Process method for extracting Mg and Li from bittern and simultaneously producing hydrotalcite
A process method, magnesium aluminum hydrotalcite technology, applied in the process field of extracting magnesium and lithium from brine and producing hydrotalcite at the same time, achieving the effect of small loss of lithium, simple equipment, and low loss rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
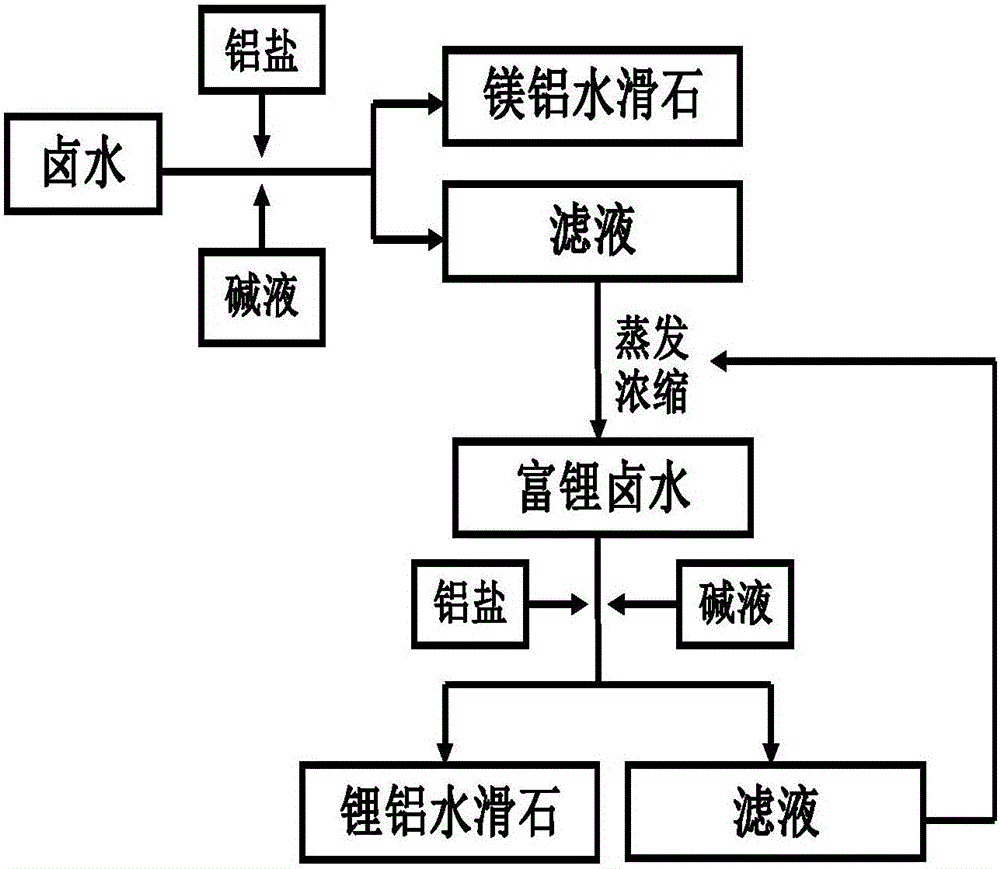
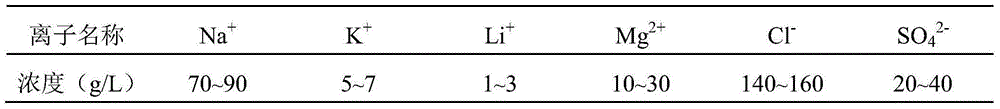
[0026] A. Weigh MgCl 2 ·6H 2 O26.0325g, MgSO 4 ·7H 2 O25.7993g, AlCl 3 ·6H 2 O18.729g, KCl3.3873g, LiCl1.8768g, NaCl8.068g were dissolved in deionized water, and the 250mL volumetric flask was constant to obtain a salt solution; NaOH19.8593g, NaCO 3 16. 4443g was dissolved in deionized water, and the 250mL volumetric flask was made to volume to obtain an alkaline solution;
[0027] Pour the salt solution and the alkali solution into the colloid mill at the same time, and rotate at a speed of 3000r / min for 3 minutes to form the MgAl-LDH crystal nucleus; transfer the crystal nucleus solution to the reactor, stir and crystallize at 80°C for 12 hours, and perform MgAl-LDH crystallization. LDH growth; filtering to obtain a MgAl-LDH filter cake, and drying the MgAl-LDH filter cake at 70°C for 12 hours to obtain a white solid MgAl-LDH product; collect the filtrate into a container.
[0028] B. Evaporate and concentrate the filtrate of step A to 250mL at 50°C, when the lithium i...
Embodiment 2
[0031] A. Weigh MgCl 2 ·6H 2 O39.0487g, MgSO 4 ·7H 2 O38.6989g, Al(NO 3 ) 3 9H 2 O29.1008g, KCl9.1837g, LiCl1.8768g, NaCl8.068g were dissolved in deionized water, and the 250mL volumetric flask was constant to obtain a salt solution; NaOH22.3368g, NaCO 3 16. 4443g was dissolved in deionized water, and the 250mL volumetric flask was made to volume to obtain an alkaline solution;
[0032] Pour the salt solution and the alkali solution into the colloid mill at the same time, and rotate at a speed of 4000r / min for 5 minutes to form the MgAl-LDH crystal nucleus; transfer the crystal nucleus solution to the reactor, and carry out the crystallization of MgAl-LDH at 80°C for 12 hours. LDH growth; filtering to obtain a MgAl-LDH filter cake, drying the MgAl-LDH filter cake at 80°C for 6 hours to obtain a white solid MgAl-LDH product; collecting the filtrate into a container.
[0033] B. Evaporate and concentrate the filtrate of step A to 250mL at 50°C, when the lithium ion concen...
Embodiment 3
[0036] A. Weigh MgCl 2 ·6H 2 O39.0487g, MgSO 4 ·7H 2 O29.139g, Al 2 (SO 4 ) 3 18H 2 O25.8488g, KCl9.1837g, LiCl1.8768g, NaCl8.068g were dissolved in deionized water, and the 250mL volumetric flask was constant to obtain a salt solution; NaOH22.3223g, NaCO 3 16. 4443g was dissolved in deionized water, and the 250mL volumetric flask was made to volume to obtain an alkaline solution;
[0037] Pour the salt solution and the alkali solution into the colloid mill at the same time, and rotate at a speed of 2000r / min for 6 minutes to form the MgAl-LDH crystal nucleus; transfer the crystal nucleus solution to the reactor, stir and crystallize at 70°C for 10 hours, and perform MgAl-LDH crystallization. LDH growth; filtering to obtain a MgAl-LDH filter cake, drying the MgAl-LDH filter cake at 80°C for 8 hours to obtain a white solid MgAl-LDH product; collecting the filtrate into a container.
[0038] B. Evaporate and concentrate the filtrate of step A to 250mL at 50°C, when the l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com