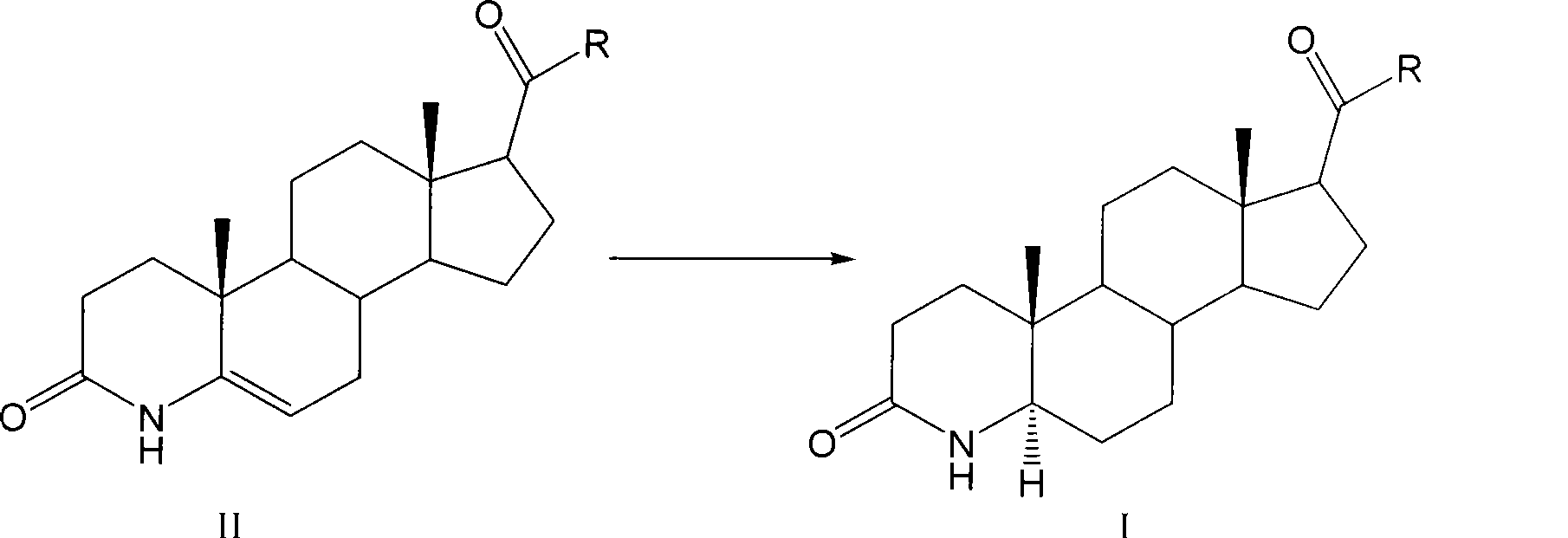
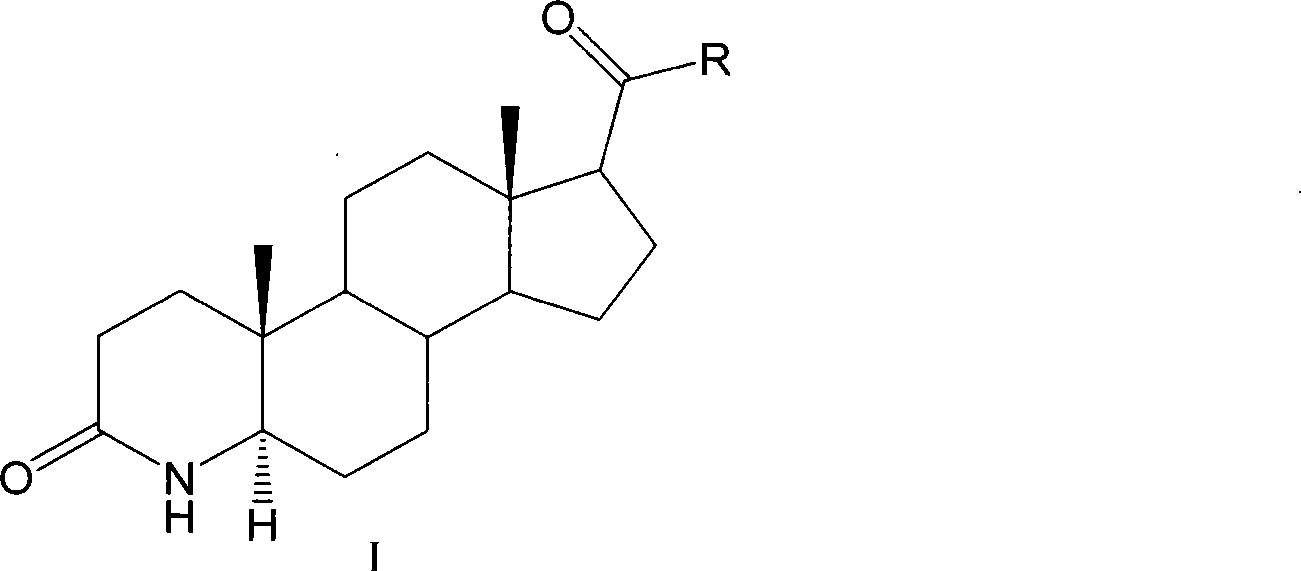
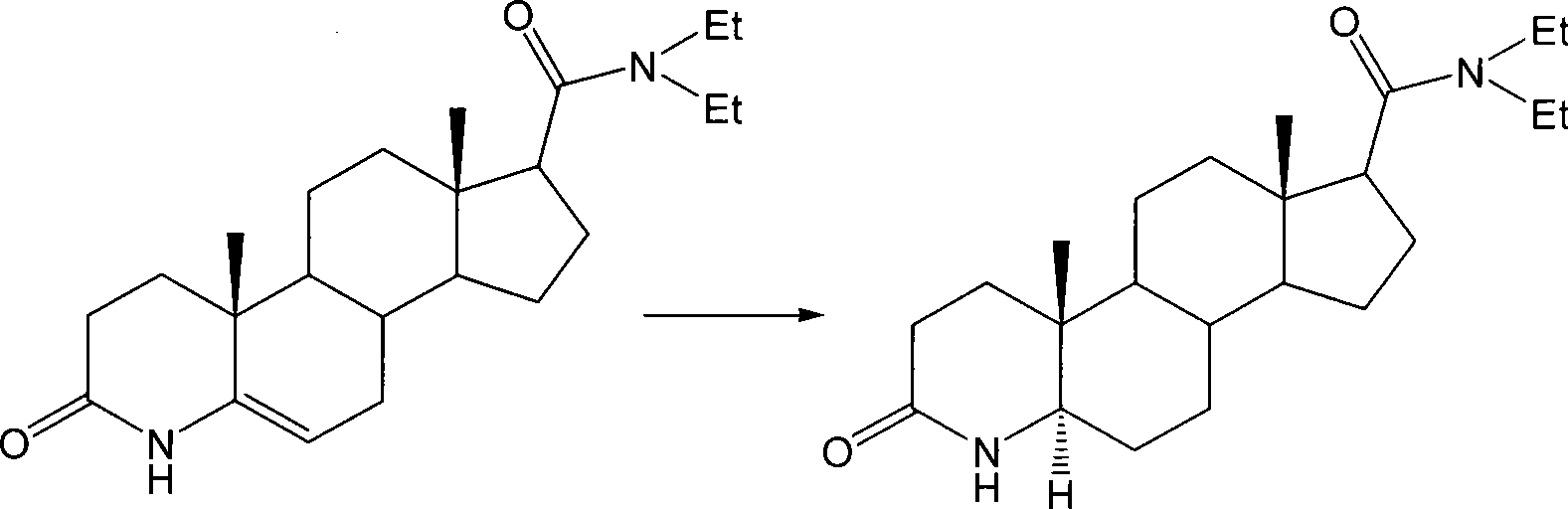
Method for preparing 3-carbonyl-4-aza-5alpha-androstanes
A compound and carbonyl technology, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of high production cost, difficulty in mass production, expensive precious metal reagents, etc., and achieve the effect of low cost and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] This embodiment is the preparation method of 3-carbonyl-4-aza-5α-androst-17β-carboxylic acid, the steps are as follows:
[0025] ①Add 10g of 3-carbonyl-4-aza-5-androstene-17β-carboxylic acid, 6g of ammonium formate and 250ml of formic acid into a dry reaction flask, stir and heat up to 60°C for hydrogenation reaction for 24h. Then concentrated under reduced pressure to remove formic acid, washed the reactant into 125ml of ice water to precipitate crystals, filtered and dried to obtain 9.6g of crude product.
[0026] ② Add the crude product to ethyl acetate for refluxing and dissolve, then recrystallize at 0-5°C to obtain 7.3 g of white crystals, and the α-body is determined to be 98.5 wt% by HPLC (high performance liquid chromatography).
Embodiment 2~ Embodiment 8
[0028] The rest are the same as in Example 1, and the differences are shown in Table 1.
[0029] Table 1
[0030] Example 1 Example 2 Example 3 Example 4 Example 5 Example 6 Example 7 Example 8 3-carbonyl-4-
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com