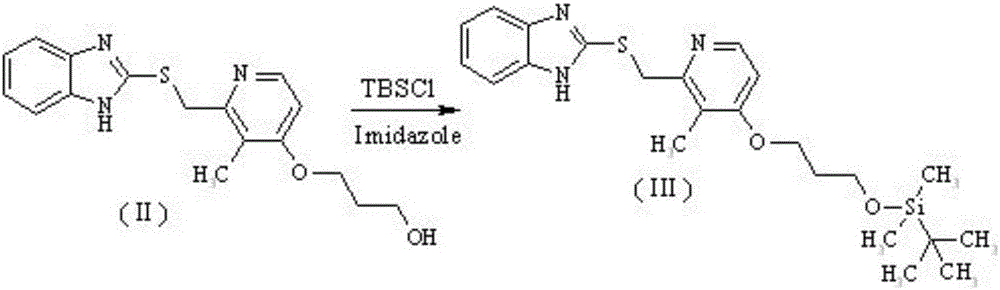
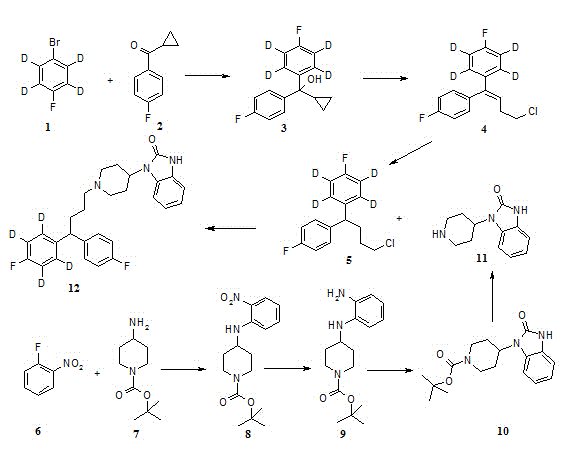
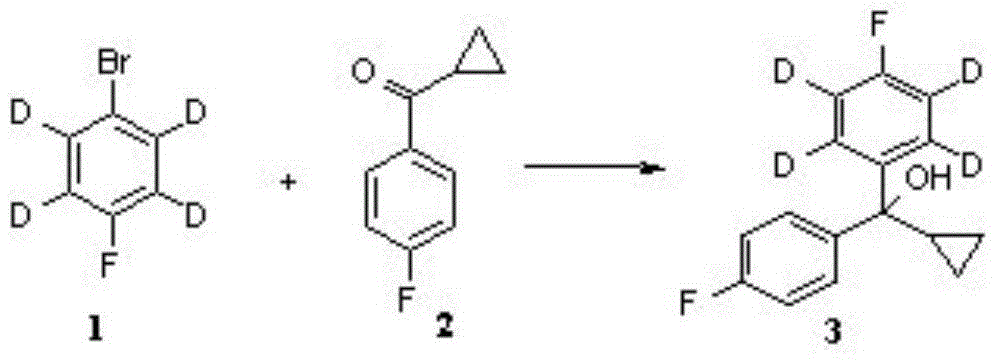
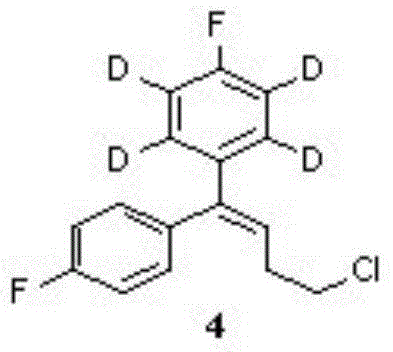
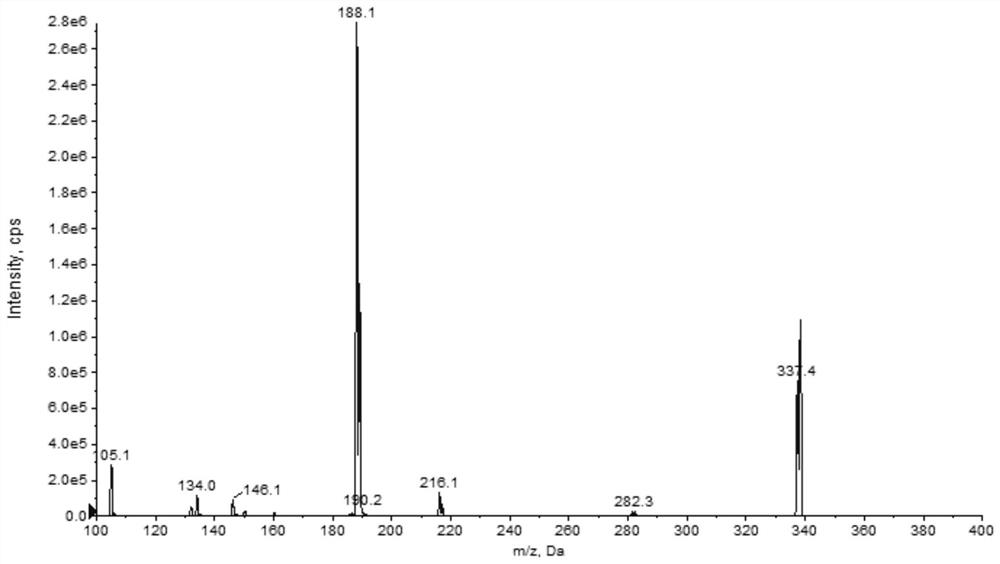
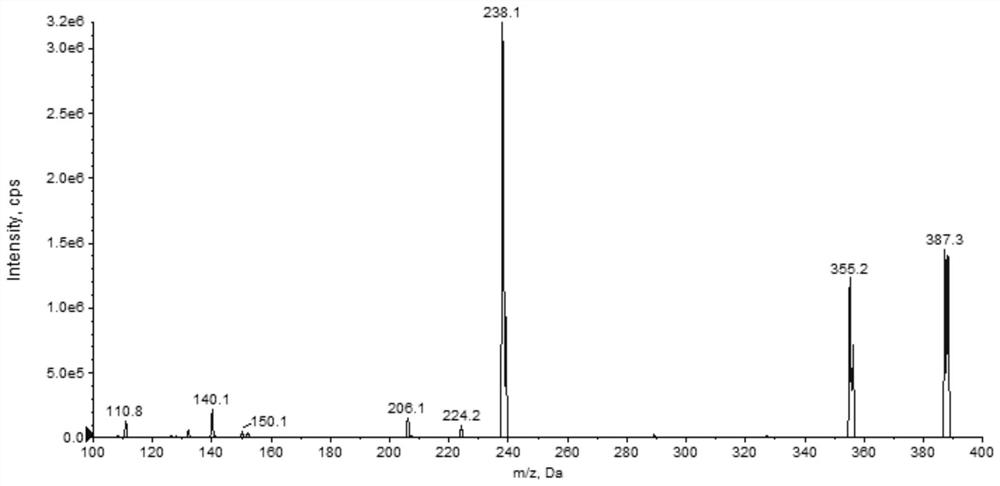
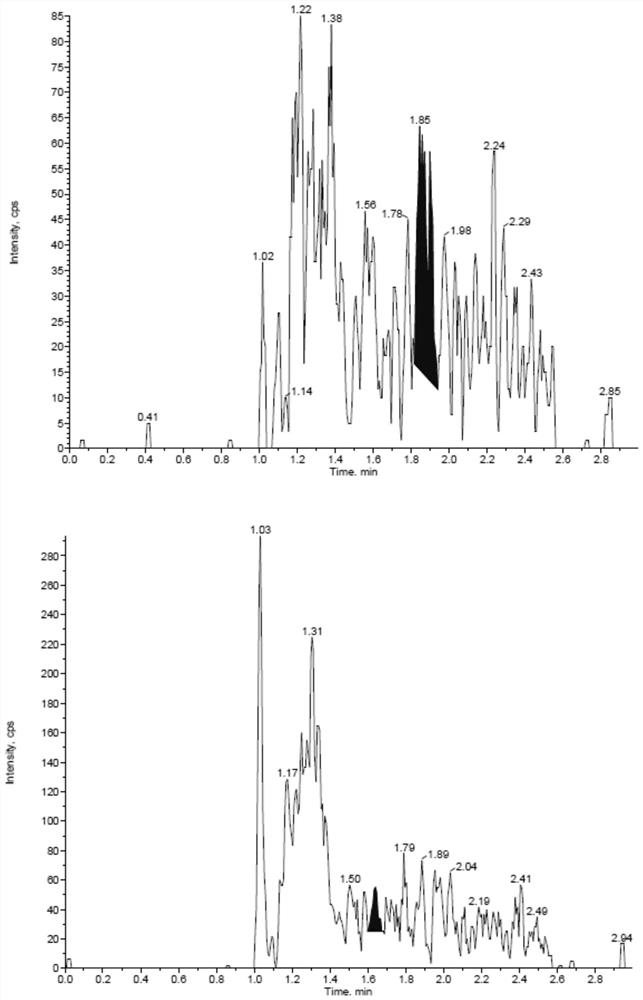
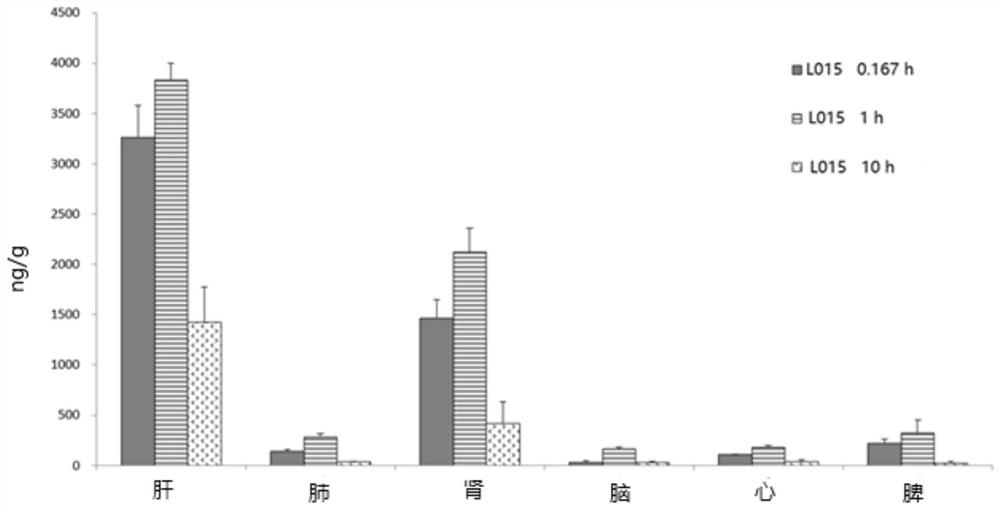
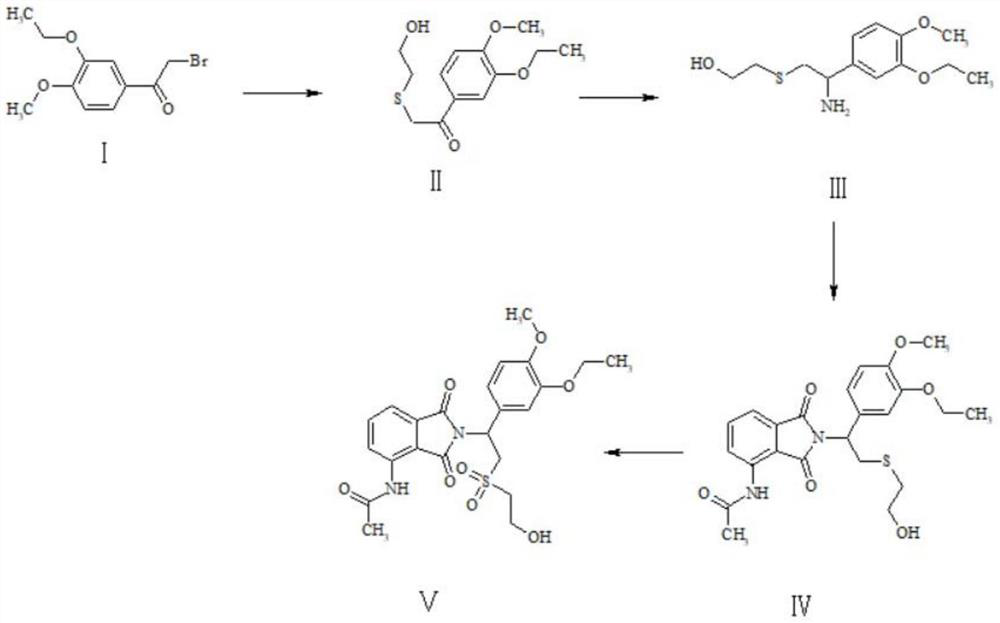
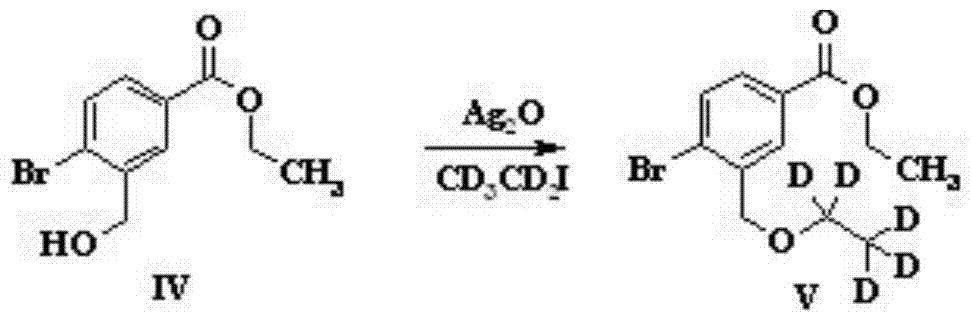Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46 results about "Clinical pharmacokinetic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
System and method for integrating and validating genotypic, phenotypic and medical information into a database according to a standardized ontology
InactiveUS20070178501A1Safest and most effective treatmentGood decisionData processing applicationsMicrobiological testing/measurementData validationMedical record
The system described herein enables clinicians and researchers to use aggregated genetic and phenotypic data from clinical trials and medical records to make the safest, most effective treatment decisions for each patient. This involves (i) the creation of a standardized ontology for genetic, phenotypic, clinical, pharmacokinetic, pharmacodynamic and other data sets, (ii) the creation of a translation engine to integrate heterogeneous data sets into a database using the standardized ontology, and (iii) the development of statistical methods to perform data validation and outcome prediction with the integrated data. The system is designed to interface with patient electronic medical records (EMRs) in hospitals and laboratories to extract a particular patient's relevant data. The system may also be used in the context of generating phenotypic predictions and enhanced medical laboratory reports for treating clinicians. The system may also be used in the context of leveraging the huge amount of data created in medical and pharmaceutical clinical trials. The ontology and validation rules are designed to be flexible so as to accommodate a disparate set of clients. The system is also designed to be flexible so that it can change to accommodate scientific progress and remain optimally configured.
