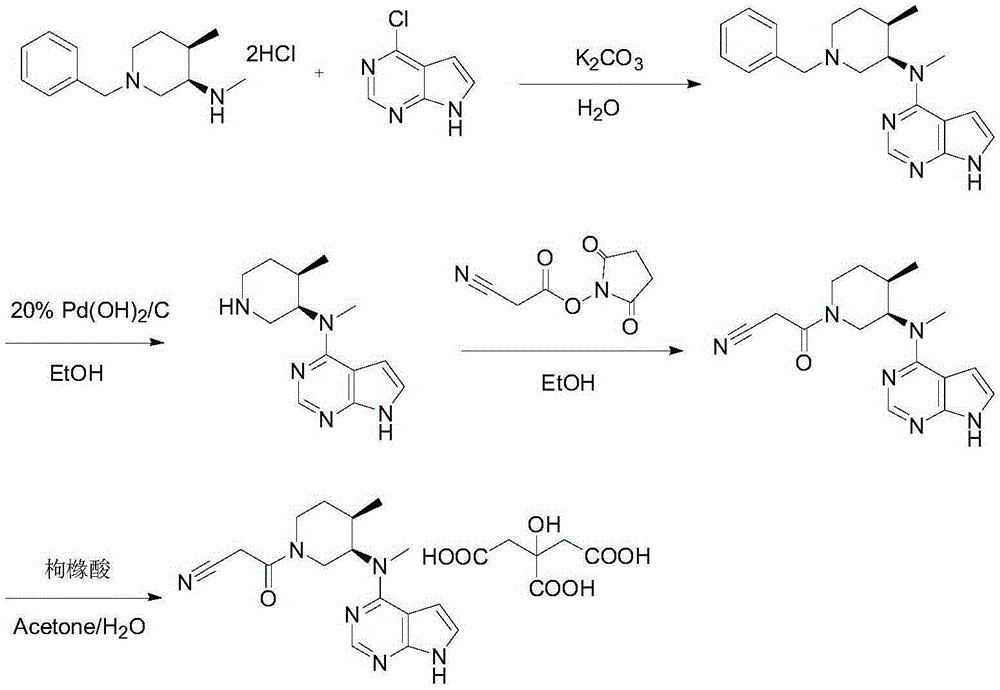
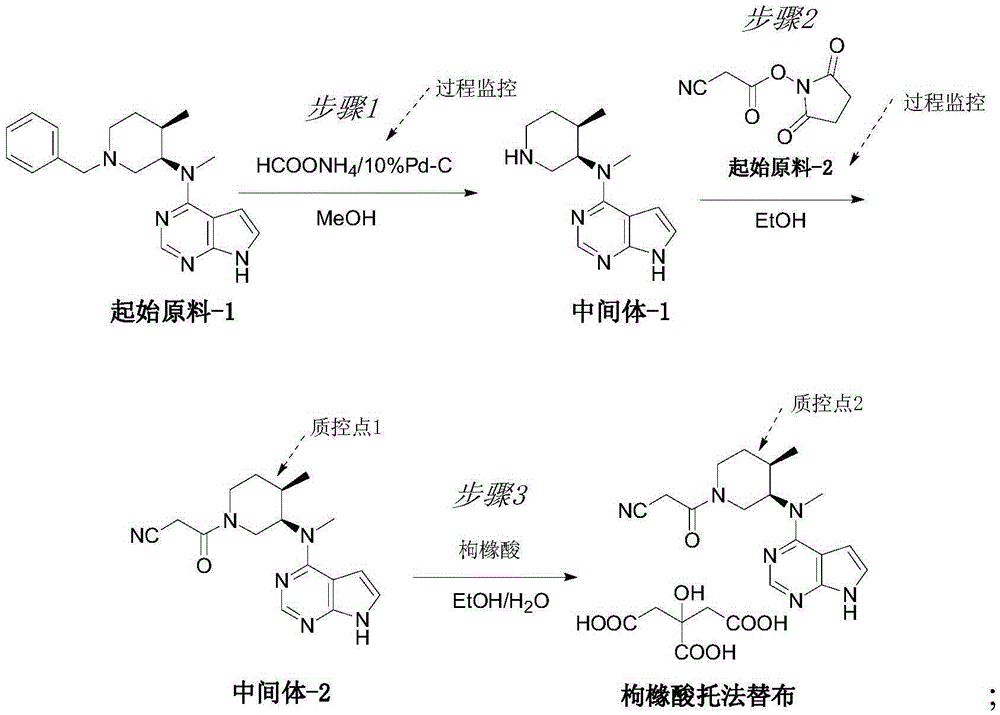
Novel synthetic process of tofacitinib citrate
A technology of tofacitinib and synthesis process, applied in the field of medicine, can solve problems such as long time and low yield, and achieve the effects of improving yield, reasonable route and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 synthetic intermediate-1:
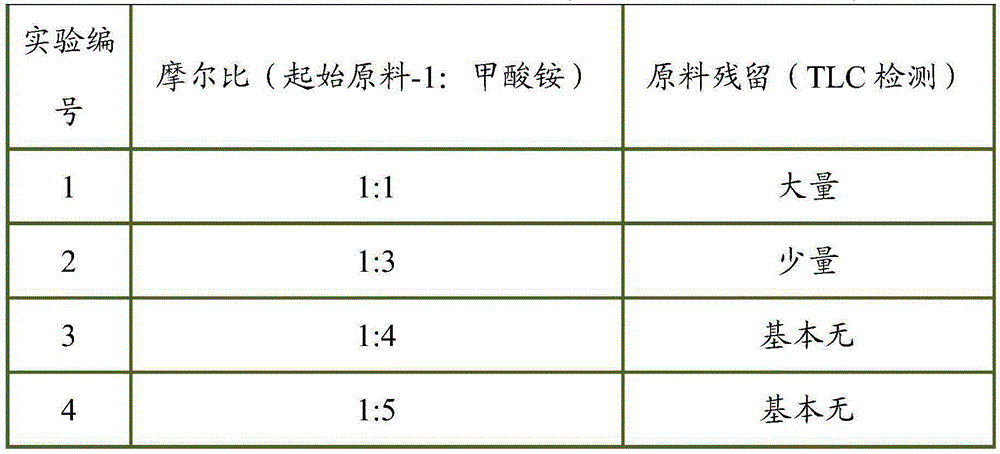
[0045] 1g of N-methyl-N-((3R,4R)-4-methyl-1-benzyl-3-piperidinyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (from Starting material-1) and 0.2g of 10% palladium carbon were added to a three-necked reaction flask, 30ml of anhydrous methanol was added to stir and disperse, and 0.187g of ammonium formate was added. The temperature was raised to reflux, and the reaction was carried out for 2 hours, and the reaction was monitored by TLC.
Embodiment 2
[0046] Embodiment 2 synthetic intermediate-1:
[0047] 1g of N-methyl-N-((3R,4R)-4-methyl-1-benzyl-3-piperidinyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (from Starting material-1) and 0.2g of 10% palladium carbon were added to a three-necked reaction flask, 30ml of anhydrous methanol was added to stir and disperse, and 0.561g of ammonium formate was added. The temperature was raised to reflux, and the reaction was carried out for 2 hours, and the reaction was monitored by TLC.
Embodiment 3
[0048] Embodiment 3 synthetic intermediate-1:
[0049] 1g of N-methyl-N-((3R,4R)-4-methyl-1-benzyl-3-piperidinyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (from Starting material-1) and 0.2g of 10% palladium carbon were added to a three-necked reaction flask, 30ml of anhydrous methanol was added to stir and disperse, and 0.748g of ammonium formate was added. The temperature was raised to reflux, and the reaction was carried out for 2 hours, and the reaction was monitored by TLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com