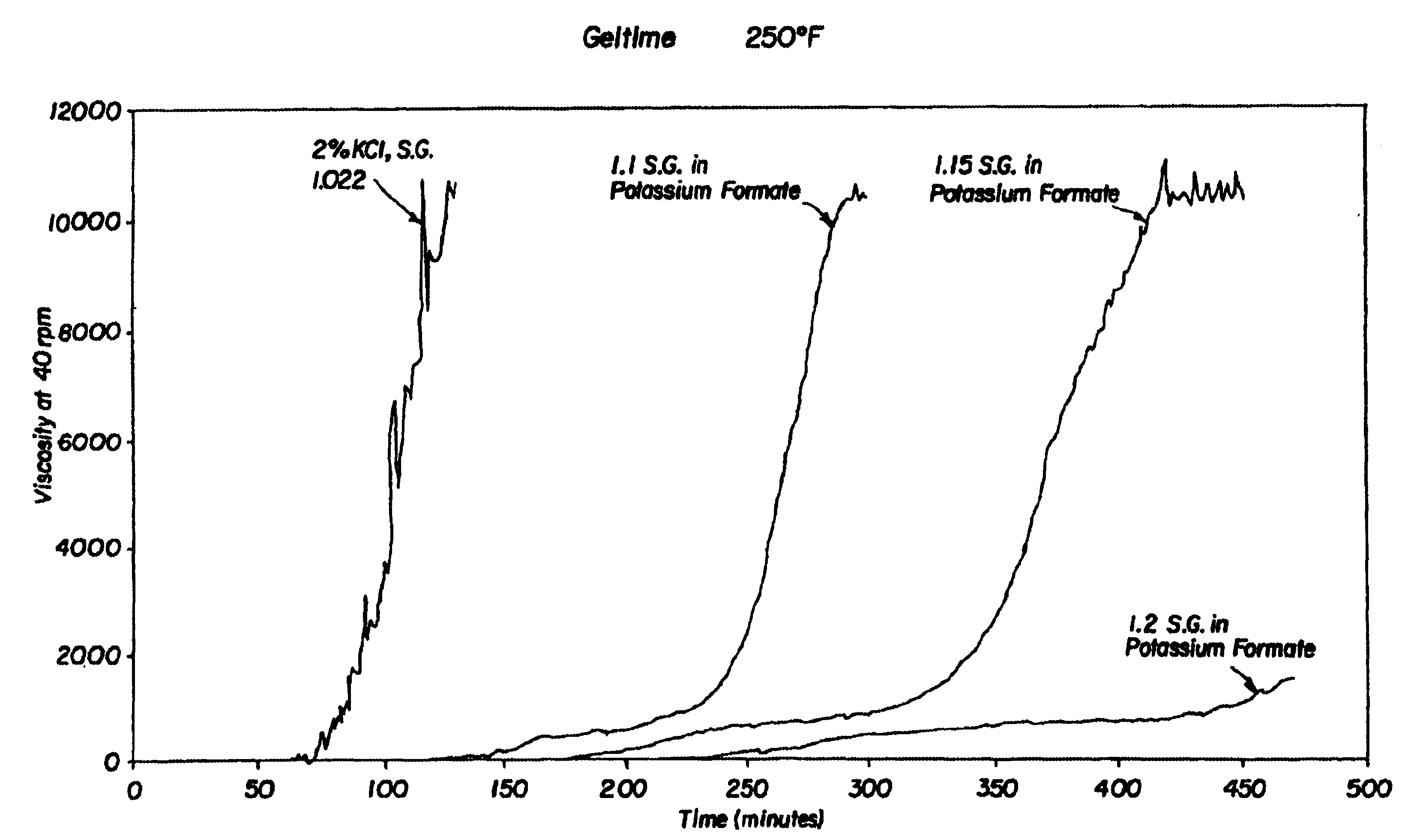
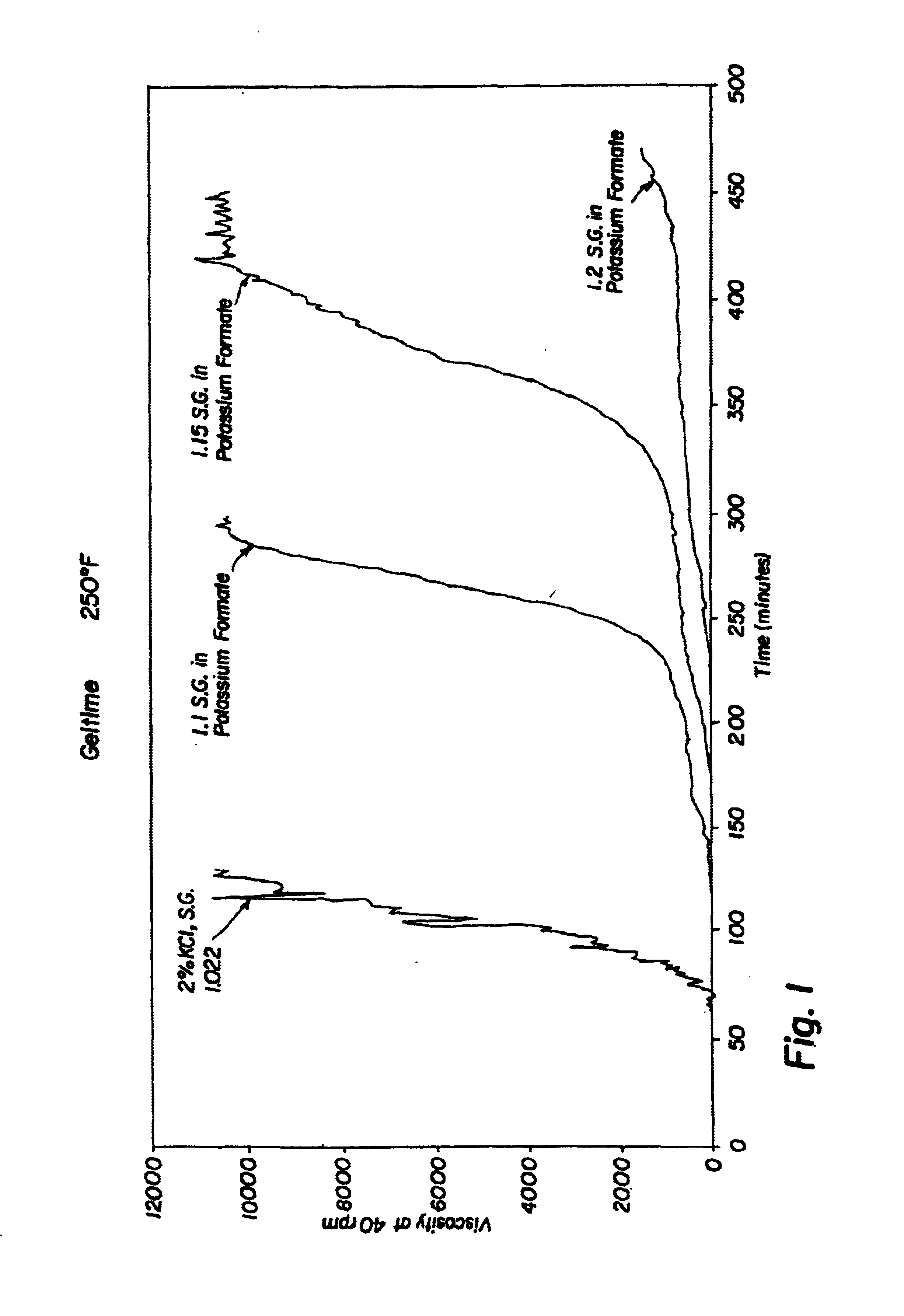
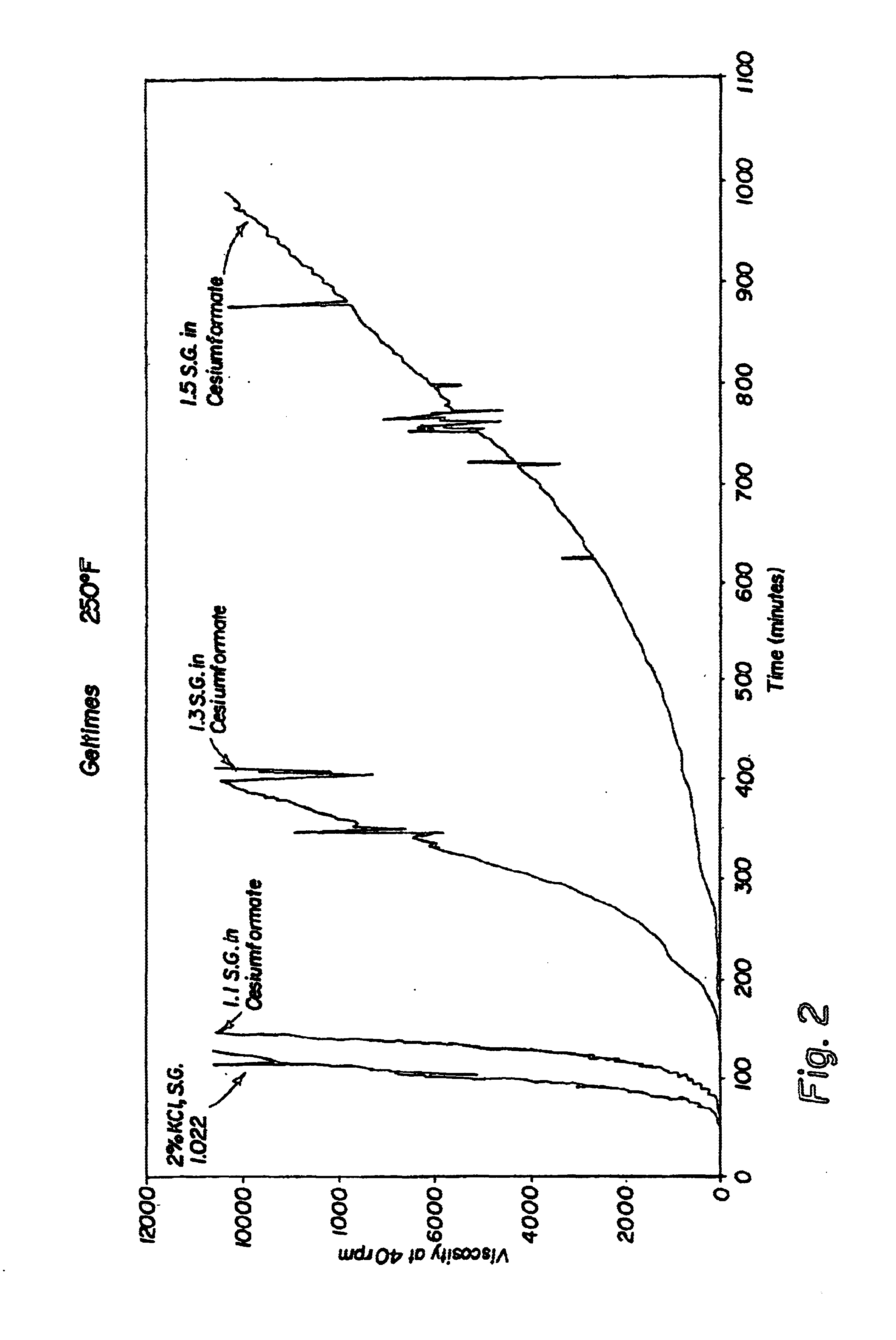
Compositions and methods including formate brines for conformance control
Compositions and methods are provided for reducing the permeability of subterranean zones. More particularly, water-soluble polymeric compositions which form cross-linked gels in the zones. In general, the composition comprises (a) at least one water-soluble polymer; (b) at least one organic gelling agent capable of cross-linking the water-soluble polymer; and (c) at least one water-soluble formate. More preferably, the water-soluble polymer is a copolymer of (i) at least one non-acidic ethylenically unsaturated polar monomer, and (ii) at least one copolymerisable ethylenically unsaturated ester. The gelling agent is preferably selected from the group consisting of a polyalkyleneimine, polyfunctional aliphatic amine, an aralkylamine, and a heteroaralkylamine. The preferred water-soluble formate is selected from the group consisting of ammonium formate, lithium formate, sodium formate, potassium formate, rubidium formate, cesium formate, and francium formate. Water is used to make an aqueous composition prior to use in a subterranean formation. The methods of this invention for reducing the permeability of a subterranean zone are comprised of the steps of introducing an aqueous composition according to the invention into a subterranean zone, and then allowing the aqueous composition to form a cross-linked gel in the zone. Preferably, the method includes the step of subsequently producing hydrocarbons from the subterranean formation.
Owner:HALLIBURTON ENERGY SERVICES INC
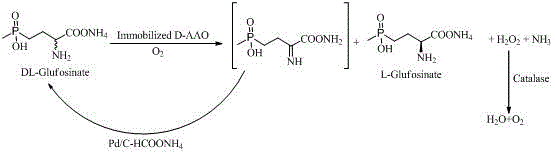
Enzyme-chemocatalysis racemization removing preparation method for L-glufosinate-ammonium
The invention discloses an enzyme-chemocatalysis racemization removing preparation method for L-glufosinate-ammonium. According to the method, a one-pot reaction manner is adopted, under the molecular oxygen, immobilization D-amino acid oxidase catalyzes D-enantiomer in an enantioselectivity mode into 2-imino-4-(hydroxy methyl phosphonyl) butyric acid in a dehydrogenation mode, and palladium-ammonium formate catalyzes 2-imino -4-(hydroxy methyl phosphonyl) butyric acid into DL-glufosinate-ammonium in an in-situ reduction mode. Hydrogen peroxide produced in the process is efficiently decomposed into water and oxygen through catalase. Complete reacemization removing of DL-glufosinate-ammonium and efficient preparing of L-glufosinate-ammonium are achieved through biological oxidation-chemical reduction circulation. The method has the advantages that the process is simple, cost is low, environmental friendliness is achieved, and energy is saved. High-concentration DL-glufosinate-ammonium can be converted into L-glufosinate-ammonium. The yield is 90%, the optical purity of the product is 99%, and the method is suitable for industrial production of L-glufosinate-ammonium.
