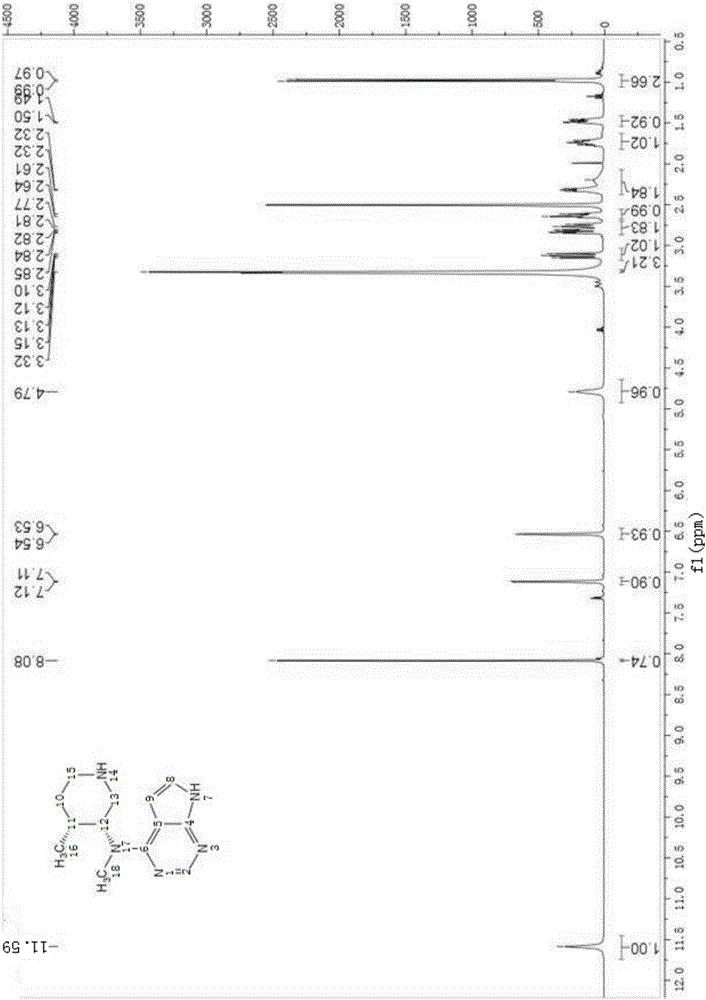
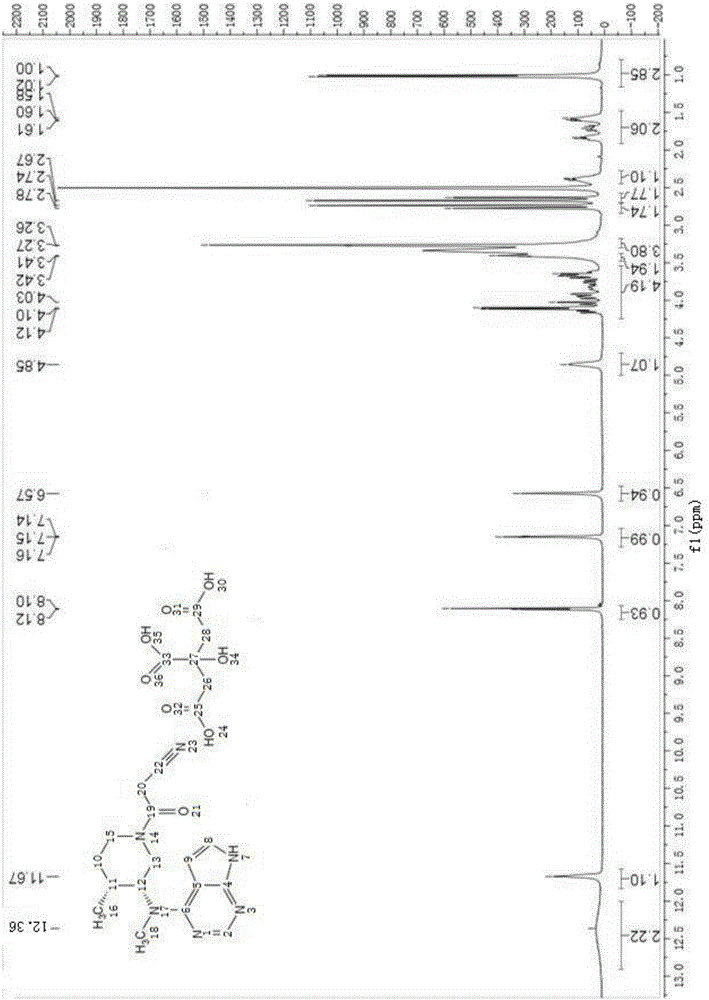
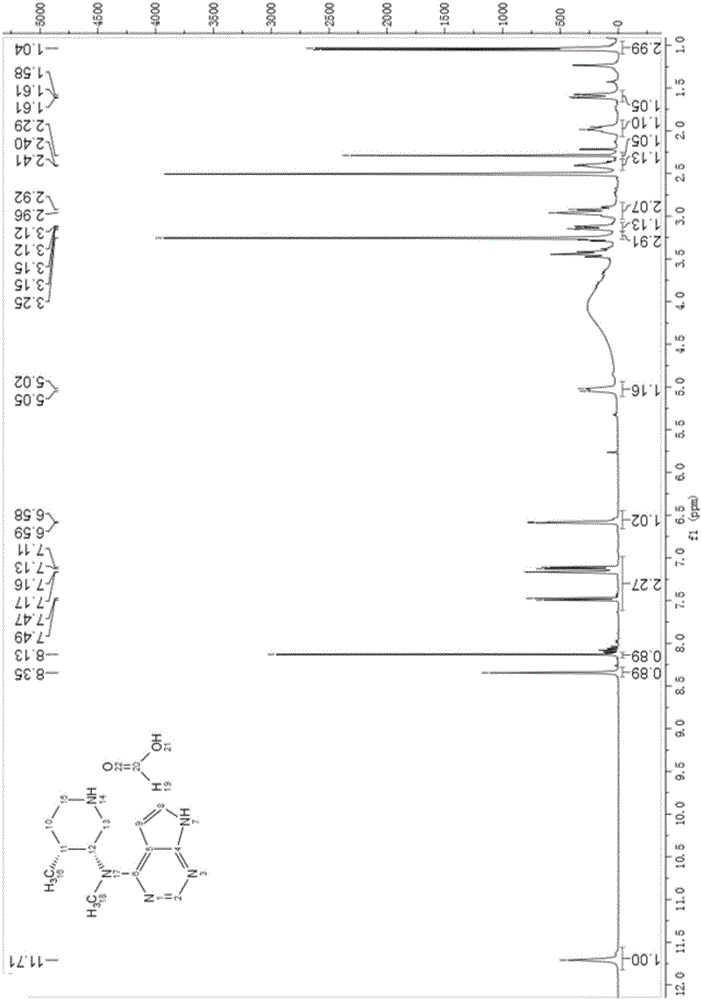
Method for synthesizing citric acid tofacitinib
A synthetic method, the technology of tofacitinib, applied in the field of synthesis of tofacitinib citrate, can solve the problems of potential safety hazards, long reaction time, incomplete reaction, etc., and achieve simplified post-treatment purification process and clean reaction Thoroughly, the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the synthesis of tofacitinib citrate
[0044] The synthetic route is:
[0045]
[0046] Concrete synthetic steps are as follows:
[0047] (1) Preparation of intermediate 4 (compound shown in formula 4)
[0048]Add 15g (45mmol) of compound 5 to a 500ml reaction flask, add 200ml of anhydrous methanol, add 3g of 10% Pd / C (water content 50%) in batches under stirring at room temperature, add 8.3g (180mmol) of formic acid dropwise, heat and reflux at 70°C for 2h , TLC monitored the completion of the reaction, cooled, filtered off Pd / C with diatomaceous earth, and concentrated to dryness under reduced pressure to obtain 13 g of white solid 4 with a yield of 100%.
[0049] (2) Preparation of intermediate 3 (compound shown in formula 3)
