Method for preparing avibactam sodium through one-pot method
A technology of avibactam sodium and reaction solution, applied in the field of medicine, can solve the problems of being unsuitable for industrial scale-up production, cumbersome reaction operation process, and high reduction risk, and achieve the advantages of increasing safety factor, reducing production cost and improving yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
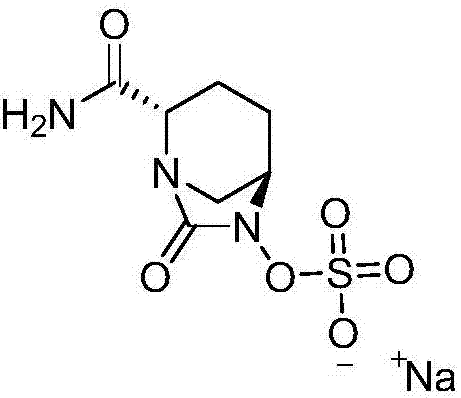
[0044] Example 1: Preparation of [(1R,2S,5R)-2-(aminocarbonyl)-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-yl]sulfuric acid monosodium salt
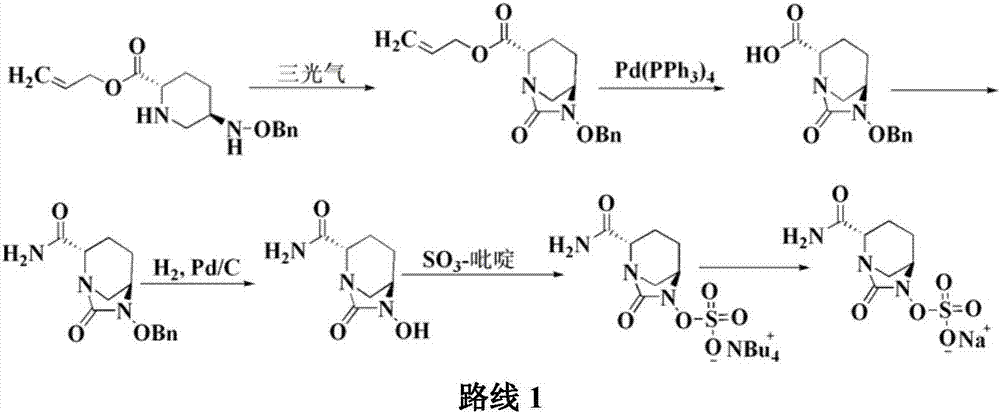
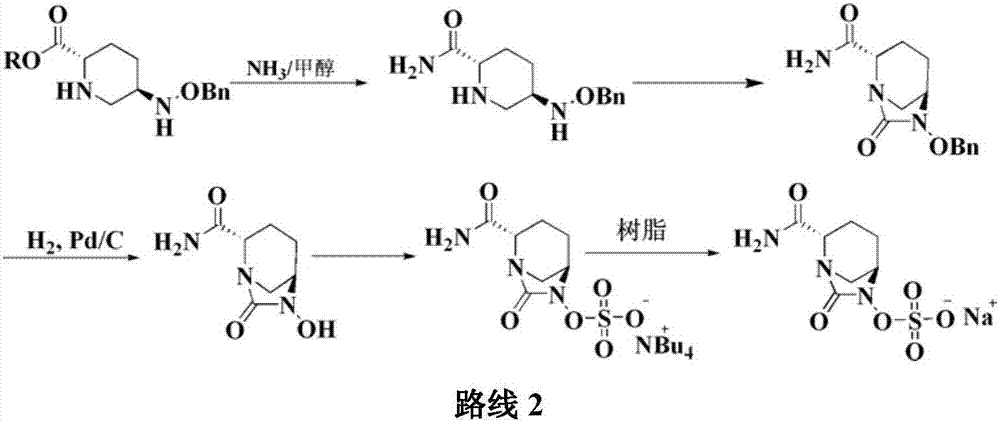
[0045]Add 20g of compound I into a 1000ml reaction flask, add 400ml of tetrahydrofuran, add 35g of diisopropylethylamine under stirring, and add 11.3g of triphosgene dropwise at a controlled temperature of -10 to 10°C. TLC detects that the reaction is complete. Warm up to room temperature, add a saturated solution of lithium hydroxide dropwise, control the pH value of the feed solution to about 10 and stir for 1 hour. After the reaction is detected by TLC, the reaction solution is adjusted to acidity with hydrochloric acid, and 300ml of dichloromethane is added for extraction. The organic layer was dried over anhydrous sodium sulfate, suction filtered, washed, 7.7g triethylamine and 8.2g n-butyl chloroformate were added, and 37g concentrated ammonia water (mass fraction 25%-28% ), TLC detects that after the completion of the reaction, the layers...
Embodiment 2
[0046] Example 2: Preparation of [(1R,2S,5R)-2-(aminocarbonyl)-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-yl]sulfuric acid monosodium salt
[0047] Add 20g of compound I into a 1000ml reaction flask, add 400ml of tetrahydrofuran, add 35g of diisopropylethylamine under stirring, and add 11.3g of triphosgene dropwise at a controlled temperature of -10 to 10°C. TLC detects that the reaction is complete. Warm up to room temperature, add a saturated solution of lithium hydroxide dropwise, control the pH value of the feed solution to about 10 and stir for 1 hour. After the reaction is detected by TLC, the reaction solution is adjusted to acidity with hydrochloric acid, and 300ml of dichloromethane is added for extraction. The organic layer was dried over anhydrous sodium sulfate, suction filtered, washed, 7.7g triethylamine and 8.2g n-butyl chloroformate were added, and 37g concentrated ammonia water (mass fraction 25%-28% ), TLC detects that after the completion of the reaction, the layer...
Embodiment 3
[0048] Example 3: Preparation of [(1R,2S,5R)-2-(aminocarbonyl)-7-oxo-1,6-diazabicyclo[3.2.1]oct-6-yl]sulfuric acid monosodium salt
[0049] Add 20g of compound I into a 1000ml reaction flask, add 400ml of tetrahydrofuran, add 35g of diisopropylethylamine under stirring, and add 11.3g of triphosgene dropwise at a controlled temperature of -10 to 10°C. TLC detects that the reaction is complete. Warm up to room temperature, add a saturated solution of lithium hydroxide dropwise, control the pH value of the feed solution to about 10 and stir for 1 hour. After the reaction is detected by TLC, the reaction solution is adjusted to acidity with hydrochloric acid, and 300ml of dichloromethane is added for extraction. The organic layer was dried over anhydrous sodium sulfate, suction filtered, washed, 7.7g triethylamine and 8.2g n-butyl chloroformate were added, and 37g concentrated ammonia water (mass fraction 25%-28% ), TLC detects that after the completion of the reaction, the layer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com