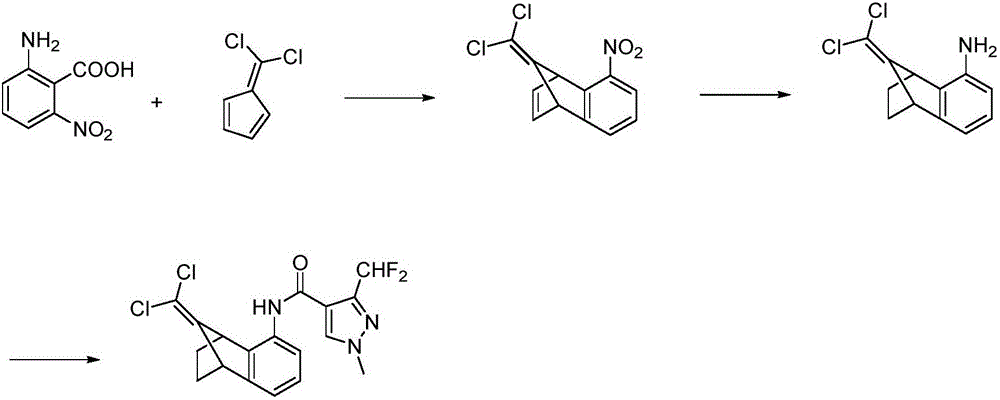
Intermediate of benzovindiflupyr and preparation method and application thereof
A technology for reaction and reaction kettle, applied in the field of intermediates of benzofluconazole, can solve the problems of long steps, difficult to obtain raw materials, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
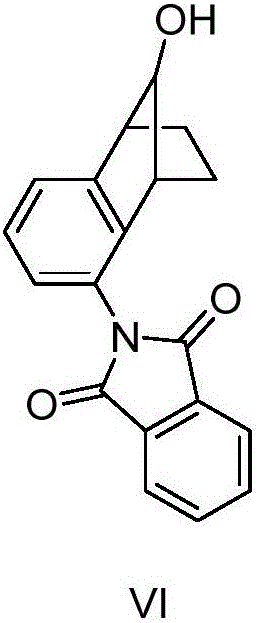
[0060] Example 1 Synthesis of N-(1,2,3,4-tetrahydro-1,4-endomethylene-naphthalene-9-phenol-5-yl-)-phthalimide (VI) .
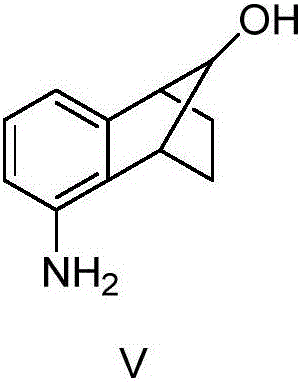
[0061] The molar ratio of the feed materials is 5-amino-1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-ol:phthalic anhydride=1:1.05.
[0062] In a 500ml four-necked bottle equipped with mechanical stirring and a thermometer, add 5-amino-1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-ol (99.8g, 0.57mol ), phthalic anhydride (88.5g, 0.59mol), the temperature was raised to 130°C, and stirred for 1.5h. After the reaction was completed, 200ml of toluene was added dropwise while hot. 1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-ol-5-yl-)-phthalimide (VI) 167.3g, yield 96.2%, The melting point is 148.9-149.7°C.
Embodiment 2
[0063] Example 2 Synthesis of N-(1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-phenol-5-yl-)-phthalimide (VI) .
[0064] The molar ratio of the feed materials is 5-amino-1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-ol:phthalic anhydride=1:1.01.
[0065] In a 500ml four-necked bottle equipped with mechanical stirring and a thermometer, add 5-amino-1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-ol (99.8g, 0.57mol ), phthalic anhydride (85.8g, 0.58mol), the temperature was raised to 150°C, and stirred for 1.5h. After the reaction was completed, 200ml of toluene was added dropwise while hot. 1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-phenol-5-yl-)-phthalimide (VI) 158.2g, yield 90.1%, The melting point is 148.8-149.9°C.
Embodiment 3
[0066] Example 3 Synthesis of N-(1,2,3,4-tetrahydro-1,4-endomethylene-naphthalene-9-phenol-5-yl-)-phthalimide (VI) .
[0067] The molar ratio of the feed materials is 5-amino-1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-ol:phthalic anhydride=1:1.2.
[0068] In a 500ml four-necked bottle equipped with mechanical stirring and a thermometer, add 5-amino-1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-ol (99.8g, 0.57mol ), phthalic anhydride (100.6g, 0.68mol), the temperature was raised to 120°C, and stirred for 1.5h. After the reaction was completed, 200ml of toluene was added dropwise while hot. 1,2,3,4-tetrahydro-1,4-methano-naphthalene-9-phenol-5-yl-)-phthalimide (VI) 162.6g, yield 93.5%, The melting point is 148.9-149.9°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com