Owner:重庆惠健生物科技有限公司
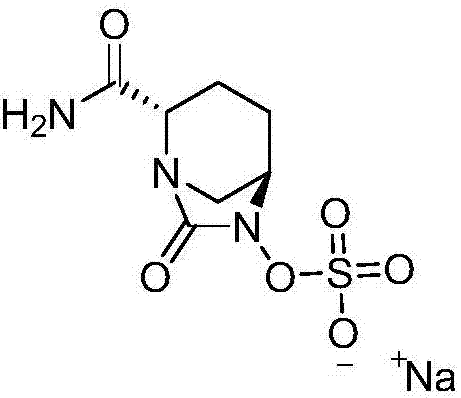
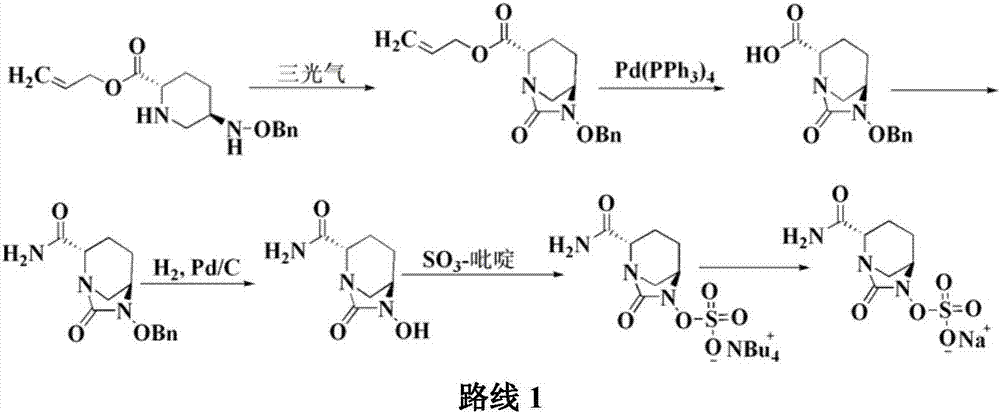
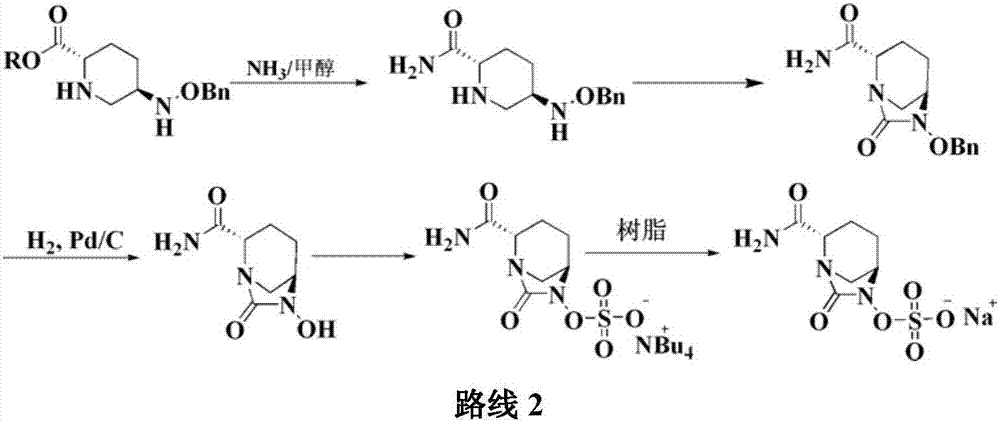
Method for preparing avibactam sodium through one-pot method
The invention discloses a method for preparing avibactam sodium through a one-pot method. The method comprises the following steps: by taking (2S,5R)-5-[(benzyloxy)amino]pyridine-2-ethyl carboxylate oxalate as a starting material, firstly generating a compound II by reacting with triphosgene, hydrolyzing, adding ammonia water to perform ammoniation, thereby obtaining a compound III, performing hydrogenation by taking formic acid, ammonium formate or hydrazine hydrate as a hydrogen donor in hydrogenation reaction, and then salifying to obtain the avibactam sodium. The avibactam sodium is prepared by use of the one-pot method, the raw material is cheap and easy to obtain, the reaction condition is mild, the operation is simple, the safety is higher, the yield is high, the purity is good, and the method is suitable for large scale industrial production. Formulae are shown in the description.
Owner:QILU TIANHE PHARMA
L-2-reanal biological preparation method
ActiveCN102605014ALow costMild transformation conditionsChemical recyclingFermentationSeparation technologyThreonine
The invention relates to an L-2-reanal biological preparation method, which includes: utilizing L-threonine as raw materials, stirring the L-threonine in water at the temperature of 15-50 DEG C under the catalytic action of L-threonine deaminase and whole cells with a leucine dehydogenase and coenzyme regenerating function, and obtaining L-2-reanal after reaction. In the L-2-reanal biological preparation method, water is used for substituting for buffer salt solution to form a water-phase reaction system, cost of raw materials is reduced, threonine deaminase and whole cells used for catalyzing can be produced in batch by fermentation of microorganisms, are low in cost and wide in sources. Biological enzymatic catalysis reaction is water-phase reaction, transformation conditions of the enzymic method are mild, raw materials are transformed thoroughly, post-treatment is simple, products are separated by isoelectric point crystallization technology and film evaporation separation technology jointly, and the L-2-reanal biological preparation method is capable of recovering water and ammonium formate is low in cost, free of discharge of waste water and waste residues, environment-friendly in process and applicable to industrialized production of the L-2-reanal.
Owner:ENZYMEWORKS
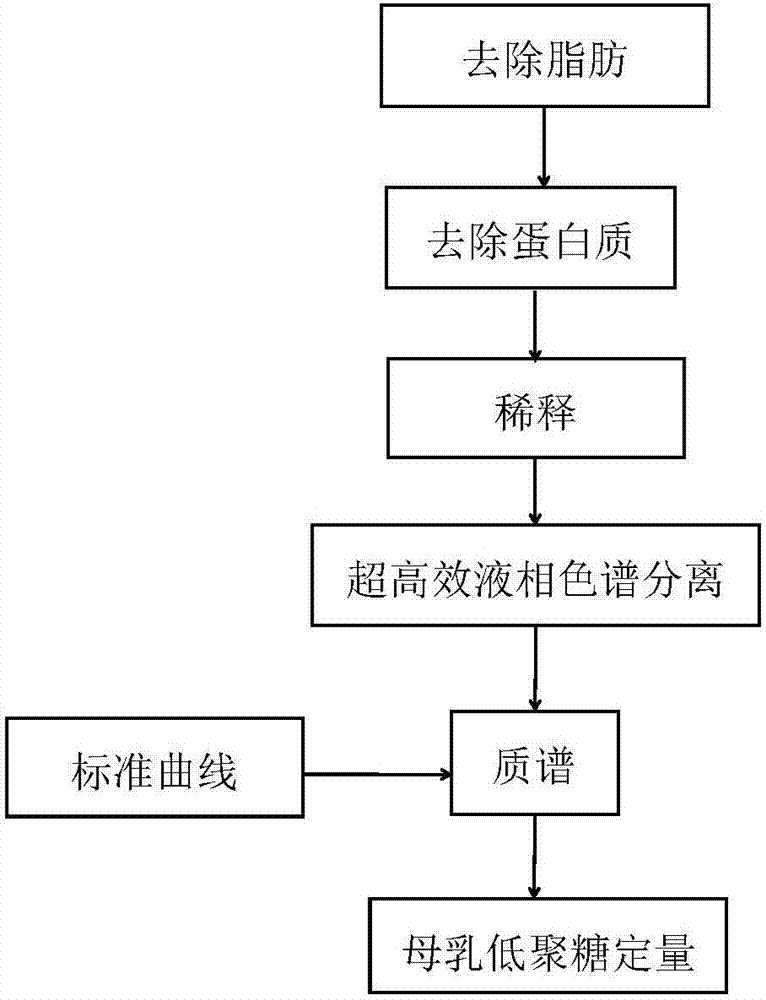
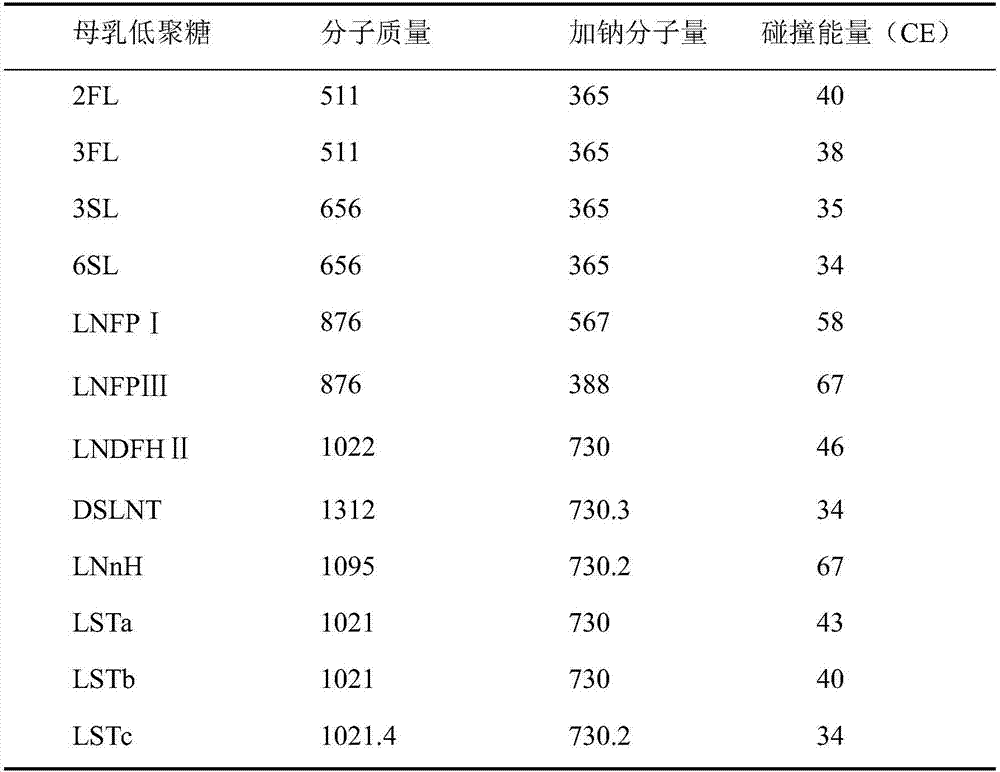
Quick qualitative and quantitative method for oligosaccharide in breast milk
ActiveCN107192771AAccurate detectionQuick checkComponent separationGradient elutionColumn temperature
The invention discloses a quick qualitative and quantitative method for oligosaccharide in breast milk. The quick qualitative and quantitative method mainly includes steps of 1, pretreating samples, to be more specific, removing fat and proteins from 150-250 micro-l of breast milk to obtain ultimate supernatant, adding ultra-pure water into the supernatant and diluting the supernatant to obtain the loaded samples; 2, establishing standard curves for standard substances by the aid of ultrahigh-performance liquid chromatography and mass spectrometry; 3, separating different components of the oligosaccharide in the breast milk in the loaded samples by the aid of ultrahigh-performance liquid mass spectrometry and carrying out quantitative analysis by the aid of mass spectrometry combined with the standard curves so as to obtain the content of the oligosaccharide in the breast milk. The ultrahigh-performance liquid chromatography is implemented by the aid of amino chromatographic columns with the sizes of 2.1*100 mm and 1.7 micrometers, 8-10 mmol / L of ammonium formate solution (A) and acetonitrile (B), and the ammonium formate solution (A) and the acetonitrile (B) are used as mobile phases; gradient elution programs are carried out by the aid of 95%-75% of B for 0-10 min or are carried out by the aid of 75% of B for 10-15 min or are carried out by the aid of 75%-65% of B for 15-20 min or are carried out by the aid of 65%-10% of B for 20-21 min or are carried out by the aid of 10% of B for 21-24 min or are carried out by the aid of 10%-95% of B for 24-25 min or are carried out by the aid of 95% of B for 25-35 min; the flow rates are 0.3 mL / min, and the column temperatures are 40-60 DEG C. The quick qualitative and quantitative method has the advantage that 12 types of oligosaccharide in the breast milk can be quickly detected by the aid of the quick qualitative and quantitative method and can be quantified by the aid of the quick qualitative and quantitative method.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
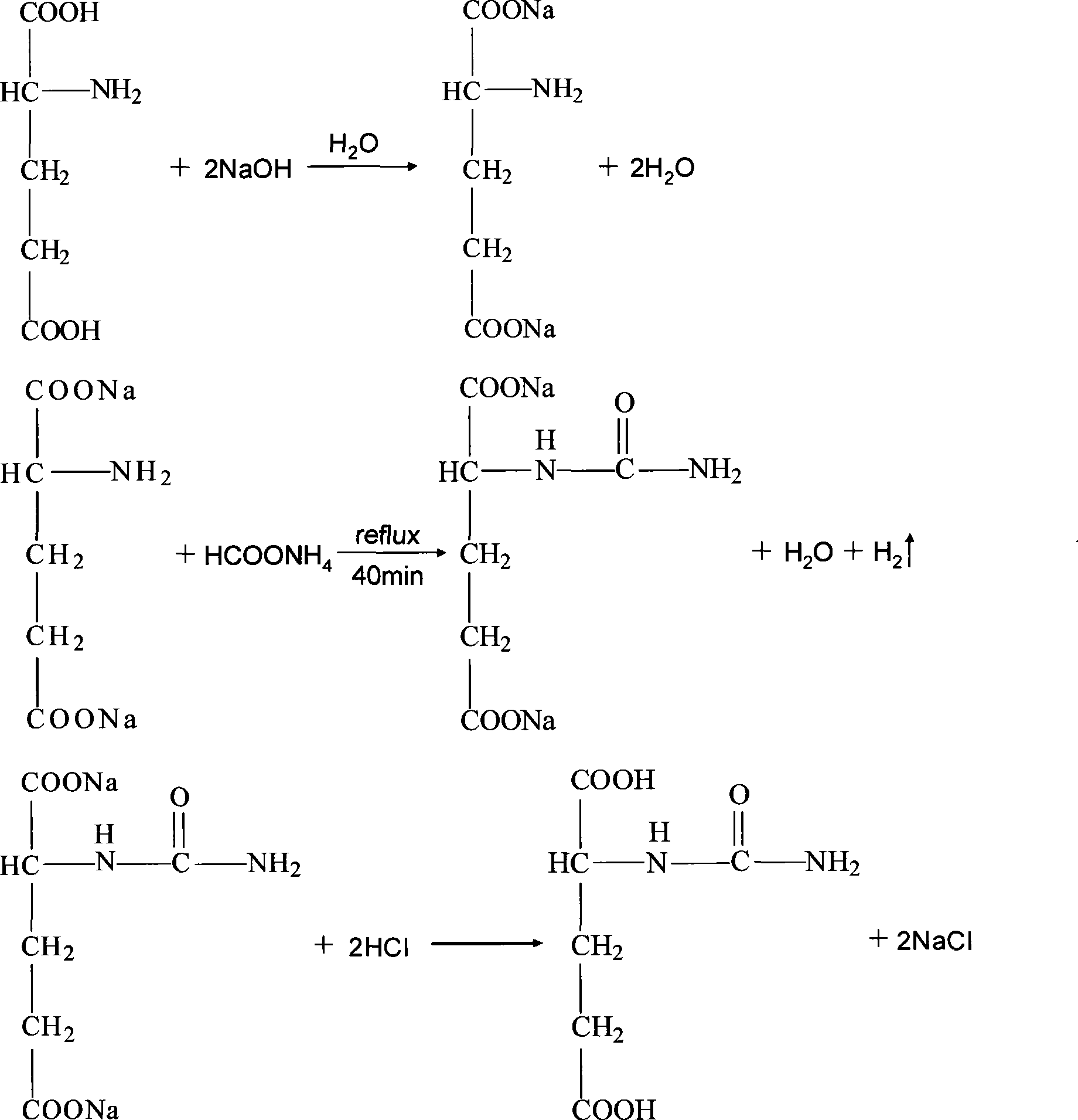
Preparation of N-carbamylglutamic
ActiveCN101440042ALow toxicityEasy to produceOrganic compound preparationAnimal feeding stuffCarbamylglutamic acidRoom temperature
The invention relates to a method for preparing N-carbamylglutamic acid and application thereof. The method comprises the following steps: (1) according to molar ratio of 0.8-1.6:0.8-1.6:1.6-3.2, glutamic acid, ammonium formate and sodium hydroxide are weighed for standby; (2) the weighed glutamic acid and sodium hydroxide are dissolved in distilled water and are stirred; the obtained mixture is added with the weighed ammonium formate and is stirred; the total mixture reflows for 30 to 55 minutes at a temperature of between 98 and 110 DEG C; and (3) the reaction mixture obtained in the step (2) is cooled to room temperature, is washed by formaldehyde, is filtered, is added with concentrated hydrochloric acid, is acidified, is kept stand at a temperature of between 4 DEG C below zero and 2 DEG C, is crystallized and is filtered to obtain the N-carbamylglutamic acid. Compared with the prior method, the method for preparing the N-carbamylglutamic acid has the following advantages: the method reduces the cost and toxicity of the raw materials, has simple and high-efficient process and is favorable for large-scale production. The N-carbamylglutamic acid prepared through the method has the application that the N-carbamylglutamic can be used as a pig feed additive.