Owner:NATERA
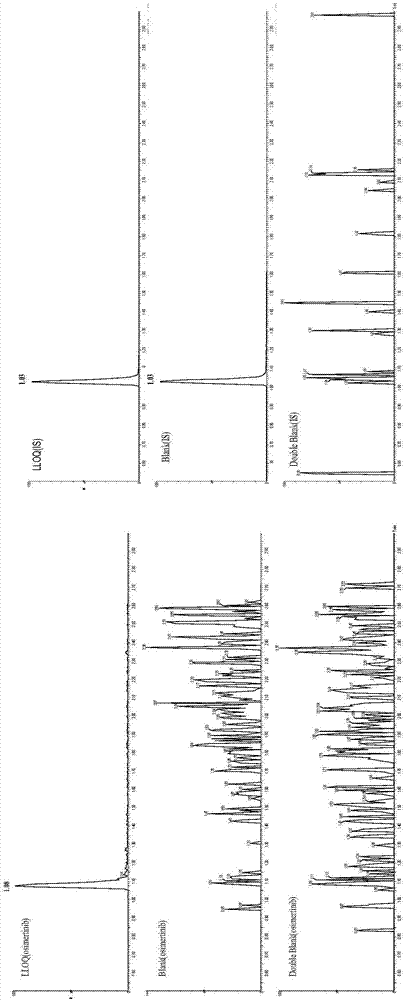
Detection of drug concentration of osimertinib in human plasma and/or cerebrospinal fluid by combining UPLC-MS/MS
The invention relates to detection of the drug concentration of osimertinib in human plasma and / or cerebrospinal fluid by combining UPLC-MS / MS. According to the present invention, the method for determining the drug concentration of osimertinib in human plasma and / or cerebrospinal fluid through an ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS / MS) is provided, wherein formic acid-containing ammonium formate water-formic acid acetonitrile is used as a mobile phase so as to perform the method; and the method has advantages of good specificity and high sensitivity, and is used for the detection of clinical pharmacokinetic samples, wherein the linearity is good when the osimertinib concentration is 2-500 ng.ml<-1> in the plasma sample and the osimertinib concentration is 0.5-20 ng.ml<-1> in the cerebrospinal fluid sample.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
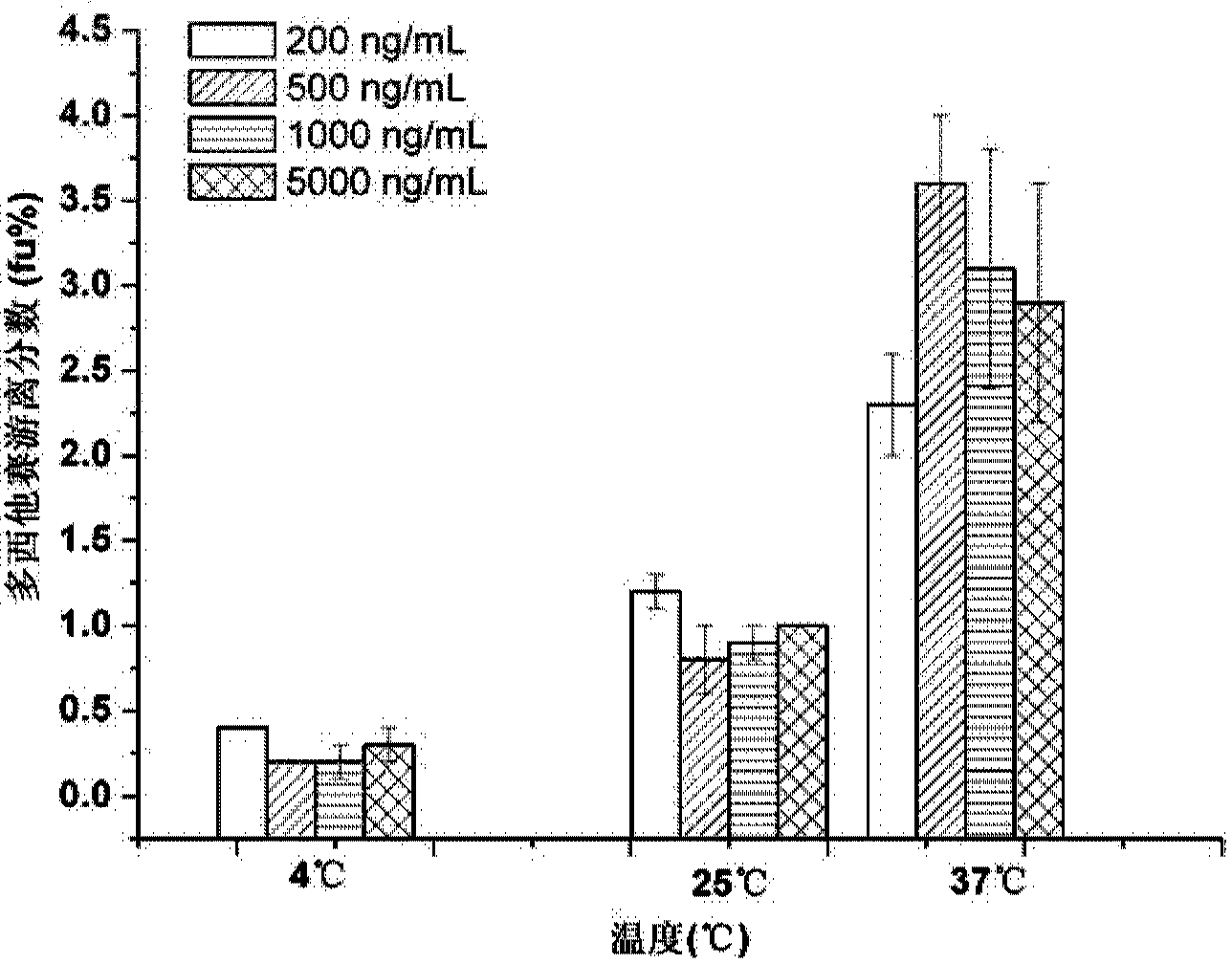
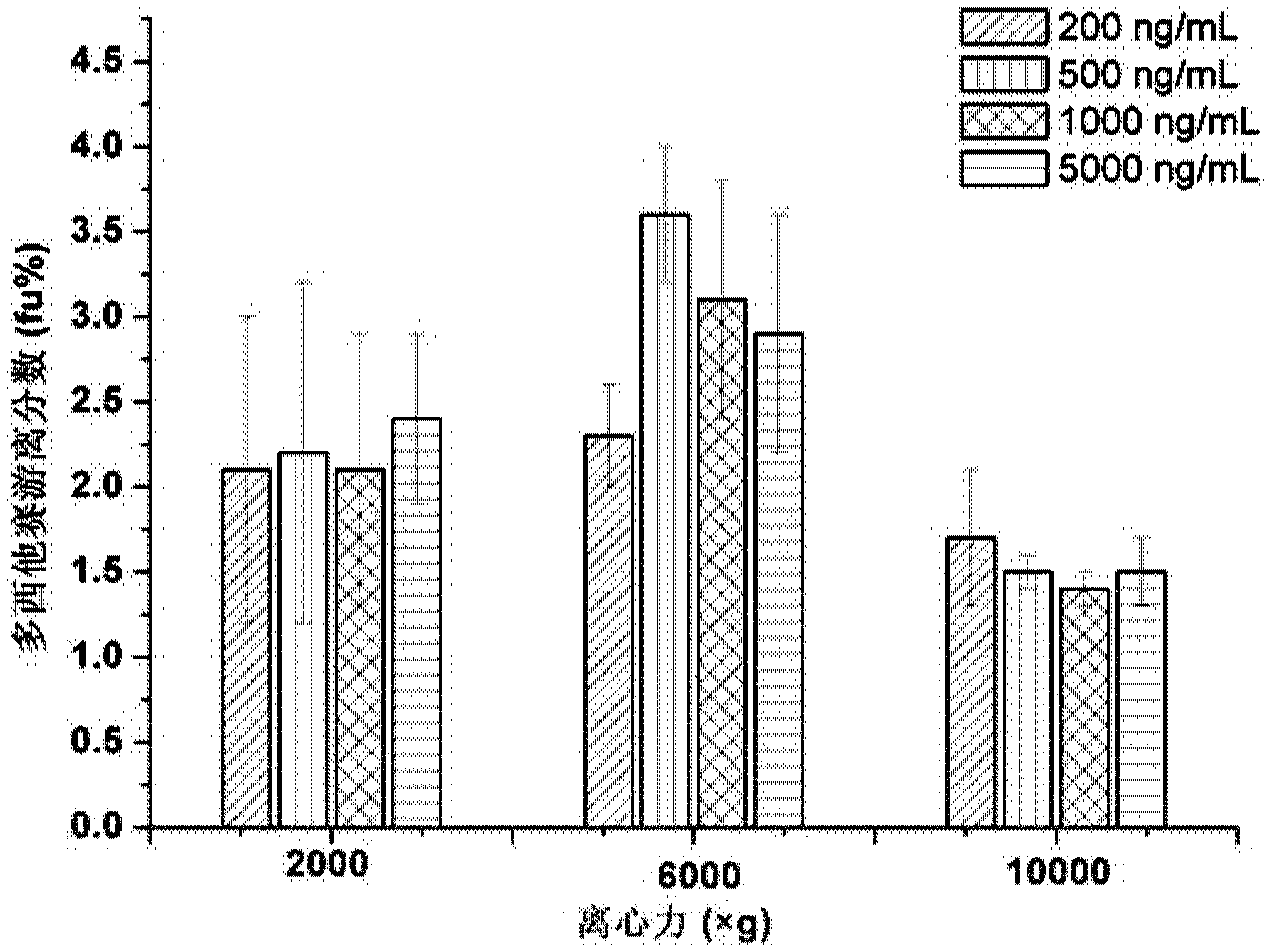
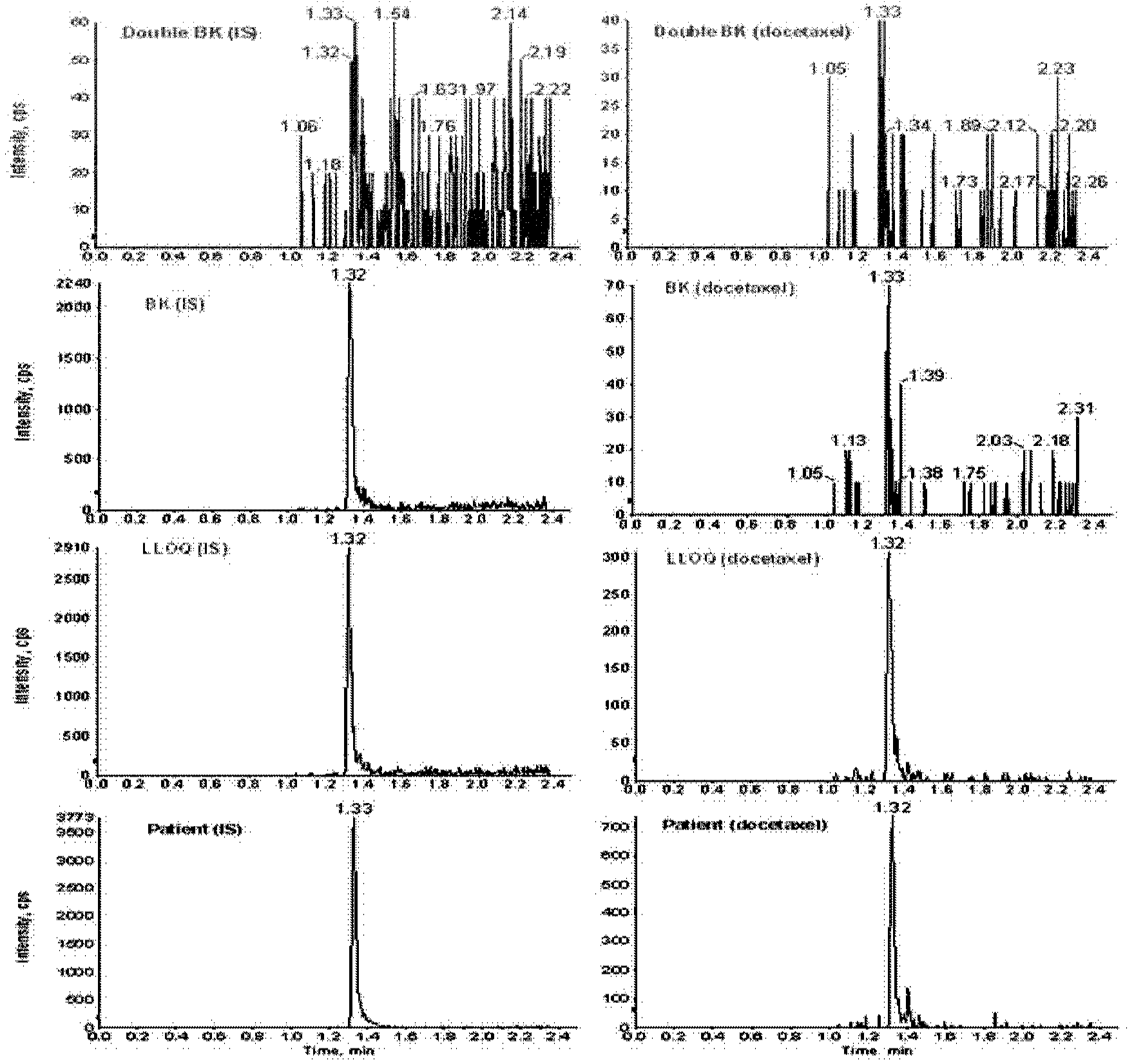
Ultrafiltration and UPLC-MS/MS (ultra-high performance liquid chromatography tandem mass spectrometry) method for measuring concentration of free docetaxel in human plasma
ActiveCN103454360AHigh sensitivityStrong specificityComponent separationLipid formationUltrafiltration