[0050] Add 13g (45mmol) of intermediate 4 to a 500ml reaction flask, add 50ml of water, add 37.26g of potassium carbonate, add 100ml of dichloromethane, stir at room temperature for 1h, extract the aqueous layer with dichlo...
Embodiment 2
[0053] Embodiment 2: the preparation of intermediate 4 (compound shown in formula 4)
[0054] Add 15g (45mmol) of compound 5 to a 500ml reaction flask, add 200ml of anhydrous methanol, add 1.5g of 10% Pd / C (water content 50%) in batches under stirring at room temperature, add 8.3g (180mmol) of formic acid dropwise, and heat to reflux at 70°C After 10 h, TLC monitored the completion of the reaction, cooled, filtered off Pd / C with celite, and concentrated to dryness under reduced pressure to obtain 12.3 g of white solid 4 with a yield of 95%.
Embodiment 3
[0055] Embodiment 3: the preparation of intermediate 4 (compound shown in formula 4)
[0056] Add 15g (45mmol) of compound 5 to a 500ml reaction flask, add 200ml of anhydrous methanol, add 4.5g of 10% Pd / C (water content 50%) in batches under stirring at room temperature, add 8.3g (180mmol) of formic acid dropwise, and heat to reflux at 70°C After 2h, TLC monitored the completion of the reaction, cooled, filtered off Pd / C with celite, and concentrated to dryness under reduced pressure to obtain 12.9 g of white solid 4 with a yield of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com