Owner:林州亚太兴牧科技有限公司
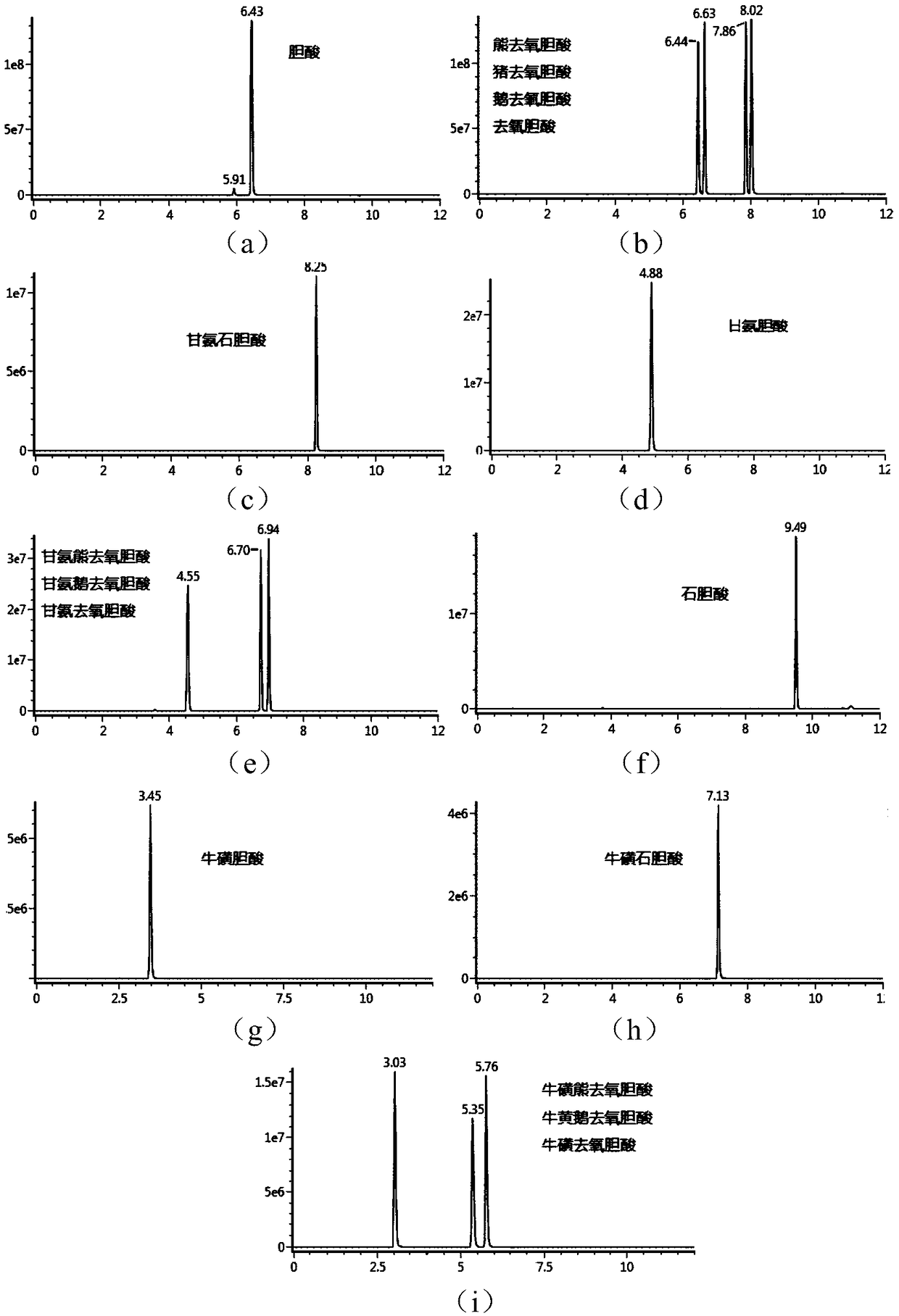
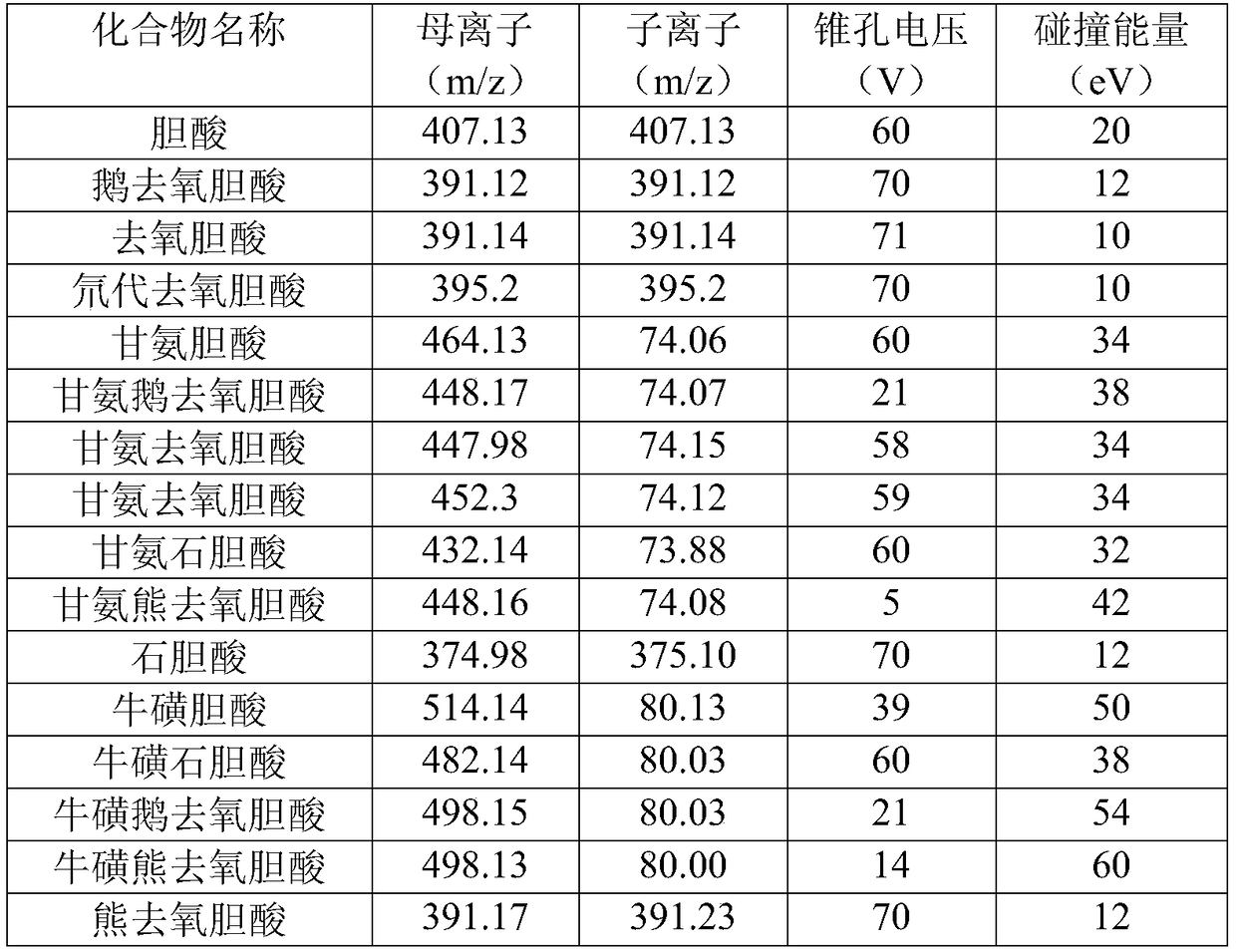
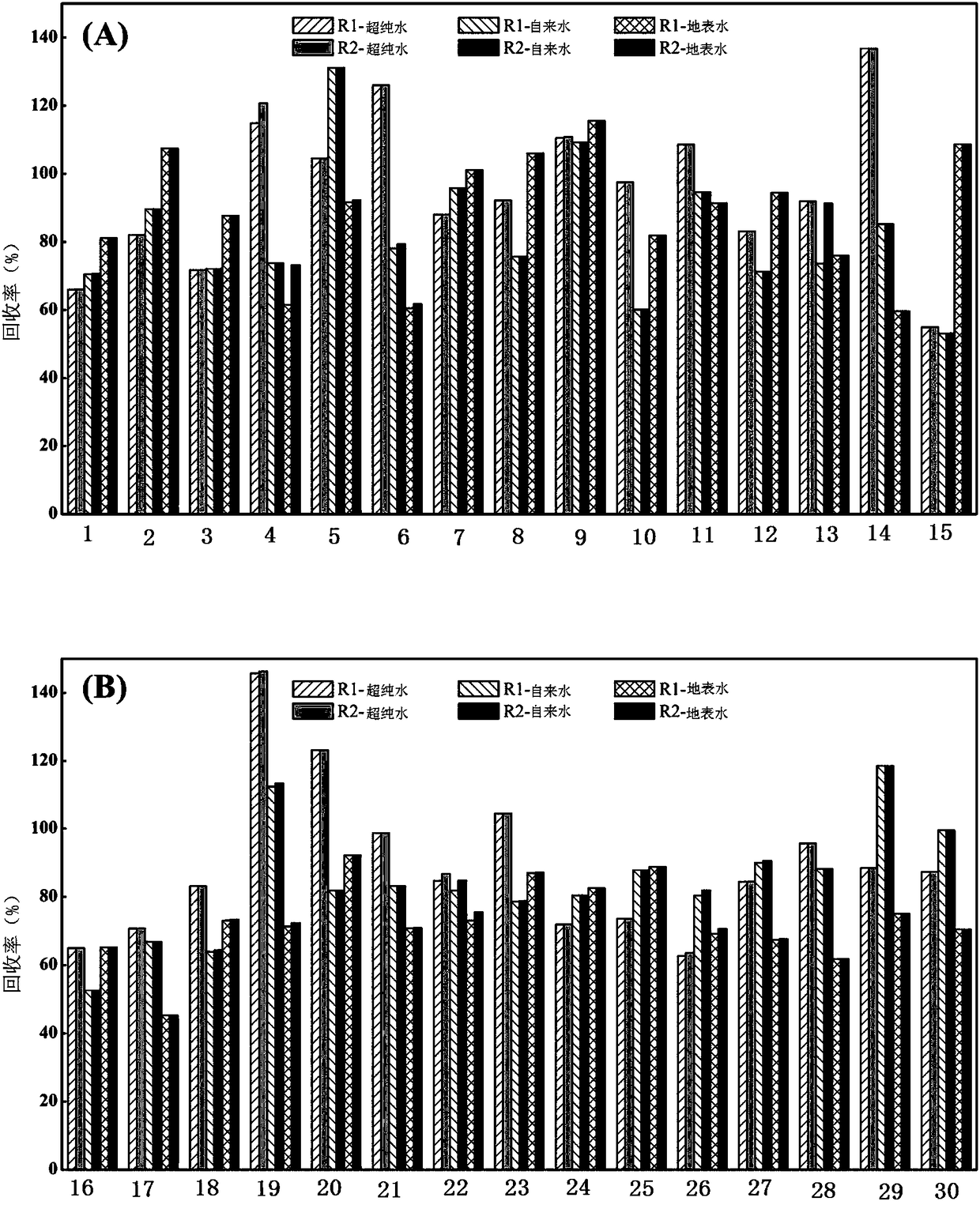
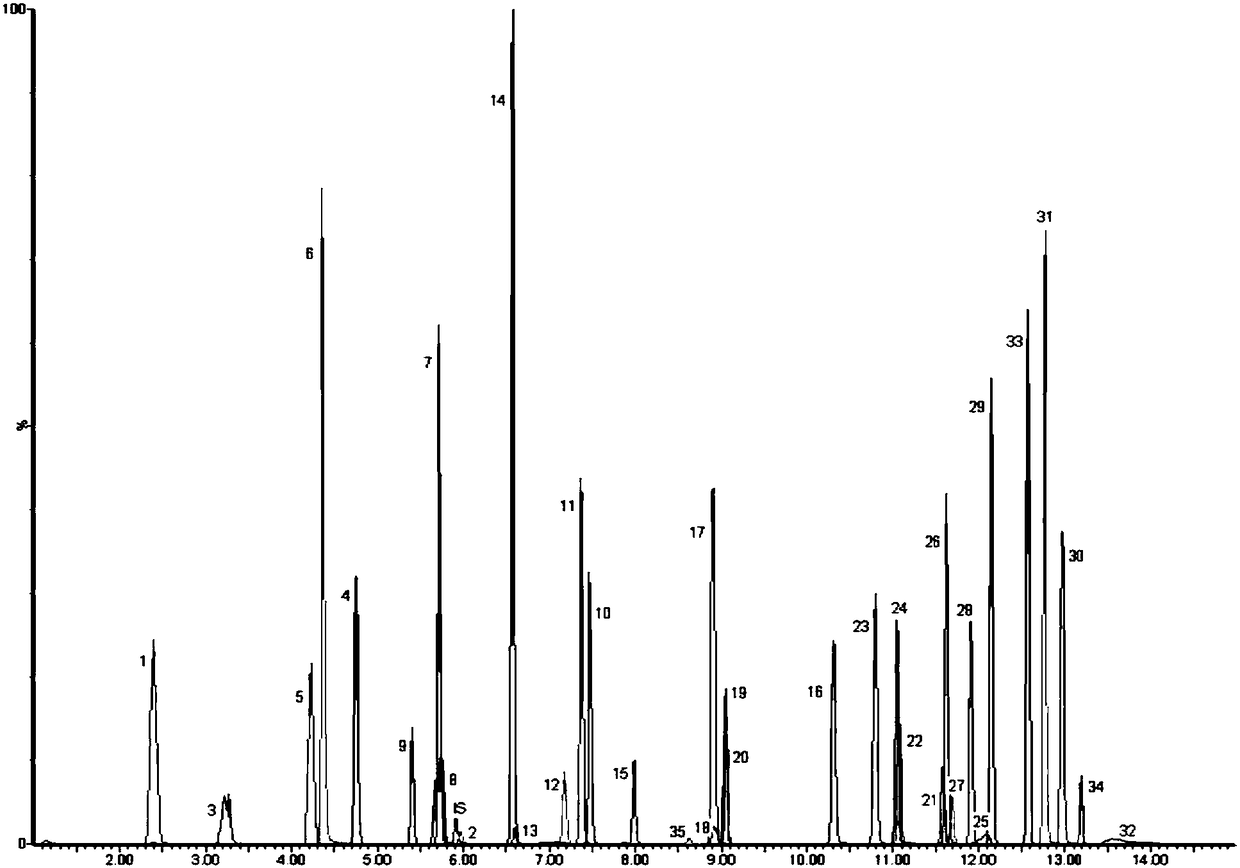
Tandem mass spectrum detection kit capable of simultaneously measuring 16 types of bile acid, and application thereof
The invention discloses a tandem mass spectrum detection kit capable of simultaneously measuring 16 types of bile acid. The kit comprises a standard substance, a quality control substance, an interiorlabel substance, precipitant, diluent and a flowing phase, wherein the precipitant is at least one of methyl alcohol, ethyl alcohol and acetonitrile; the diluent consists of water, methyl alcohol, ethyl alcohol and acetonitrile, wherein the volume ratio of water, methyl alcohol, ethyl alcohol to acetonitrile is (1-50):(0-90):(0-50):(0-50), and methyl alcohol, ethyl alcohol and acetonitrile are not zero at the same time; and the flowing phase comprises a flowing phase A and a flowing phase B, wherein the flowing phase A is the aqueous solution of ammonium formate or ammonium acetate-methanoicacid or acetic acid, and the flowing phase B is methyl alcohol and / or acetonitrile. The invention also discloses the application of the tandem mass spectrum detection kit. The tandem mass spectrum detection kit disclosed by the invention has the characteristics of high sensitivity, good specificity and the like, can accurately, simply and quickly simultaneously detect 16 types of bile acid in a matrix, and has a high application value.
Owner:易达精准(杭州)科技有限公司
Method for rapid detection of multi-class pharmaceutical and personal care products and pesticides in water
ActiveCN108254481ARule out false positivesThe test result is accurateComponent separationAmmonium formateTandem mass spectrometry
The invention provides a method for rapid detection of multi-class pharmaceutical and personal care products and pesticides in water. The method includes: taking a to-be-detected water sample, and subjecting the to-be-detected water sample to pretreatment to obtain a to-be-detected sample; employing one or more groups of the following five groups of detection conditions for liquid chromatography-tandem mass spectrometry detection on the to-be-detected sample respectively so as to realize qualitative analysis and quantitative detection of multi-class pharmaceutical and personal care products and pesticides in the to-be-detected water sample: (a) the mobile phase is a formic acid-ammonium formate aqueous solution and acetonitrile, and the ionization mode is electrospray ionization positive mode; (b) the flow phase is a formic acid aqueous solution and acetonitrile, and the ionization mode is electrospray ionization positive mode; (c) the mobile phase is an acetic acid-ammonium acetate aqueous solution and acetonitrile, and the ionization mode is electrospray ionization negative mode; (d) the mobile phase is water and acetonitrile, and the ionization mode is electrospray ionization negative mode; and (e) the mobile phase is a formic acid-ammonium formate solution and acetonitrile, and the ionization mode is electrospray ionization positive mode.
Owner:SHENZHEN INST OF ADVANCED TECH
Hydrophilic interaction chromatography-tandem mass spectrometry detection method of phospholipids in Metapenaeus ensis