The invention relates to an ultrafiltration and UPLC-MS / MS (ultra-high performance liquid chromatography tandem mass spectrometry) method for measuring concentration of free docetaxel in human plasma and provides a method for measuring the concentration of the free docetaxel in the plasma after docetaxel lipid microsphere injection. The method comprises an ultrafiltration (UF) step and a UPLC-MS / MS analysis step. The UF-UPLC-MS / MS method which is high in sensitivity and specificity and good in reproducibility is established on the premise that the balance of a combination medicine-free medicine in the plasma is not damaged. The method used for measuring the concentration of the free medicine and established for first time meets the biological sample analysis requirements of Guide for the Research of Chemical Drug and Clinical Pharmacokinetics issued by CFDA in 2005 in accuracy, precision, specificity, stability, extracting recovery rate and matrix effect.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Preparation method of sparsenatan for stabilizing isotope labeling
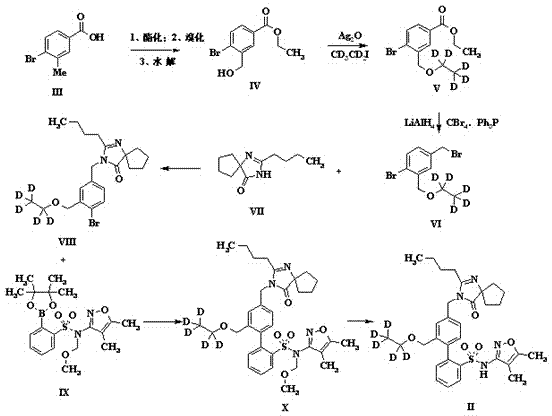
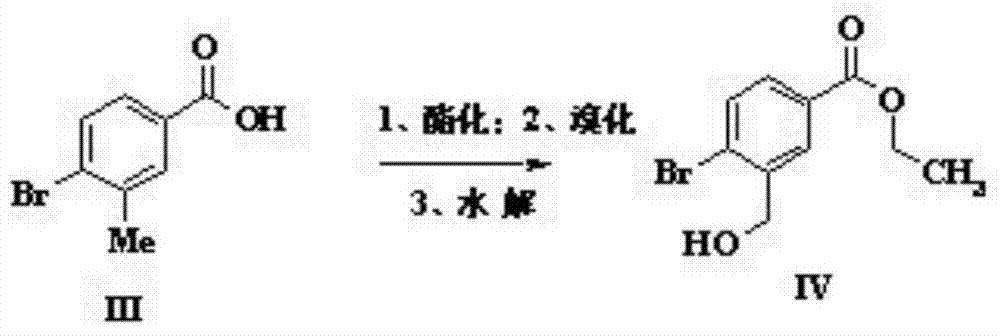
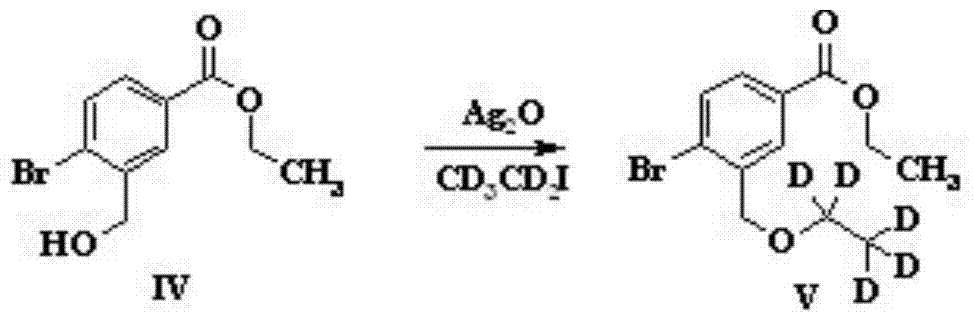
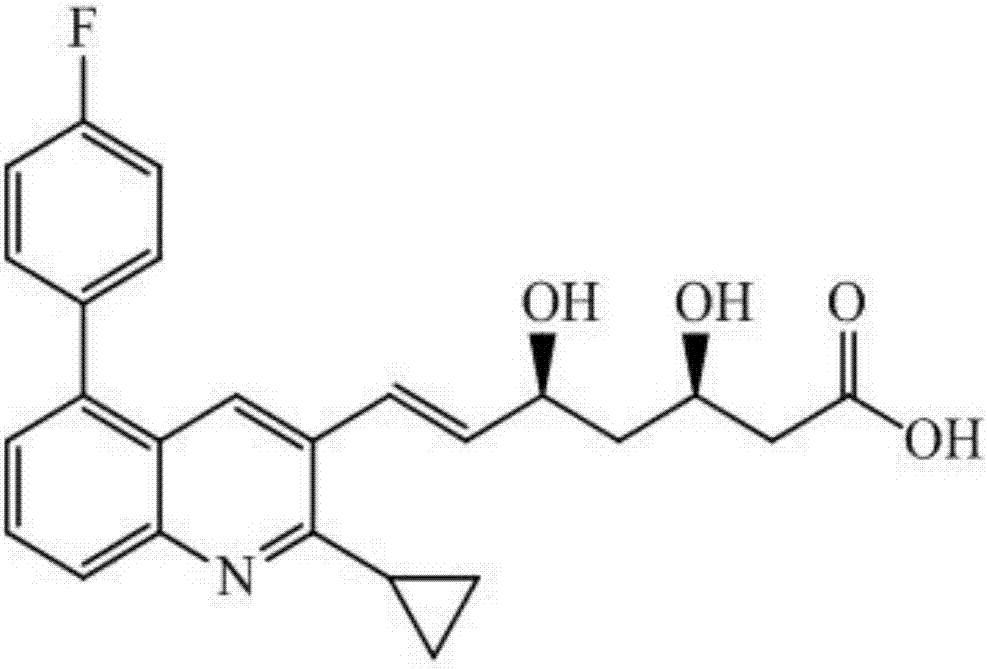
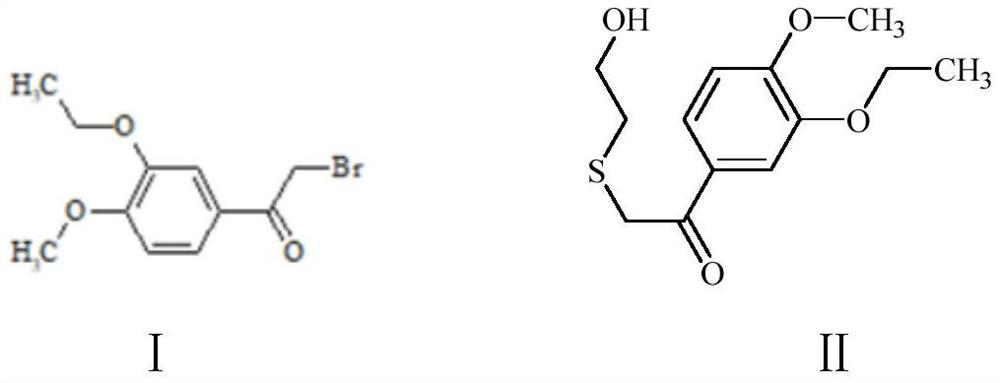
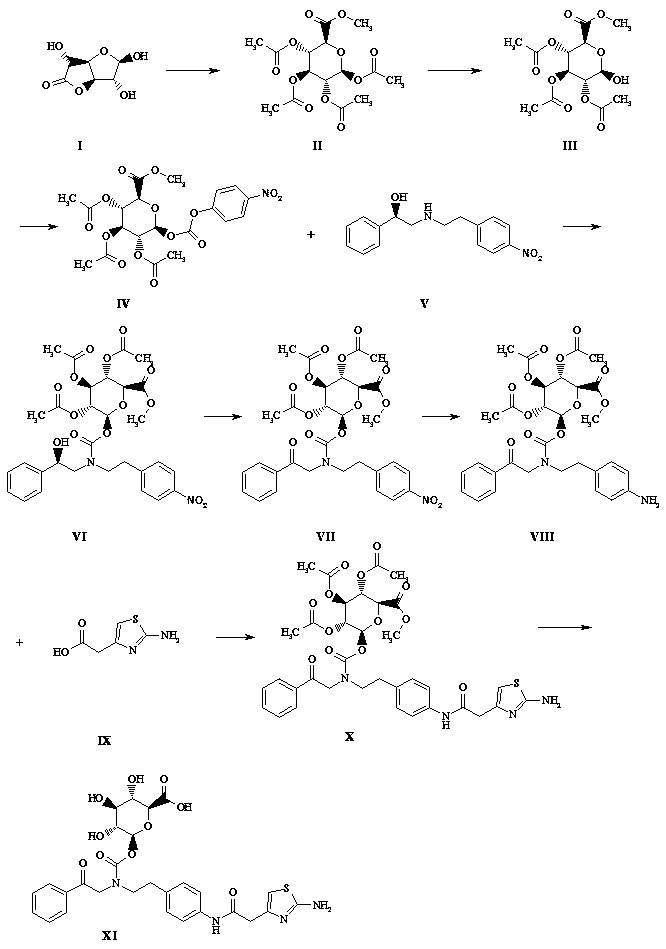
