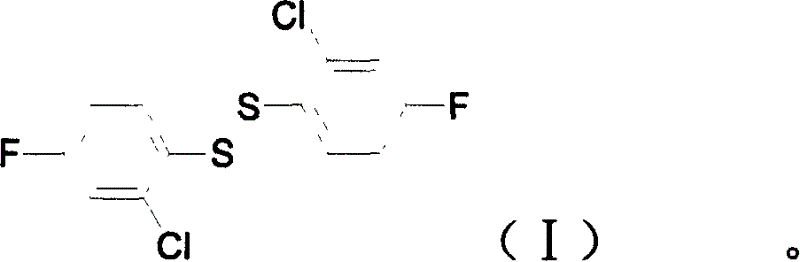
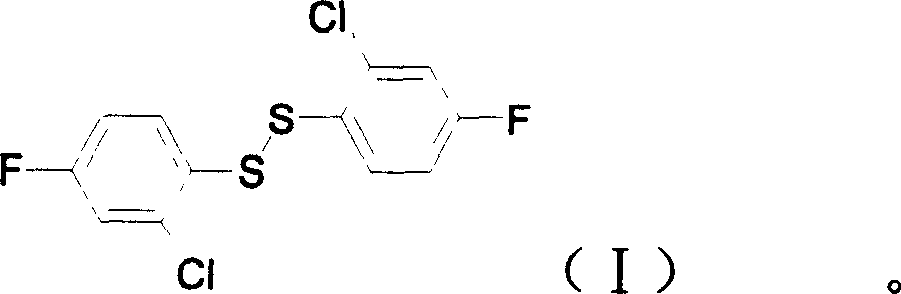
2(2-chlorine-4-phenyl fluoride) bisulfide, preparation and aplication
A technology of disulfide and fluorophenyl, which is applied to the preparation method and application field of new substances, can solve problems such as difficult preparation and difficult industrial production, and achieve the effects of simple operation, mild reaction conditions and reasonable route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of bis(4-amino-2-chlorophenyl) disulfide
[0022] Add 3,4-dichloronitrobenzene (96.0g, 0.5mol) and water (150mL) into a 1000mL four-necked flask, raise the temperature to 50~55℃, add sodium sulfide nonahydrate (360.0g, 1.5 mol), sulfur (24.0g, 0.75mol) and water (210mL), add it in 10min, then heat to reflux for 10-12h, HPLC analysis has completed the reaction; azeotropic distillation separated a small amount of by-products 3,4- Dichloroaniline (2.5g, yield 3.1%), at this time about 150mL of water is distilled out; add 150mL of water to the reaction solution and adjust the temperature to 45℃, cool in a water bath, add 30% hydrogen peroxide dropwise at 40~50℃ (56.7g, 0.5mol), cool to room temperature after adding, filter with suction, wash with water, and recrystallize with ethanol to obtain 76.5g of orange-yellow crystals, yield 95.0%, content 98.3%, melting point 190.5-192.0°C.
Embodiment 2~5
[0023] Examples 2~5: Preparation of bis(4-amino-2-chlorophenyl) disulfide
[0024]
Embodiment 6
[0025] Example 6: Preparation of bis(2-chloro-4-fluorophenyl) disulfide
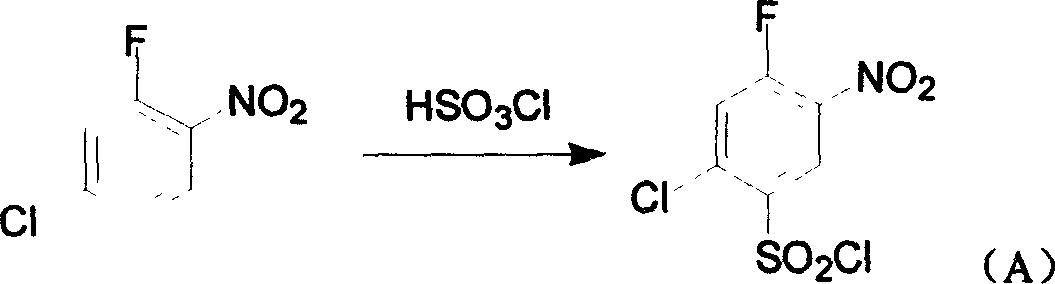
[0026] In a 500ml three-necked flask, add bis(2-chloro-4-aminophenyl) disulfide (20.0g, 62mmol) and 30% dilute sulfuric acid (250g), stir at room temperature for 1 hour, and cool to 5-10°C in an ice-water bath. Then, 30% sodium nitrite aqueous solution (29.7g, 129mmol) was added dropwise within 30min at this temperature, and after the addition, the reaction was continued with stirring at 5-10°C for 1.5h. A small amount of insoluble matter was removed by suction filtration. The filtrate was slowly added with 40% fluoroboric acid aqueous solution (33g, 150mmol) under cooling in an ice-water bath, and the reaction temperature was controlled at 5~10℃ and stirred for 30min. 1:10) The solution was washed with ether and dried in vacuum to obtain a pale yellow powdery diazonium fluoroborate (30.5g); the obtained diazonium salt was placed in a dry 250mL three-necked flask, and the temperature was carefully raised to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com