ActiveCN106153763ASimple and fast operationEasy to trainComponent separationLipid formationPhospholipid
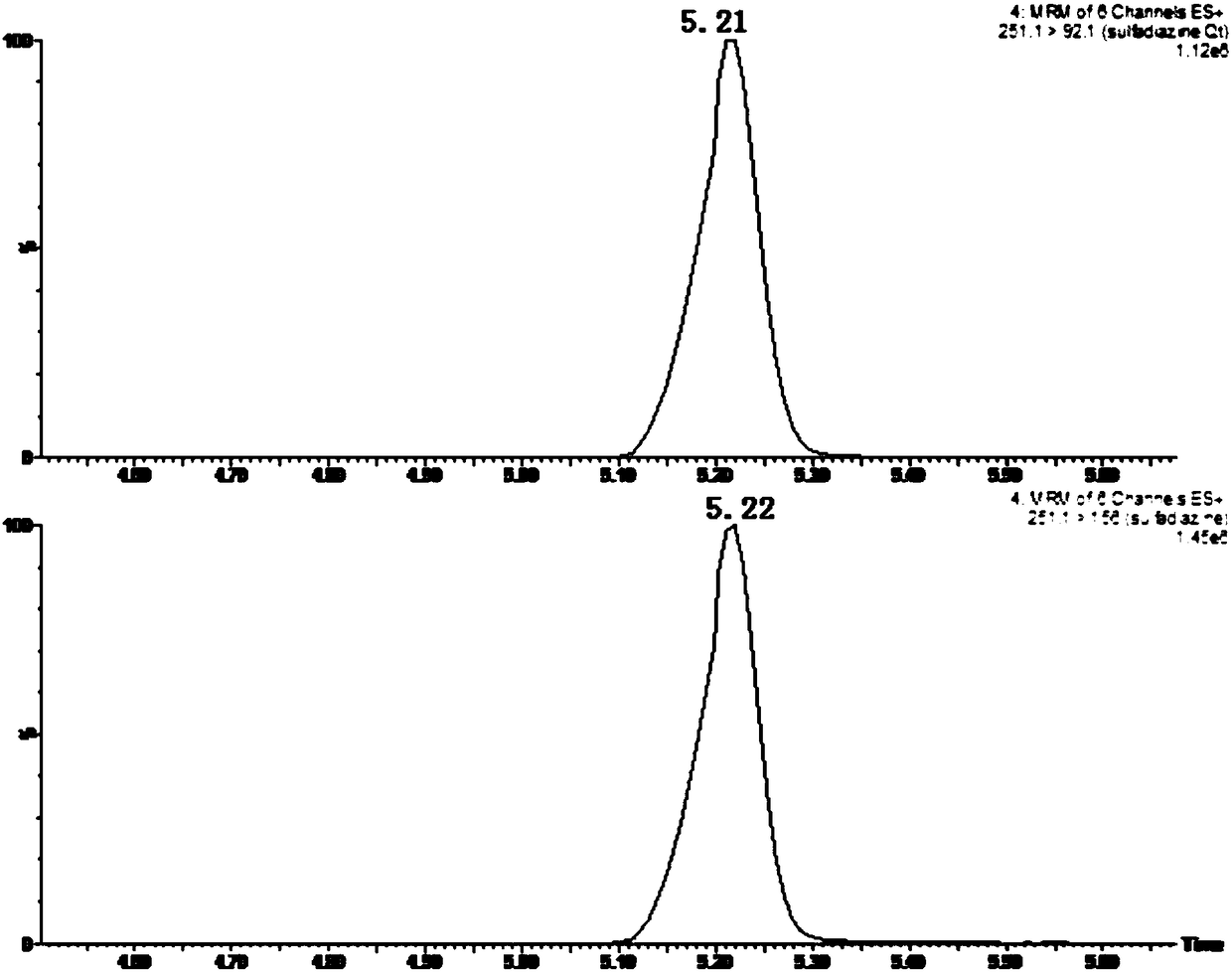
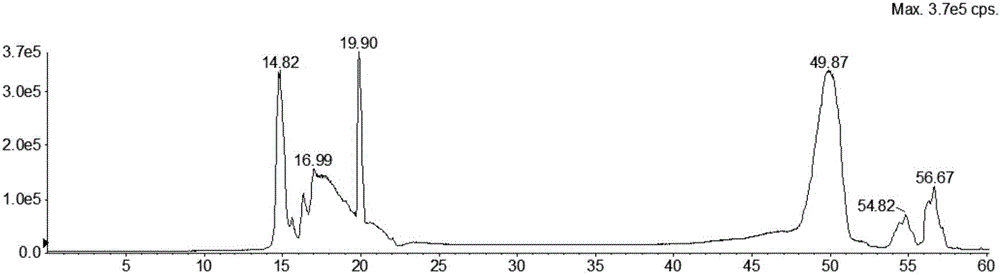
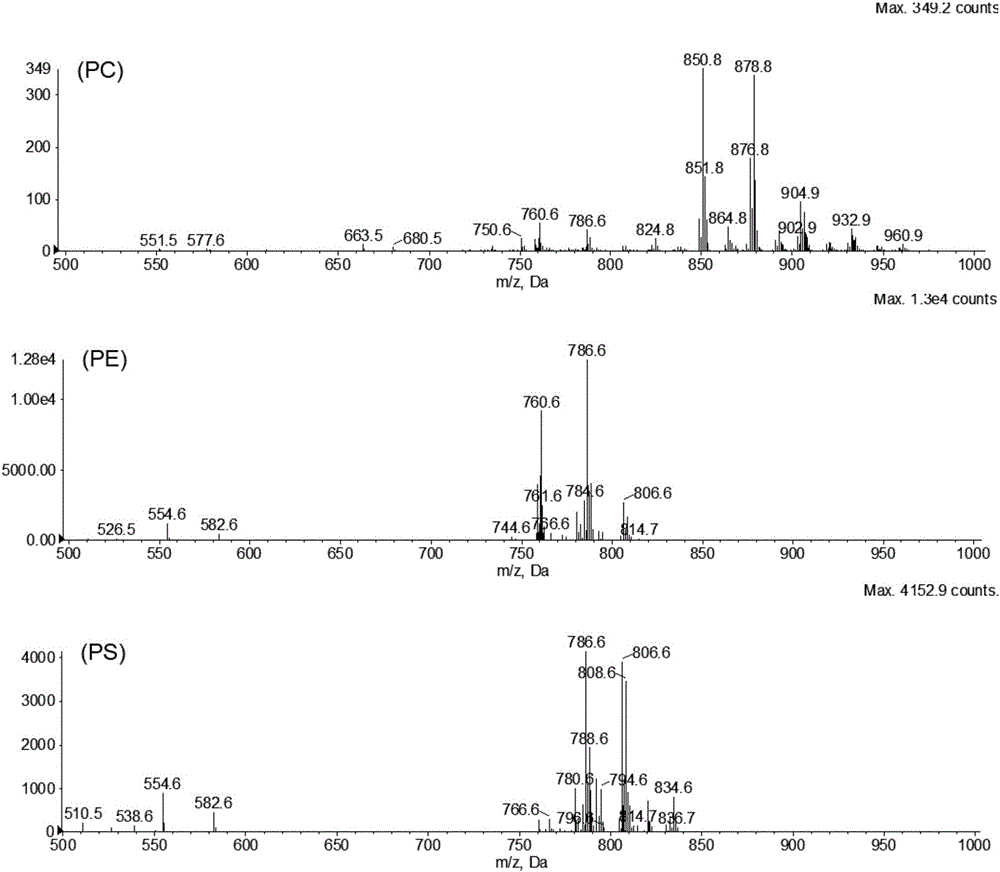
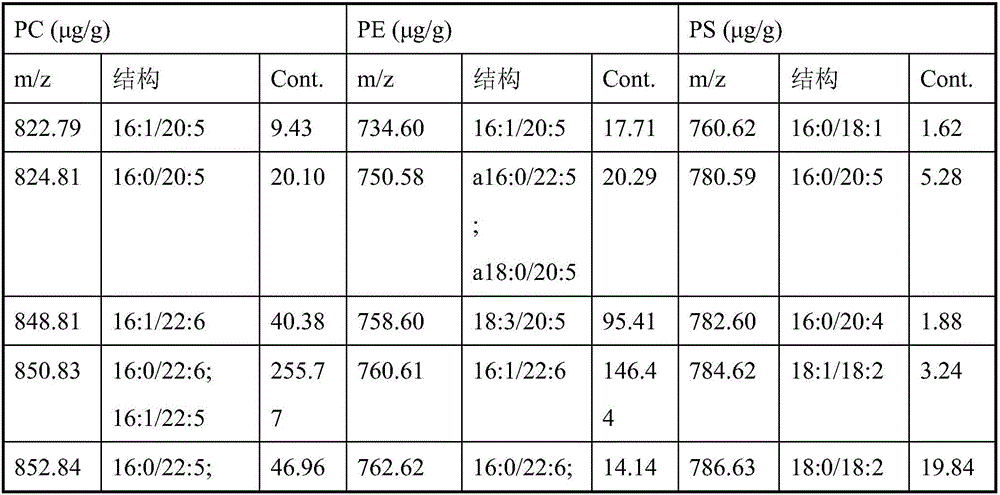
The invention discloses a hydrophilic interaction chromatography-tandem mass spectrometry detection method of phospholipids in Metapenaeus ensis. The method includes the steps of firstly, performing corresponding preprocessing on ground Metapenaeus ensis to obtain crude lipid extract; secondly, performing liquid phase separation on the crude lipid extract, wherein a chromatographic column is a YMC Triart diol HILIC column, a gradient elution method is used, a gradient system comprises a flowing phase A and a flowing phase B, the flowing phase A is an acetonitrile solution containing 53mmol / L formic acid, and the flowing phase B is an aqueous solution containing 60mM ammonium formate and 53mM formic acid; thirdly, performing mass spectrometric detection analysis on the elution liquid obtained in the second step. By the method, the total content of PE, PS and PC in the Metapenaeus ensis can be detected accurately, and mass spectrometry identification of various molecular species in the PC, PE and PS phospholipids can be achieved.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
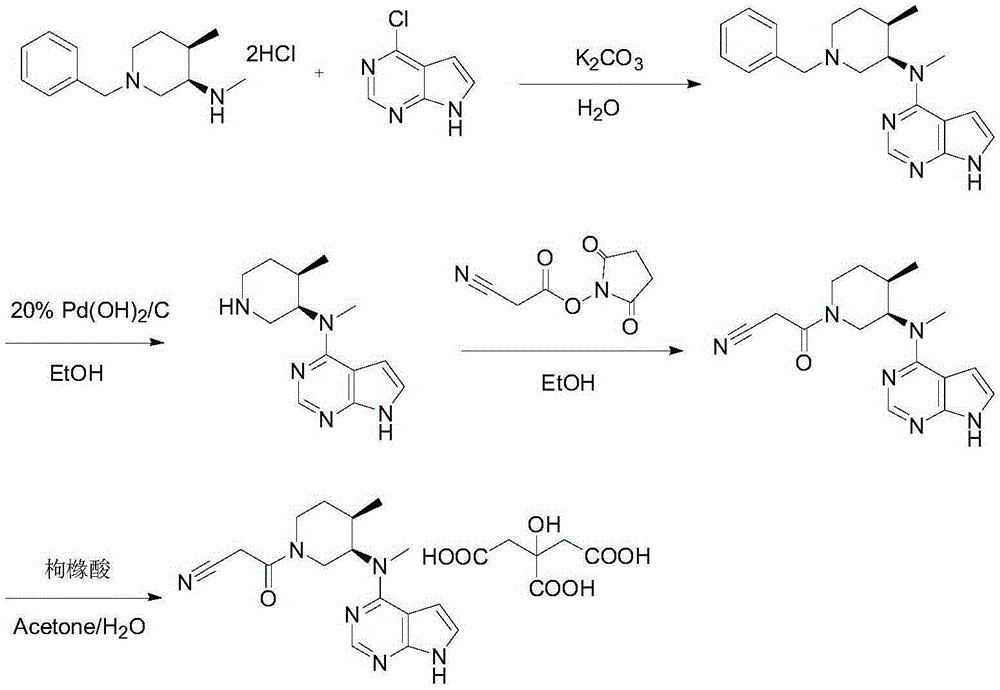
Method for synthesizing citric acid tofacitinib
ActiveCN106146517AEliminate potential safety hazardsShort reaction timeCarboxylic acid salt preparation4-methylpiperidineTofacitinib
The invention discloses an efficient and safe method for synthesizing citric acid tofacitinib. N-[(3R,4R)-1-benzyl-4-methylpiperidine-3-base]-N-methyl-7H-pyrrolo[2,3-d]pyrimidine-4-amine is used as the raw material, Pd / C and HCOOH reduction debenzylation is conducted, condensation is conducted under the catalysis of an EDCI or EDCI, HOBT and triethylamine compound system with cyanoacetic acid, and salifying is conducted with citric acid in acetone to obtain citric acid tofacitinib. By the adoption of the synthesis method, potential safety hazards caused by hydrogen and ammonium formate are avoided, debenzylation reaction and amidation are thorough, no side reaction is caused basically, reaction time is shortened greatly, yield is high, aftertreatment is easy, and citric acid tofacitinib can be prepared efficiently and safely.
Owner:ZHEJIANG LEPU PHARMA CO LTD
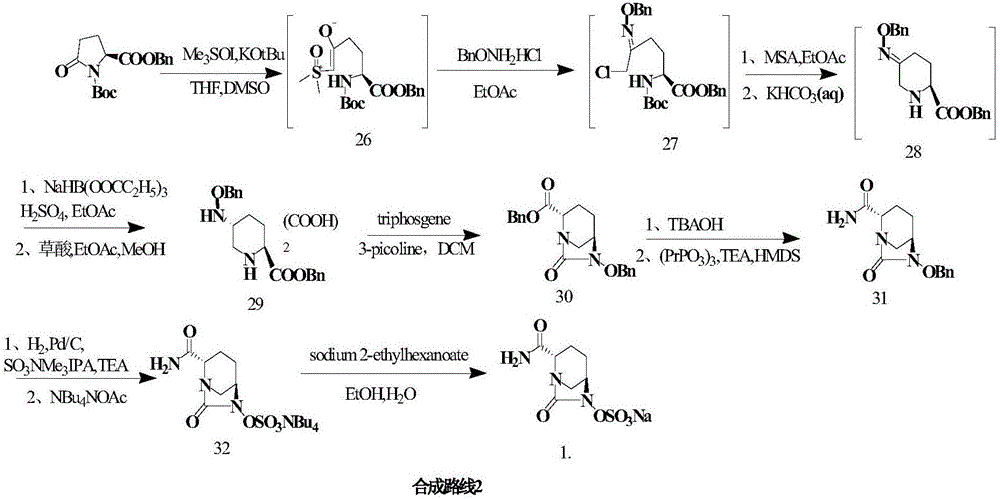
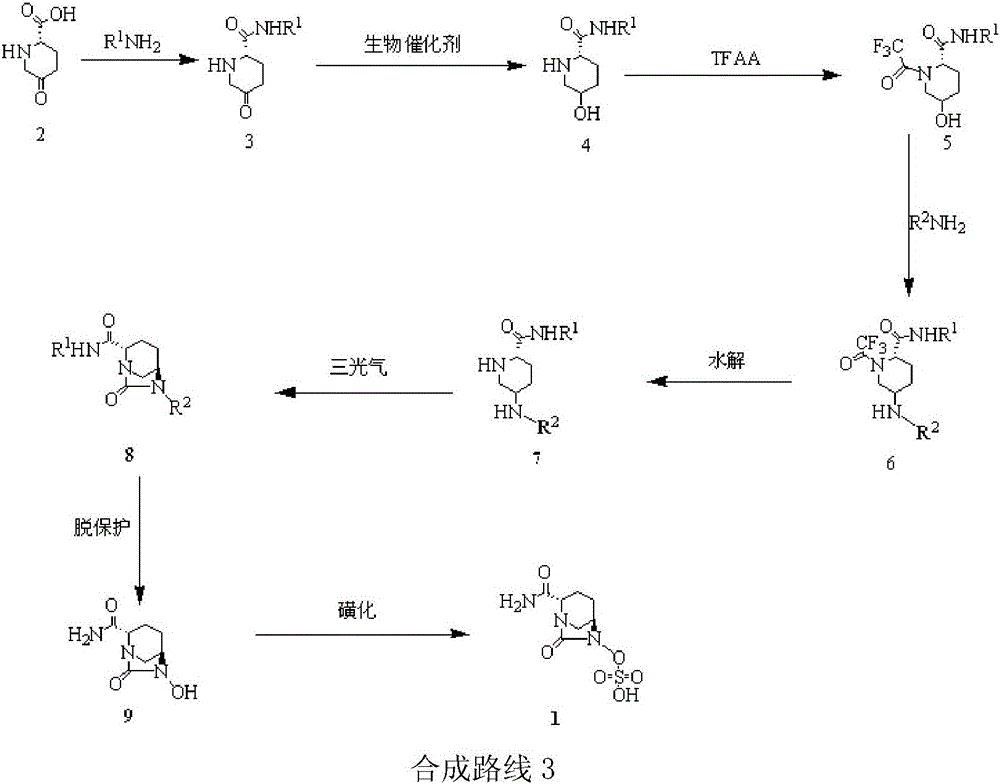
Method for synthesizing beta-lactamase inhibitor Avibactam
The invention relates to a method for synthesizing a beta-lactamase inhibitor Avibactam and belongs to the technical field of preparation of beta-lactamase inhibitors. The method disclosed by the invention comprises the following steps: (1) taking a compound 2 as a raw material, and reacting with R<1>NH2 so as to produce a compound 3; (2) enabling the compound 3 and a biocatalyst to produce a compound 4 in an organic solvent in the presence of glucose or sucrose; (3) enabling the compound 4 to react with trifluoroacetic anhydride so as to obtain a compound 5; (4) enabling the compound 5 to react with R<2>ONH2 so as to produce a compound 6; (5) hydrolyzing the compound 6 under alkaline conditions so as to produce a compound 7; (6) enabling the compound 7 to react with triphosgene so as to produce a compound 8; (7) enabling the compound 8 to react with ammonium formate in the presence of a catalyst so as to produce a compound 9 in the organic solvent, wherein the catalyst is a Pd / C system; and (8) enabling the compound 9 to react with a sulfonating agent, thereby obtaining the product 1. The method disclosed by the invention is stable in process, high in yield, simple and safe in operation and low in production cost.