The invention discloses a preparation method of sparsenatan for stabilizing isotope labeling. According to the method, 3-methyl-4-bromobenzoic acid is used as a raw material, iodoethane for deuterium labeling serves as a deuterium labeling initiator, and the sparsenatan is obtained through six-step reaction. Optimal preparation steps and reaction conditions are screened out through a large number of experiments, and the whole preparation method is reasonable in design and good in operability. The purity of the sparsenatan prepared by means of the method and used for stabilizing the isotope labeling can be higher than 99%, and the isotope abundance is higher than 99%. The sparsenatan prepared by means of the method and used for stabilizing the isotope labeling is a standard product for research on the metabolic mechanism of the sparsenatan, can be used for tracing the metabolic process of the sparsenatan in a living body and has great application and research value on clinic pharmacokinetic study.
Owner:TLC NANJING PHARMA RANDD CO LTD
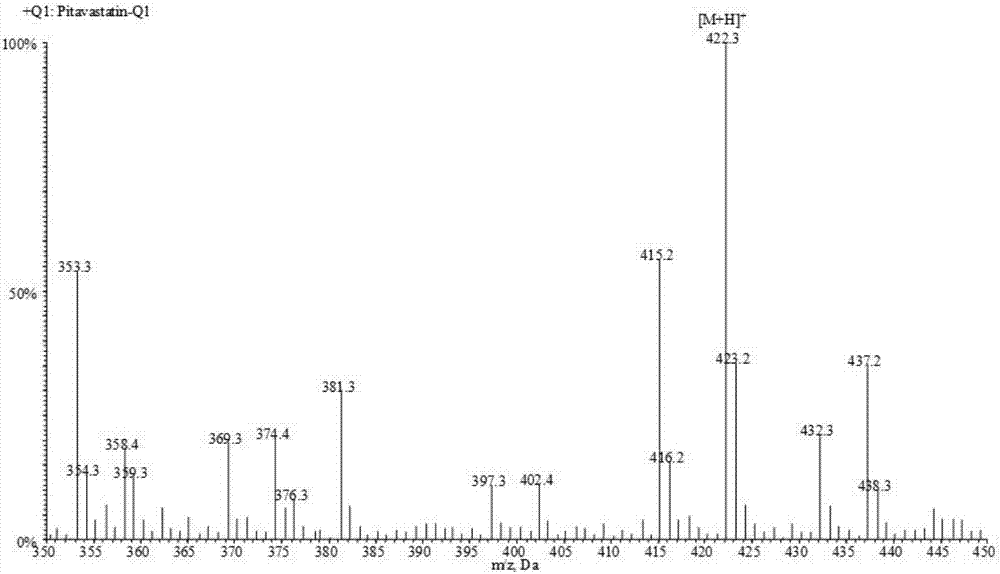
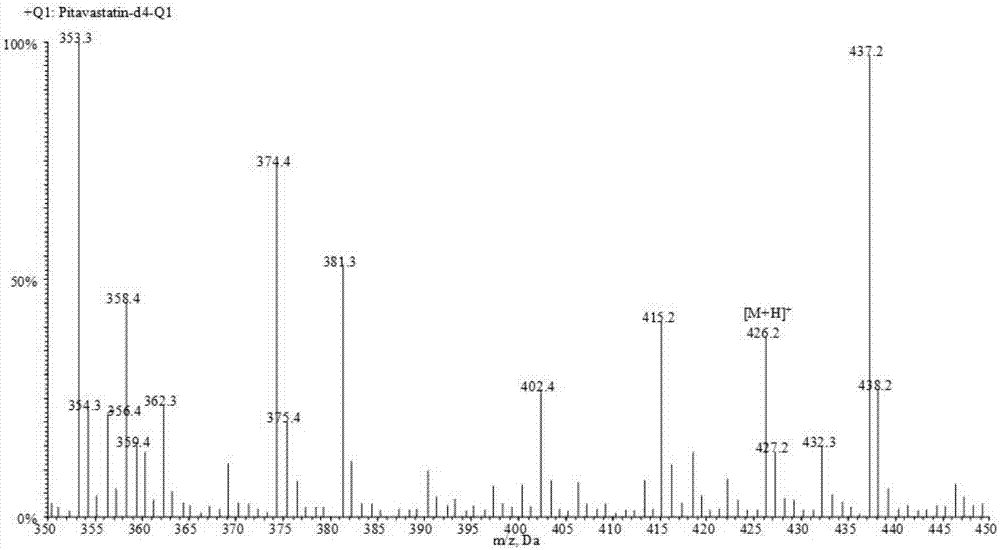
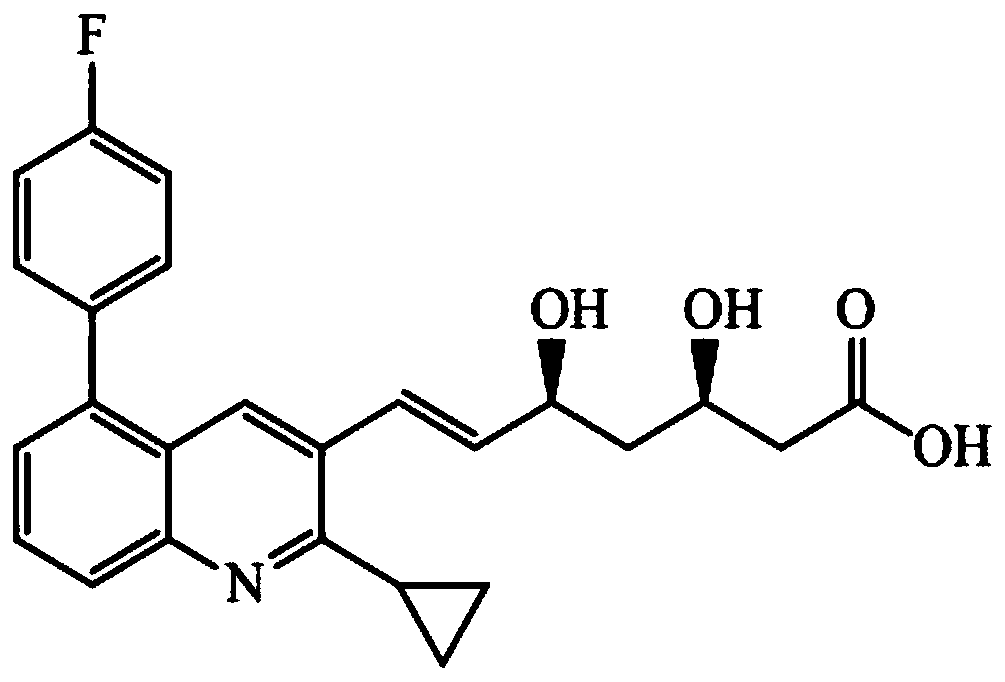
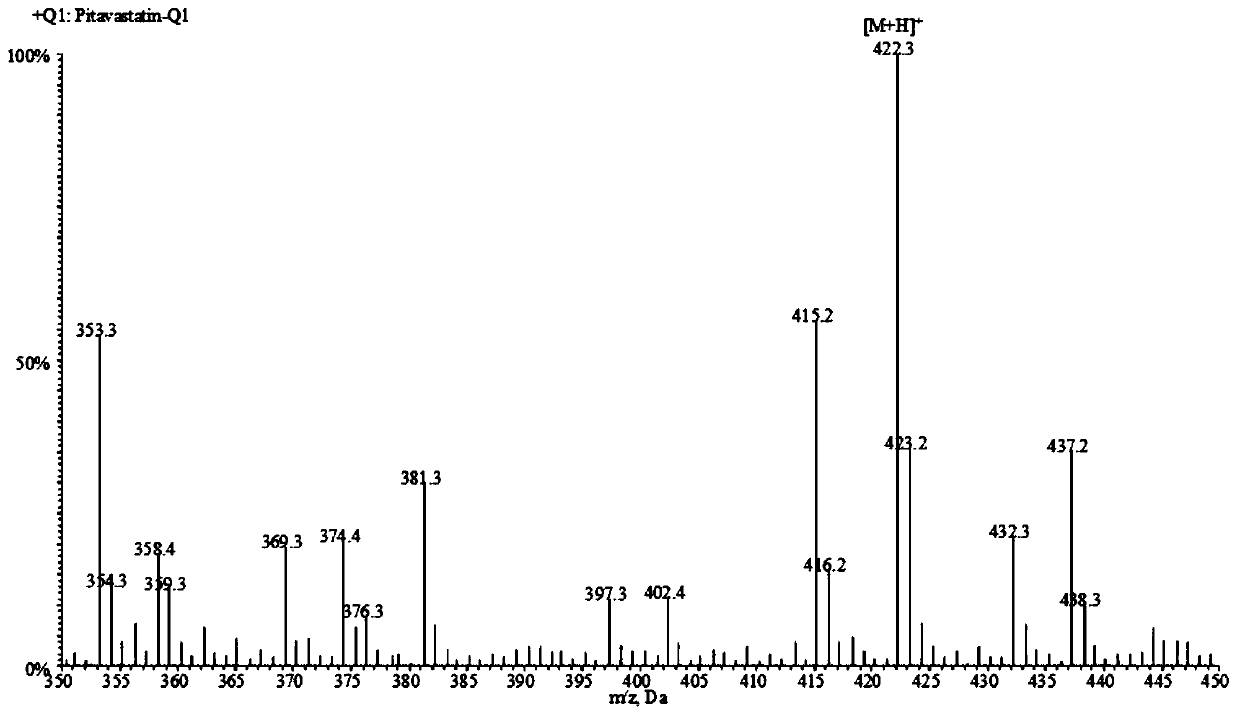
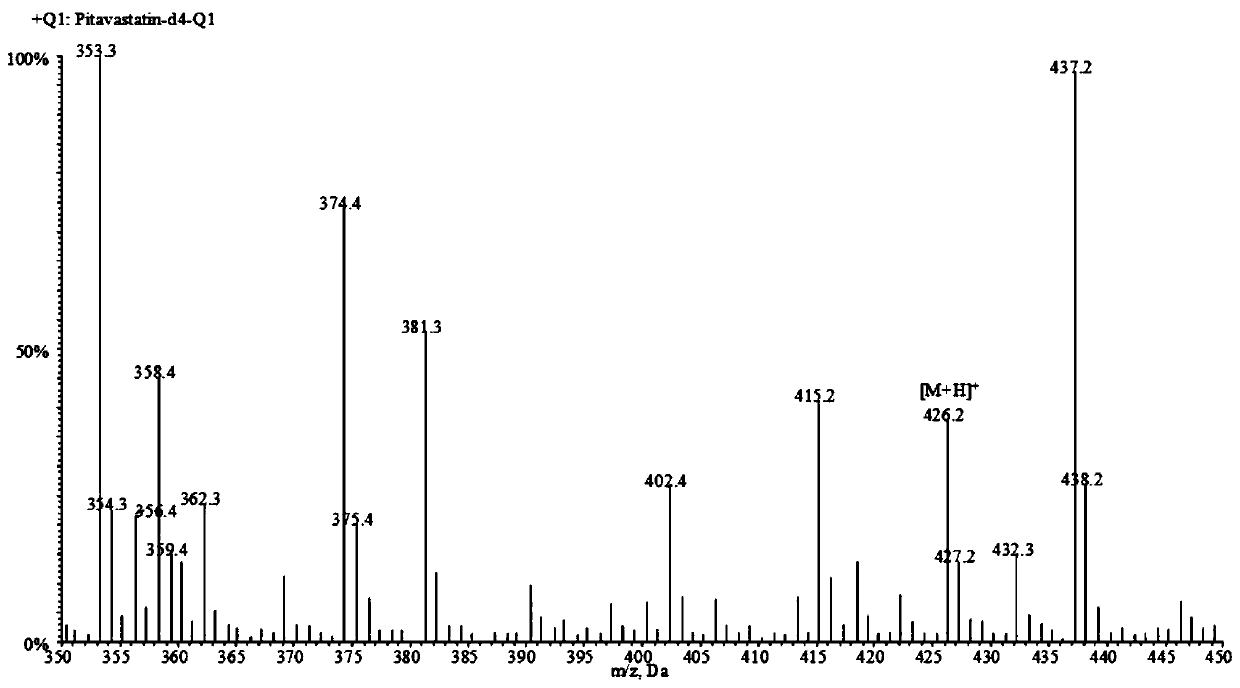
Liquid chromatogram-tandem mass spectrum method for detecting pitavastatin in human plasma, and application to clinical pharmacokinetic research
ActiveCN107144648AGood reproducibilityImprove accuracyComponent separationPretreatment methodEclipse
The invention relates to a liquid chromatogram-tandem mass spectrum method for detecting pitavastatin in human plasma, and application to clinical pharmacokinetic research. The invention provides a method for detecting pitavastatin concentration of plasma. By the method, the pitavastatin concentration of the plasma can be analyzed through LC-MS / MS. According to the method provided by the invention, a protein precipitate pretreatment method is preferably adopted, deuterated pitavastatin serves as internal standard, Eclipse Plus Phenyl-Hexyl column isocratic elution is adopted, and electrospray ionization (ESI) tandem mass spectrum detection is adopted. By adoption of the method provided by the invention, the extraction and recovery rate of the plasma sample is 93 percent or higher and is not influenced by matrix effect, the stability of the pitavastatin is inspected by counting the pitavastatin concentration RSD% before pretreatment, the accuracy of the measured data is guaranteed, the method is high in specificity and selectivity, high in sensitivity, rapid in detection and small in use amount, and simple, reliable, high-flux and condition-controllable clinical mass-batch sample analysis requirements are met. The specificity, the stability and the like of the method provided by the invention are verified, and the method can be used for evaluating the bioequivalence of various dosage forms of pitavastatin successfully.