Owner:YIYUAN XINQUAN CHEM
Method for preparing biological aviation kerosene by using swell-cooked dirty oil
InactiveCN102994138AResolve tensionAvoid pollutionLiquid hydrocarbon mixture productionBio-feedstockSlagKerosene
The invention relates to a method for preparing biological aviation kerosene by using swell-cooked dirty oil. The method comprises the following steps: filtering out slag, desalting, dewatering and decoloring, then conducting fractional distillation, circulating three to five times together with ammonium formate in circulating type microwave reaction equipment under the action of a Pd / Al2O3 catalyst, extracting together with ethyl acetate, then desalting and dewatering, adding into a fractionating tower for fractionating to obtain biological aviation kerosene. The biological aviation kerosene prepared by adopting the method reaches the existing biological aviation kerosene standard, the shortage of biological aviation kerosene can be effectively overcome, the recovered swell-cooked dirty oil can avoid polluting the environment, and the resources can be saved.
Owner:陈秀海
Detection of drug concentration of osimertinib in human plasma and/or cerebrospinal fluid by combining UPLC-MS/MS
The invention relates to detection of the drug concentration of osimertinib in human plasma and / or cerebrospinal fluid by combining UPLC-MS / MS. According to the present invention, the method for determining the drug concentration of osimertinib in human plasma and / or cerebrospinal fluid through an ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS / MS) is provided, wherein formic acid-containing ammonium formate water-formic acid acetonitrile is used as a mobile phase so as to perform the method; and the method has advantages of good specificity and high sensitivity, and is used for the detection of clinical pharmacokinetic samples, wherein the linearity is good when the osimertinib concentration is 2-500 ng.ml<-1> in the plasma sample and the osimertinib concentration is 0.5-20 ng.ml<-1> in the cerebrospinal fluid sample.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Ultra-low temperature urea solution used for vehicle
ActiveCN104607041ASimple compositionEasy to manufactureDispersed particle separationPropylene glycolAmmonium formate
The invention discloses an ultra-low temperature urea solution uses for a vehicle. The urea solution is prepared from the following raw materials by weight: 27 to 31 parts of a 75 wt% urea solution, 20 to 25 parts of ammonium formate, 10 to 15 parts of propylene glycol and 34 to 40 parts of high-purity water. The ultra-low temperature urea solution provided by the invention is simple in composition and convenient in preparation, has a freezing point lower than -45 DEG C, is applicable to northern cold areas in winter in and has high practical value.
Owner:HENAN HONGKANG ENVIRONMENTAL PROTECTION TECH
Method for directly detecting amino acid in biological tissue uniform sample by adopting high performance liquid chromatography-tandem mass spectrometry
ActiveCN107300594ASimple processing methodReduce dosageComponent separationAcetic acidBuffer solution
The invention discloses a method for directly detecting amino acid in a biological tissue uniform sample by adopting high performance liquid chromatography-tandem mass spectrometry. The method comprises the step of treating a sample with an extracting solution, wherein the extracting solution is composed of a liquid chromatogram moving phase, including acetonitrile and buffer solution; the concentration of acetonitrile is 50-95%; the buffer solution is composed of formic acid with concentration of 0.1%-0.3% and ammonium formate with concentration of 1-10mM or is composed of acetic acid with concentration of 0.1%-0.3% and ammonium acetate in concentration of 1-10mM; the pH of the extracting solution is within 3-5.5. The method disclosed by the invention can be used for directly detecting the biological tissue uniform sample, such as, blood, urine and cerebrospinal fluid; the sample treating method is simple; the sample dosage is less and can be as low as 4mu l; the detection sensitivity is high and can reach up to pg / ml; the detectable amino acid concentration range is wide; the matrix interference is less; the detection accuracy is high; up to 29 amino acids can be simultaneously detected and the detection time is short.
Owner:EXPERIMENTAL RES CENT CHINA ACAD OF CHINESE MEDICAL SCI
Dendritic golden nanophase material and preparation method thereof
InactiveCN101538736ASimple processProcess safetyPolycrystalline material growthFrom normal temperature solutionsAmmonium formatePolyvinylpyrrolidone
The invention relates to a dendritic golden nanophase material and a preparation method thereof. The gold nanophase material is dendritic, and all golden branches are formed by mutually stuck and fused golden nano particles or formed in a twin-crystal structure for growing. The preparation method comprises the following steps: pure water is added with chloroauric acid or chloraurate, ammonium formate and polyvinyl-pyrrolidone stabilizer and then transferred to a hydro-thermal reaction vessel after even ultrasonic mixing; after heating is carried out for 1-6 hours, a black mixturei is obtained after natural cooling; and the obtained product naturally subsides for 24-48 hours with ethyl alcohol or acetone or centrifugally subsides for at least 30 minutes, and after the liquid on the upper layer is removed, the product is added with absolute ethyl alcohol or acetone for dilution, and the black dendritic golden nanophase material deposition is obtained after centrifugal settling; and the ethyl alcohol or acetone washing process is repeated for 2-3 times, and finally, the black dendritic golden nanophase material coated with the polyvinyl-pyrrolidone is obtained. The dendritic golden nanophase material can be used as a commercial catalyst, a surface enhanced Raman substrate material, and the like. The method has the advantages of simple process and device and safe operation; and large-scale production can be performed by the method.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
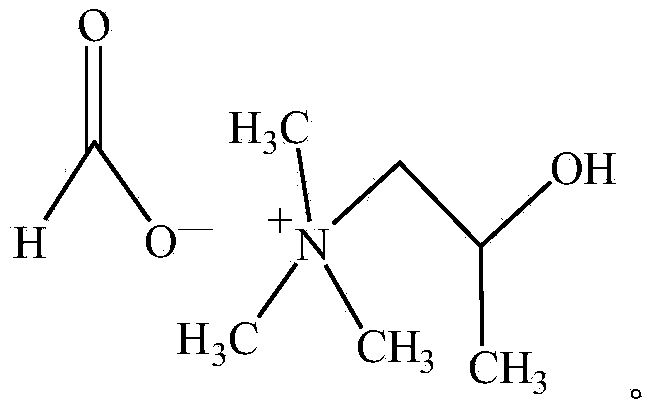
Composition, hard polyurethane foam material and refrigerating equipment
The invention discloses a composition, a hard polyurethane foam material and refrigerating equipment. The composition is prepared from the following components including premixed polyether polyol and isocyanate, wherein the premixed polyether polyol contains polyol, a foaming agent and a catalyst composition, the foaming agent contains trans 1-chloro-3,3,3-trifluoropropene, and the catalyst composition contains 1,2-dimethylimidazole, N,N-dimethyl cyolohexlemine and (2-hydroxypropyl) trimethyl ammonium formate. The composition has stability during long-term storage. In addition, the foam material obtained by adopting the composition has the characteristics of good demoulding, strong adhesion, low thermal conductivity and the like, so that the obtained product is good in energy saving effect.
Owner:HEFEI HUALING CO LTD
Goserelin acetate solid-phase synthesis method
ActiveCN104910257ATo solve such a drawback that cannot be monitoredLow costLuteinising hormone-releasing hormonePeptide preparation methodsSide chainEthylic acid
The invention relates to a goserelin acetate solid-phase synthesis method, which comprises the following steps: HBTU / DIPEA is employed as a condensation system, Fmoc-Ser-OH, Fmoc-Trp-OH are successively coupled; a whole-protection lysate with corresponding voluminal amount according to 10 times of resin weight is added, a carrier 2-CTC Resin in an intermediate is removed, all side chain protective groups are reserved; the whole-protection lysate is adjusted to slight alkaline by using DIPEA(N,N-diisopropylethylaine), semicarbazide hydrochloride and PyBop(1H-benzotriazole-1-oxygen tripyrrole alkyl hexafluorophosphate) (used for a coupling agent of peptide) are added in the whole-protection lysate for reaction coupling, a goserelin peptide solution with the side chain protective group is obtained; the lysate with 20% of TFA / DCM is added in a freezing ether for settling to obtain the white solid crude peptide; the white solid crude peptide is dried under vacuum for solving by methyl alcohol, ammonium formate and Pa / c are added for a hydrogenation reaction to remove the side chain protective group in a peptide sequence. According to the invention, side reaction phenomena can be avoided, target peptide purity is increased, yield is high, operation is convenient and feasible, the intermediate can be tracked and controlled, and the whole process is benefit for enlarged production.
Owner:苏州天马医药集团天吉生物制药有限公司
Process for preparing dipeptidyl peptidase IV inhibitors and intermediates therefor
InactiveUS20050260712A1Procedure of process can be improvedReduce processing timeSugar derivativesBacteriaPhenylalanine dehydrogenaseDipeptidyl peptidase
A process for production of cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV is provided which employs a BOC-protected amine of the structure prepared by subjecting an acid of the structure to reduce amination by treating the acid with ammonium formate, nicotinamide adenine dinucleotide, dithiothreitol and partially purified phenylalanine dehydrogenase / formate dehydrogenase enzyme concentrate (PDH / FDH) and without isolating treating the resulting amine of the structure 2 with di-tert-butyl dicarbonate to form the BOC-protected amine.
Owner:ASTRAZENECA AB
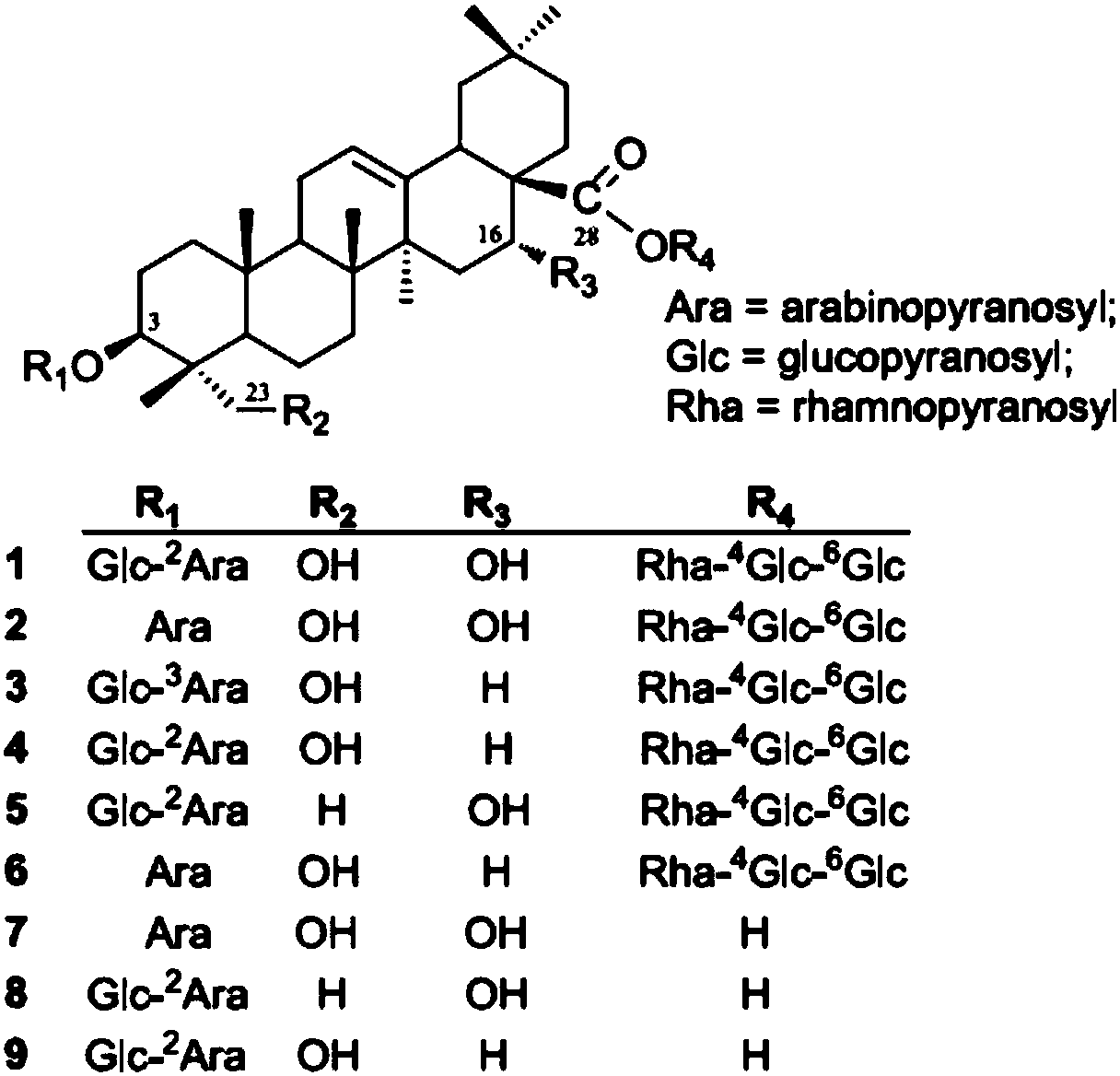
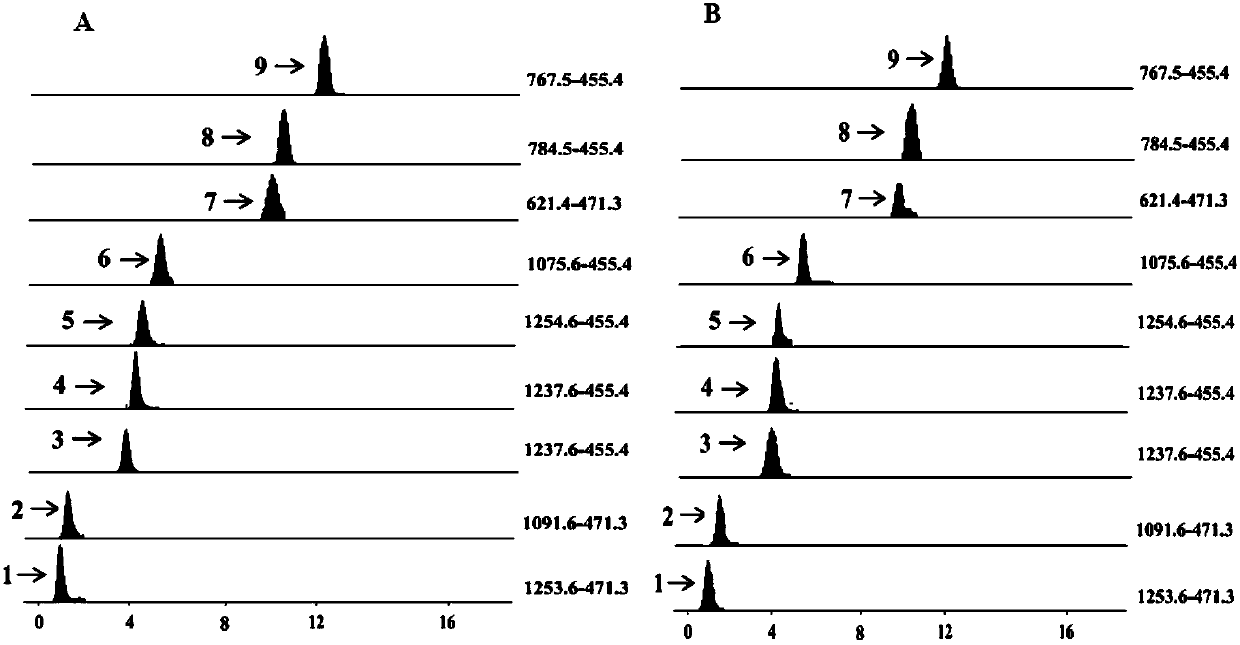
Method for performing qualitation and quantification on triterpenoid saponin in traditional Chinese medicine by utilizing electrospray protonation pyrolysis-mass spectrum multi-reaction detection mode
The invention discloses a method for performing qualitation and quantification on triterpenoid saponin in traditional Chinese medicine by utilizing an electrospray protonation pyrolysis-mass spectrummulti-reaction detection mode. The triterpenoid saponin can form a stable [M+H]<+> and / or [M+NH4)<+> molecular ion peak in a flow phase containing formic acid or ammonium formate. According to the method disclosed by the invention, a liquid chromatogram-tandem quadrupole mass spectrometry combined instrument is utilized, an electrospray positive ion tandem mass spectrum detection mode is utilized,and mass spectrum parameters of declustering potential, impact energy, impact chamber injection voltage and the like can be quickly optimized in an online mode by a single factor and response surfacemethod; thus, high-strength triterpenoid saponin protonation pyrolysis MRM target ion pairs can be formed; a Q1 calculation formula is [M+H]<+> or [M+NH4]<+>, and a Q3 calculation formula is [aglycone +H-nH2O]<+>. By means of the series of characteristic MRM target ion pairs, the purpose of quick qualitative and quantitative detection on the triterpenoid saponin in the traditional Chinese medicine is realized. The method disclosed by the invention provides an effective technology method for the qualitative and quantitative detection on the triterpenoid saponin in the traditional Chinese medicine and has been successfully applied to content measurement on the triterpenoid saponin of caulophyllum robustum and aralia elata.