Owner:苏州海科医药技术有限公司
Synthesizing method of single-configuration rabeprazole metabolite
ActiveCN105801560AReasonable process designEasy to operateOptically-active compound separationBulk chemical productionRabeprazoleMetabolite
Owner:TLC NANJING PHARMA RANDD CO LTD
Preparation method of deuterated pimozide
ActiveCN104829591AOptimal reaction stepReasonable process designOrganic chemistryStable Isotope LabelingMechanism of action
The invention discloses a preparation method of deuterated pimozide. The pimozide-d4 is synthesized from 4-bromopentafluorobenzene-d4 through eight steps. The provided preparation method has the advantages of reasonable technological design, simple operation, easy product separation and purification, easily-available raw materials, high yield, and high purity. The isotope abundance of the obtained target product is high. The prepared stable isotope-labeled pimozide-d4 can be well applied to clinical pharmacokinetics researches, so people can acknowledge the metabolism process and action mechanism of pimozide in human body more precisely and conveniently, and thus the deuterated pimozide has an important application value.
Owner:TLC NANJING PHARMA RANDD CO LTD
Preparation method of cinacalcet impurity
ActiveCN110041206AImprove protectionReasonable process designOrganic compound preparationCarboxylic acid amides preparationPhenylacetic acidTest sample
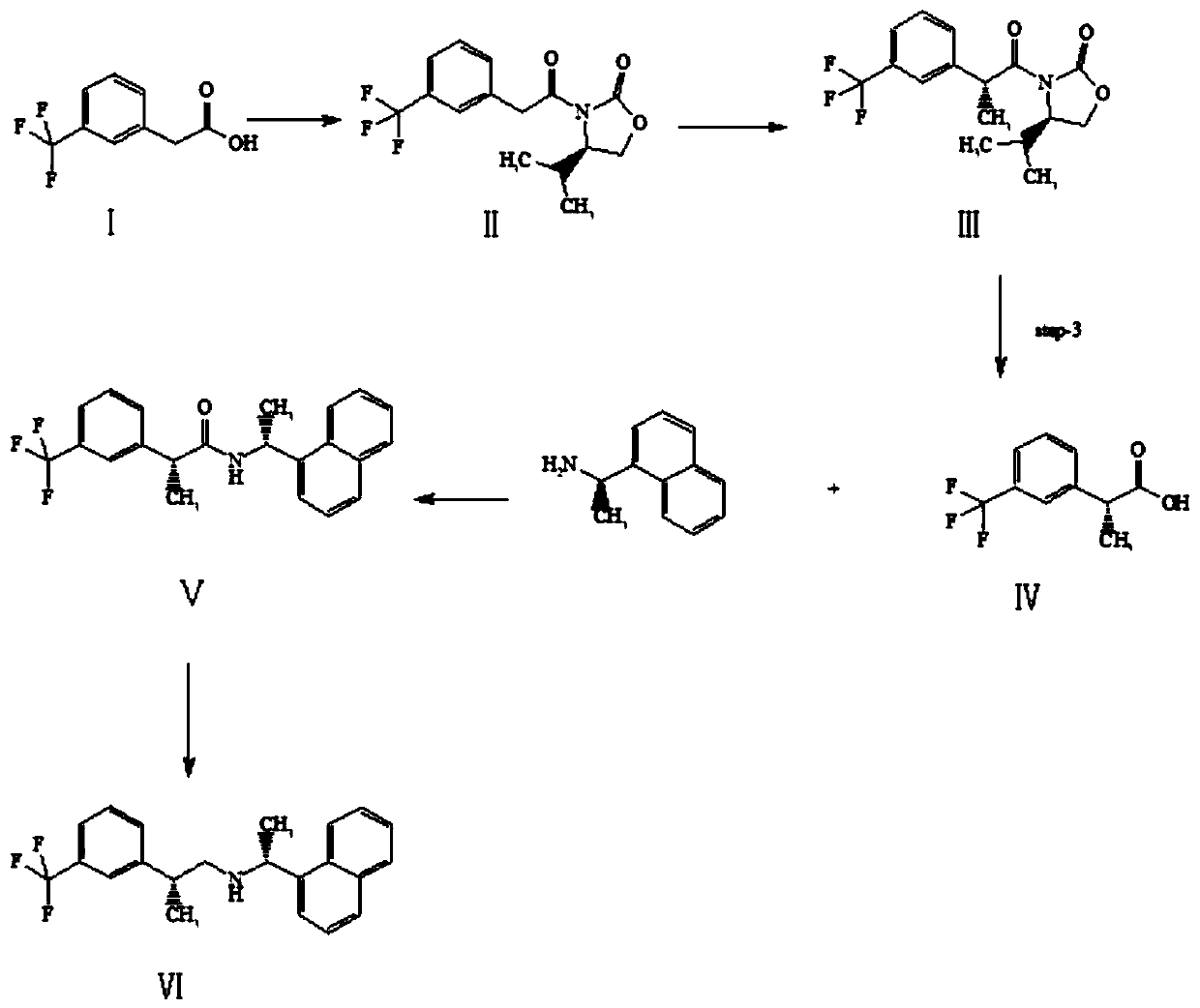
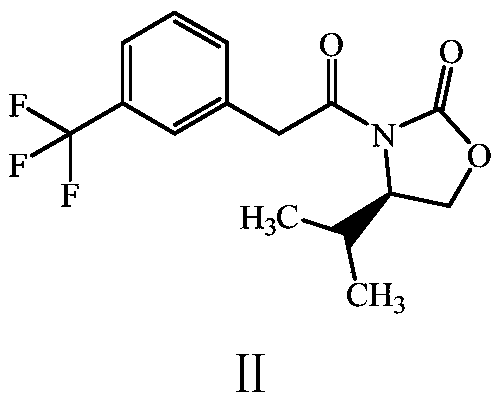
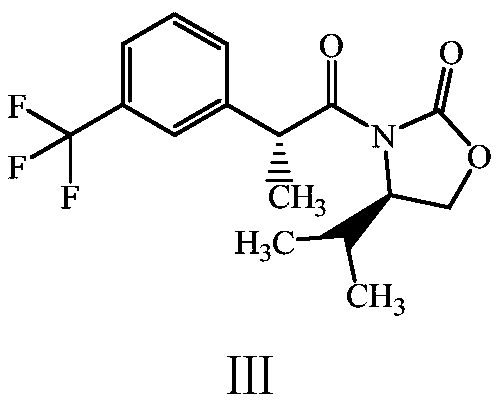
The invention discloses a preparation method of a cinacalcet impurity and belongs to the field of medicine synthesis. A whole technology is reasonable in design, strong in operability and convenient for purification. The method takes m-(trifluoromethyl)phenylacetic acid as a starting raw material to synthesize the cinacalcet impurity through five-step reaction. According to the preparation methoddisclosed by the invention, optimal preparation steps and reaction conditions are screened through an experiment; the purity of the cinacalcet impurity prepared by the preparation method reaches 98.8percent or more and a test sample is provided for researching cinacalcet; the cinacalcet impurity has important research value in clinical pharmacokinetic researches.