Owner:匡海学 +2
Method for detecting 205 kinds of pesticide residues in rice
InactiveCN104698114AAchieving Simultaneous DetectionImprove extraction efficiencyComponent separationGradient elutionAmmonium formate
The invention relates to the analytical chemistry and food safety field and in particular relates to a method for detecting 205 kinds of pesticide residues in rice. According to the method, a sample is extracted by acetonitrile, extraction solution is purified by a QuEChERS method, a chromatographic column shown as below is adopted as an analytical column, methyl alcohol-ammonium formate aqueous solution is used as a moving phase, then gradient elution, electrospraying and positive ion scanning are carried out to separate and detect 205 kinds of pesticide residues; mass spectrum conditions are optimized and the optimal mass spectrum analysis condition is established while the sensitivity of all ingredients is considered; the detection limits of 205 kinds of pesticides are all lower than 10mu g / kg, and the range of the recovery rate of 205 kinds of pesticides is within 50-130%. According to the method, the detection limit, the recovery rate, the precision and other technical indexes all meet the domestic and foreign requirements for detection of pesticide residues in rice.
Owner:陈溪 +2
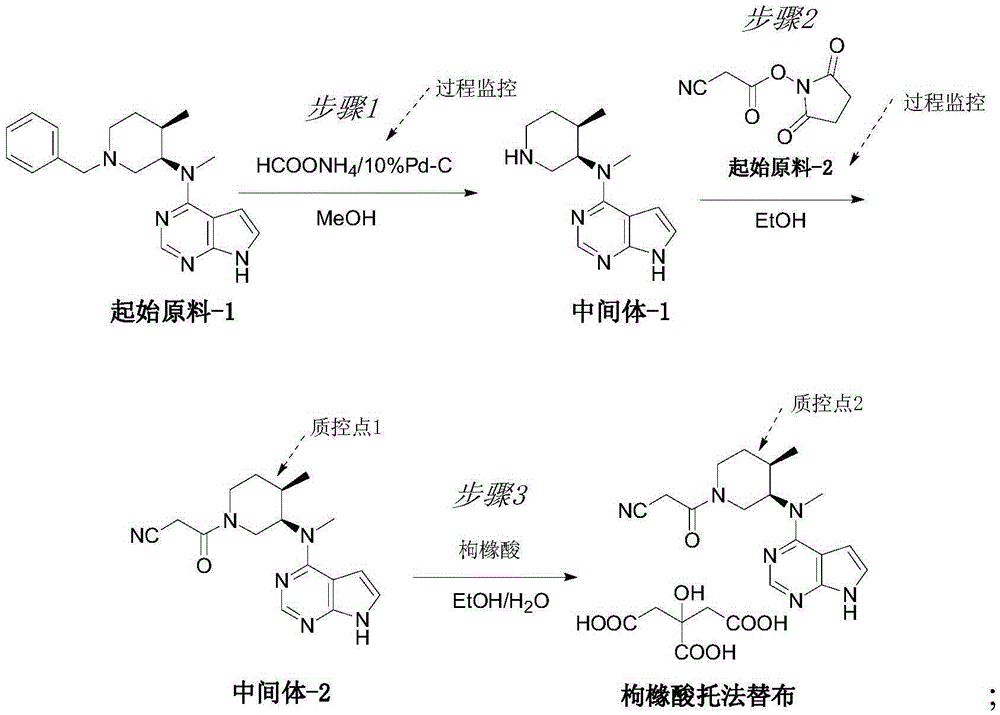
Novel synthetic process of tofacitinib citrate
InactiveCN105348287AReasonable routingMild reaction conditionsOrganic chemistryPalladium on carbonChemical synthesis
The invention discloses a novel synthetic process of tofacitinib citrate. The novel synthetic process comprises the steps that 1, an initial raw material-1, 10% of palladium on carbon, absolute methanol and ammonium formate are mixed in a reaction container, and reacting is carried out to obtain a midbody-1; 2, the midbody-1 prepared in the step 1 is dissolved into absolute ethyl alcohol, an initial raw material-2 is added, reacting is carried out at the reaction temperature of 20 DEG C to 50 DEG C, reaction liquid is purified to obtain a crude midbody-2 after reacting is finished, and the crude midbody-2 is refined to obtain a refined midbody-2; 3, the refined midbody-2 is subjected to heating reflux and dissolved clarification through absolute ethyl alcohol, a citric acid water solution is dropwise added, and reacting continues to be carried out at the temperature of 50 DEG C to 90 DEG C; then the mixture is slowly cooled to 20 DEG C to 45 DEG C, and stirring and devitrification are carried out; crystals are filtered and washed with ethyl alcohol, drying is carried out under reduced pressure at the temperature of 40 DEG C to 60 DEG C, and white crude tofacitinib citrate is obtained. The chemical synthetic process is more reasonable in route, and the reaction conditions are milder.
Owner:NINGBO LIWAH PHARM CO LTD
Polyurethane foaming white material and polyurethane composition
The invention discloses a polyurethane foaming white material. The polyurethane foaming white material comprises combined polyether, a foaming agent and a trimerization type catalyst, wherein the foaming agent comprises trans-1-chloro-3, 3, 3-trifluoropropene, and the trimerization type catalyst is (2-hydroxypropyl) trimethyl ammonium formate. The invention further provides a polyurethane composition. The polyurethane foaming white material has stability in storage, and after the polyurethane foaming white material is placed at room temperature for 1 month, the foaming reaction speed of the polyurethane composition and the heat conduction coefficient of foam are basically unchanged.
Owner:HEFEI HUALING CO LTD
Low-temperature condition release ammonia reducing agent composition and preparation method thereof
The invention relates to a low-temperature condition release ammonia reducing agent composition and a preparation method thereof, and belongs to the low-temperature condition release ammonia reducing agent composition and the preparation method thereof. The composition is prepared by the following raw materials in parts by mass: 15-21 parts of ammonium carbonate, 0.79-1 part of urea, 0.80-1.57 parts of ammonium formate, 2.0-8.3 parts of low molecular alcohols, and 25-31 parts of high-purity water. Compared with a traditional urea reducing agent, the composition can release an ammonia source under a lower temperature. The obtained product can be used under the environment below -15 DEG C, and can totally satisfy normal operation of an SCR system. The freezing point of the product is -15 DEG C; and the product can be used in four reasons in the south and in spring, summer, autumn and early winter in the north.
Owner:CHANGCHUN YONGCHANG PETROCHEM
Black nanophase ceramics pigment and preparation method thereof
The invention discloses a black nanophase ceramics pigment and a preparation method thereof, the method comprises: dissolving ammonium oxalate, ammonium formate, ammonium acetate and ammonium propionate in water and then mixing evenly with chromic nitrate, manganous nitrate, ferric nitrate and cobalt nitrate, heating the mixture at the temperature of 400-500 DEG C, and combusting until no air gives out to obtain the black nanophase ceramics pigment. The invention has simple reaction conditions, and the raw materials such as the nitrates, the ammonium acetate and the like are medicaments with low price and abundant resource; and the invention is benefit to large-scale industrialized production. The prepared black nanophase ceramics pigment has black color, high temperature-resistance, stable performance and good dispersibility.
Owner:SOUTH CHINA UNIV OF TECH
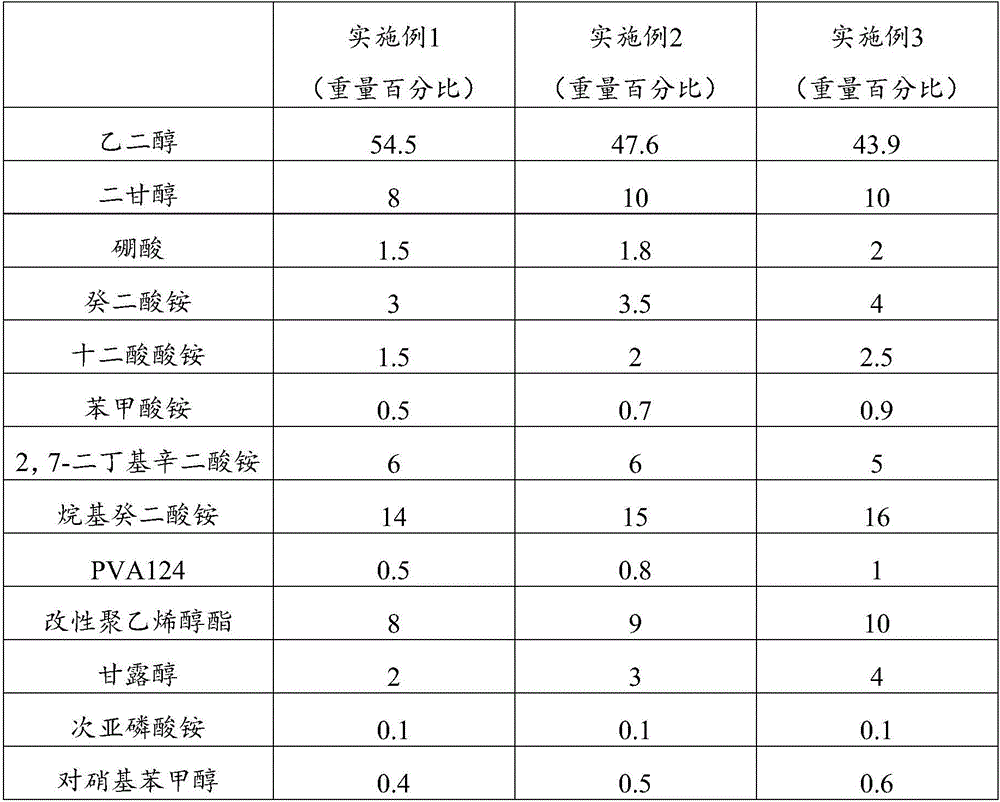
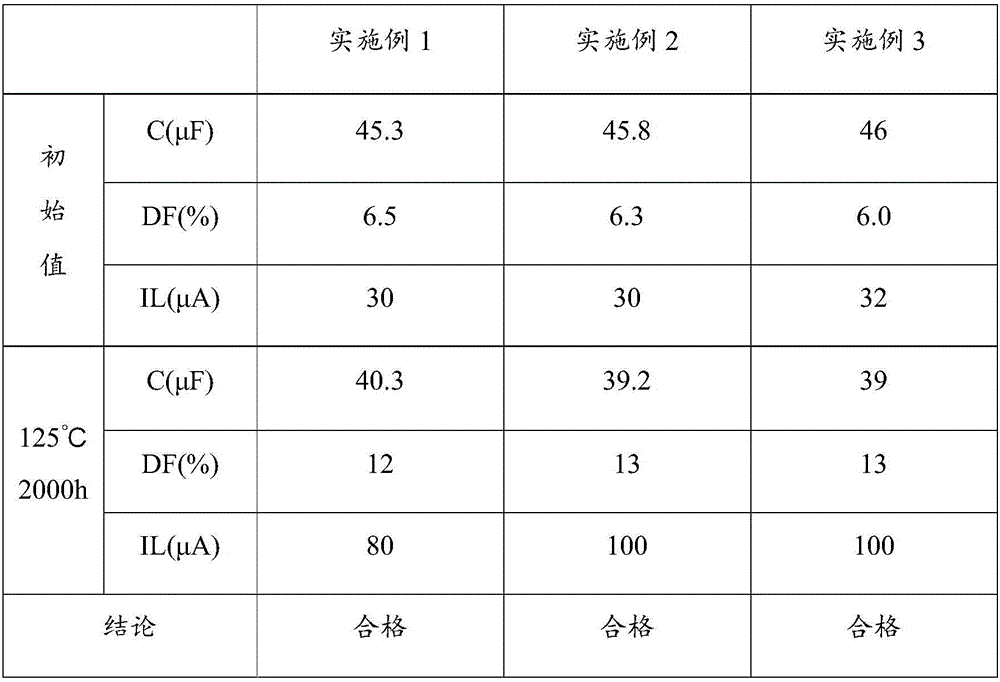
Electrolyte, electrolyte preparation method and aluminum electrolytic capacitor
ActiveCN106098379AImprove temperature resistanceImprove stabilityLiquid electrolytic capacitorsSolventTemperature resistance
The invention discloses an electrolyte, an electrolyte preparation method and an aluminum electrolytic capacitor. The electrolyte comprises, by weight percentage, 50-65w% of solvent, 23-33w% of solute, and 8-19w% of additive. The solute includes boric acid, ammonium benzoate, at least one material selected from ammonium dodecanedioate and 2,7-dibutyl octanedioic acid ammonium, and at least one material selected from ammonium sebate and alkyl ammonium sebate. Through the technical scheme, the temperature resistance performance and stability of the aluminum electrolytic capacitor are improved.
Owner:惠州市智胜新电子技术有限公司
Electrolyte of high-voltage aluminum electrolytic capacitor and preparation method of electrolyte
The invention discloses electrolyte of a high-voltage aluminum electrolytic capacitor. The electrolyte is characterized by comprising a main solvent, an auxiliary solvent, a solute and a spark voltage increasing agent, wherein the main solvent comprises one or more of ethylene glycol, gamma-butyrolactone, DMF and diglycol monobutylether; the auxiliary solvent comprises two or more of polyethylene glycol 200, polyethylene glycol 400, a nanosilicon dioxide solution, glycerol, propylene carbonate and ethylene carbonate; the solute comprises two or more of ammonium sebacate, ammonium adipate, adipic acid, boracic acid, ammonium pentaborate, ammonium azelate, ammonium dodecanedioate, ammonium formate, ammonium benzoate, mannitol, sorbitol, citric acid and quadrol. The electrolyte can be applied to 600V ultrahigh voltage, is stable in performance, low in viscosity, high in conductivity and beneficial to volume production, and can reduce the production cost.
Owner:HUNAN AIHUA GRP
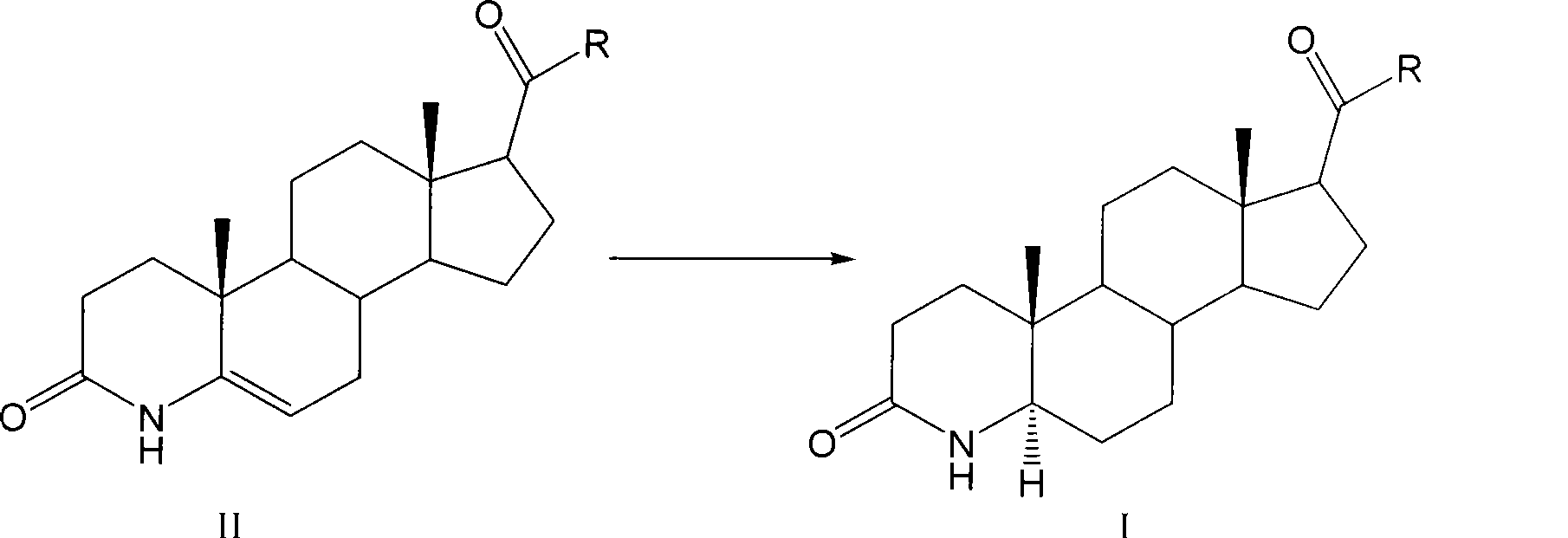
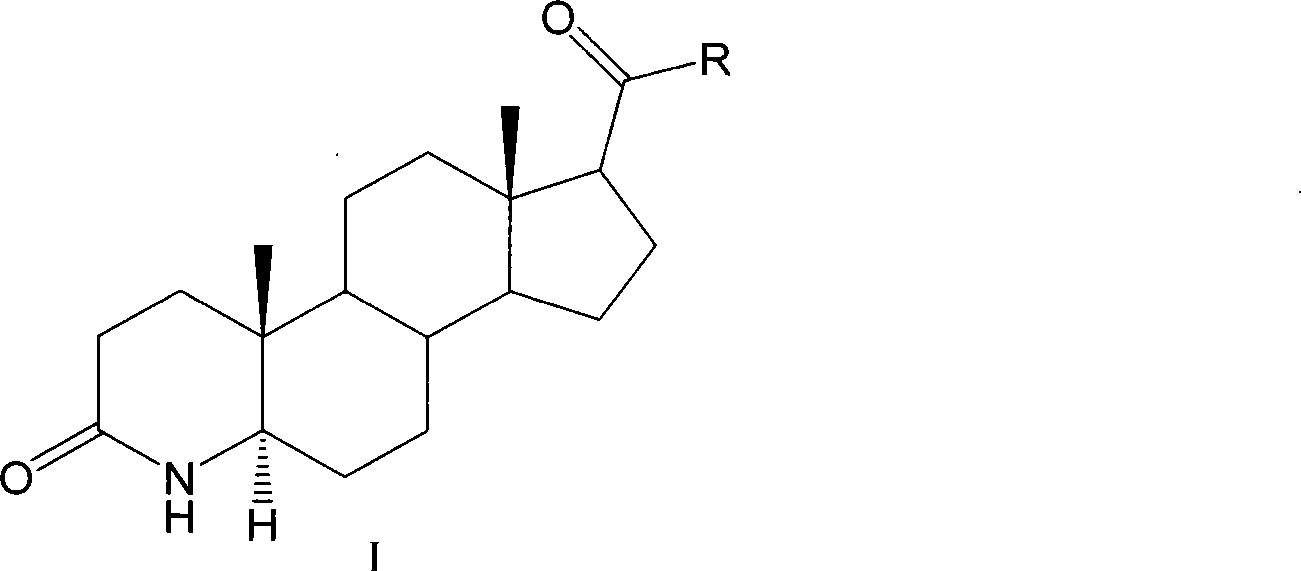
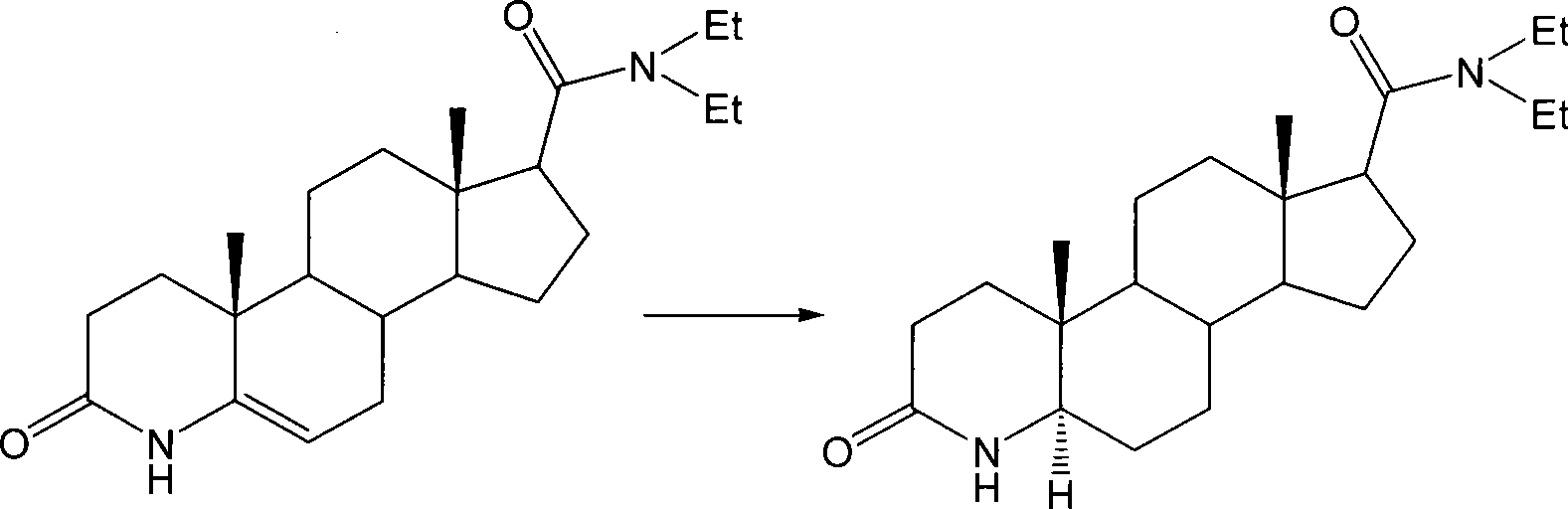
Method for preparing 3-carbonyl-4-aza-5alpha-androstanes
The invention discloses a preparation method for a 3-carbonyl-4-aza-5alpha-androstane compound. The method comprises the following steps: 1, carrying out the hydrogenation action to a 3-carbonyl-4-aza-5 -androstane alkenyl compound, formic acid and a hydrogen donor agent for 5 to 24hours at 60 to 101 DEG C, then carrying out decompression and concentration to remove the formic acid, flushing the reactants into ice water for separating crystals, and obtaining a crude product after filtration and drying; and 2, adding the crude product into a recrystallization solution for recrystallization and obtaining the product. The hydrogen donor agent can be ammonium formate, potassium formate, sodium formate or triethylammonium formate. The preparation has the moderate reaction and low cost, and is suitable for the industrialized volume production.
Owner:JIANGSU JIAERKE PHARMA GRP CORP
Apparatus for Improved Immunosuppressant Drug Monitoring
InactiveUS20140299765A1Easy to separateComponent separationSolid sorbent liquid separationEverolimusMass analyzer
A liquid chromatography / mass spectrometry system includes: a source of a first mobile phase solvent consisting essentially of water plus 10 mM ammonium formate plus 0.05% formic acid; a source of a second mobile phase solvent consisting essentially of methanol plus 10 mM ammonium formate plus 0.05% formic acid; a chromatography column comprising a length of 30 mm or less of a stationary phase comprising an 8-carbon alkyl chain material bonded to 2.6 μm diameter particles having solid silica cores surrounded by porous silica outer layers; an electrospray ion source of a mass spectrometer fluidically coupled to the chromatography column so as to generate ions therefrom; a mass analyzer of the mass spectrometer operable to quantitatively detect the ions; and a programmable processor electronically coupled to the mass analyzer and comprising instructions operable to determine, based on the ion detection, a concentration of everolimus, sirolimus, tacrolimus, or cyclosporin A.
Owner:THERMO FINNIGAN
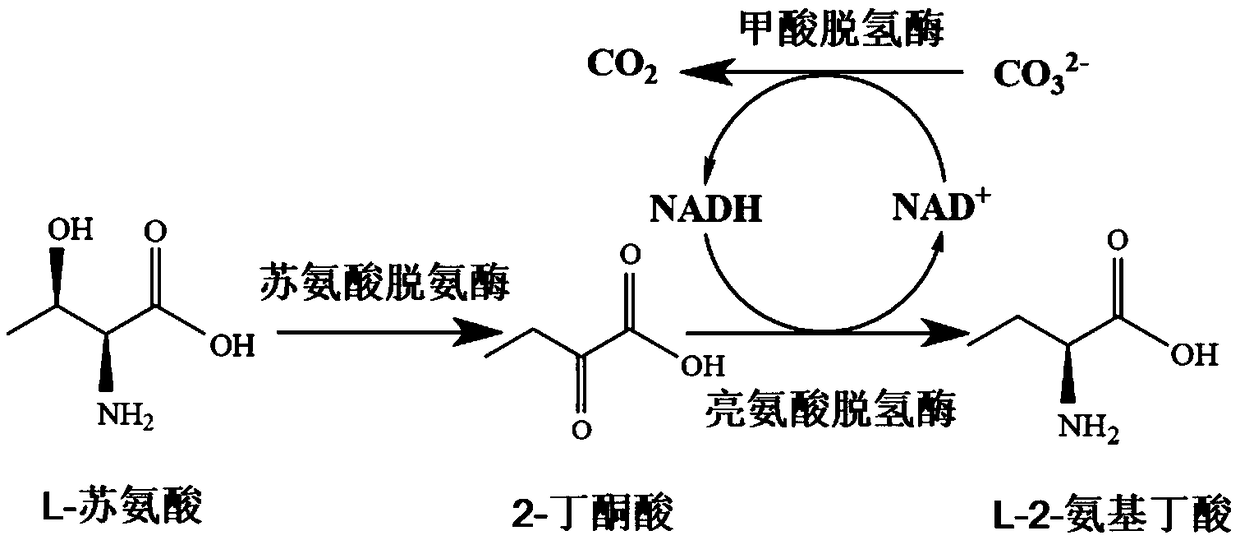
Construction and application of recombinant strain converting L-threonine to L-2-aminobutyric acid
ActiveCN109266595ALow costEasy to scale up industrial productionCarbon-nitrogen lyasesBacteriaL-threonineSal ammoniac
The invention discloses a construction and application of a recombinant strain converting L-threonine to L-2-aminobutyric acid, belonging to the field of bioengineering technology. The production method of the invention utilizes a recombinant bacterium expressing two plasmids to simultaneously realize the high-efficient expression of three enzymes, and comprises the steps of: conversion of L-threonine to L-2-aminobutyric acid, coupled with a coenzyme regeneration system, converts NAD+ into NADH, so that the concentration of NADH in the system is relatively stable, and the conversion can be carried out efficiently. Furthermore, the CO<2> converted from ammonium formate can be dissolved in ammonia water, which has little environmental pollution and industrial application value. The method has the advantages of mild conversion condition, strong specificity, low cost and short conversion time. The L-2-aminobutyric acid is prepared adopting the method, 40g / L L-threonine is added, The concentration of the obtained product L-2-aminobutyric acid is 43.3 g / L, and the conversion ratio was over 99.9%.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com