Owner:TLC NANJING PHARMA RANDD CO LTD
Method for determination of impurity A in Cetilistat in biological sample
ActiveCN109298081AAdvantages of assay methodImprove stabilityComponent separationBiological testingChemistryBiological organism
The invention relates to a method for determination of an impurity A in Cetilistat in a biological sample, and belongs to the technical field of medicines. The method adopts ultra-high performance liquid chromatography-electrospray ionization tandem mass spectrometry (UPLC-MS / MS) to detect the concentration of the Cetilistat impurity A in the biological sample. The established LC-MS / MS determination method for a Cetilistat impurity A plasma sample meets the analyzing requirement of 2015 edition of Chinese pharmacopoeia and (chemical drug non-clinical pharmacokinetics research technique governing principle) issued by SFDA in 2014 for the biological sample in the aspects of accuracy, precision, specialization, stability, the extraction recovery rate, the matrix effect and the like; and a powerful guarantee is provided for development of new drugs of Cetilistat and guidance of clinical rational drug use.
Owner:LUNAN PHARMA GROUP CORPORATION
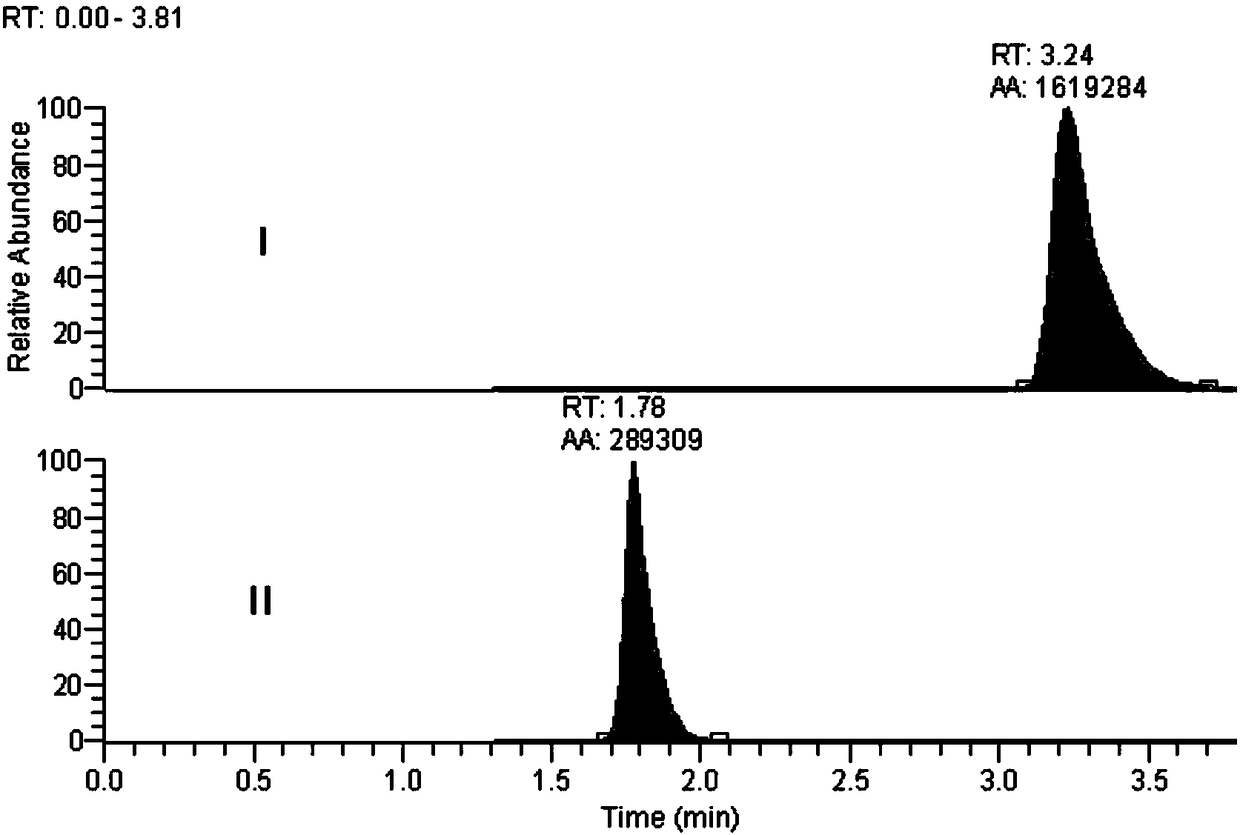
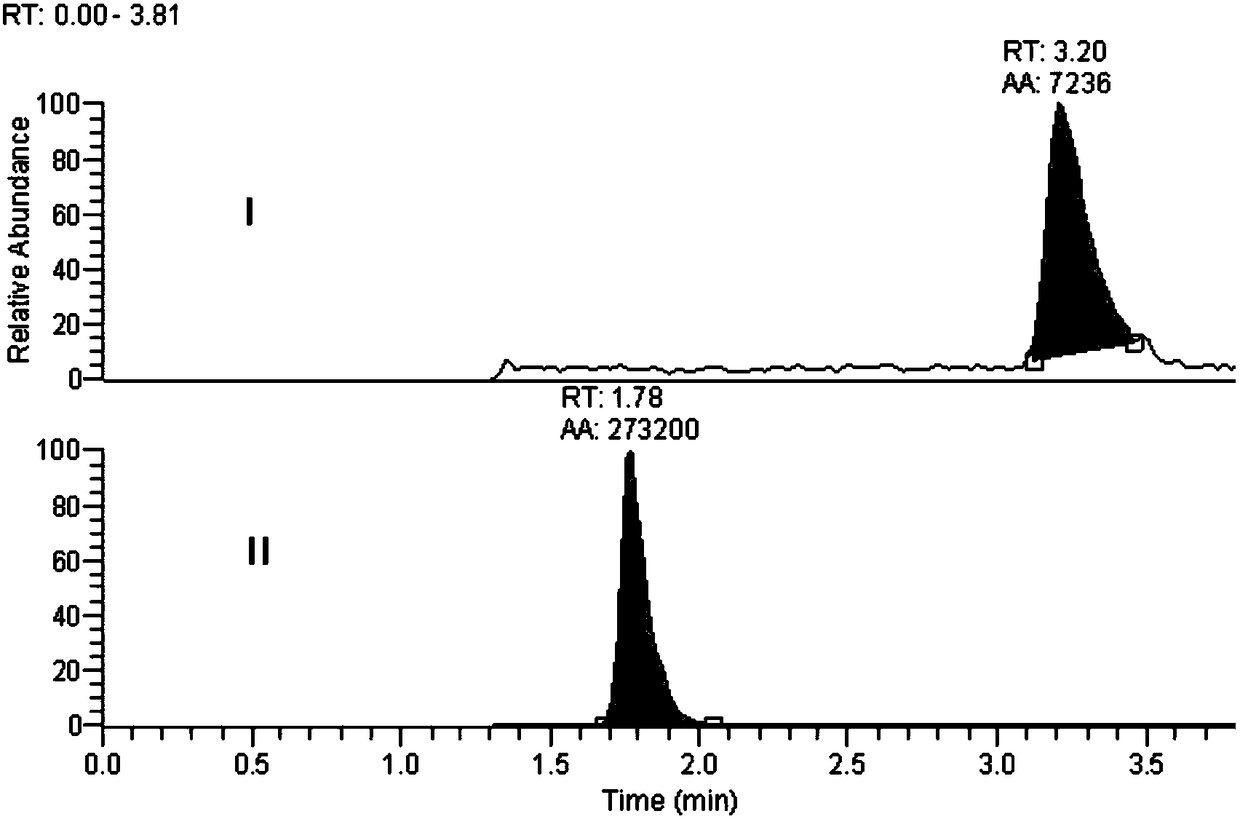
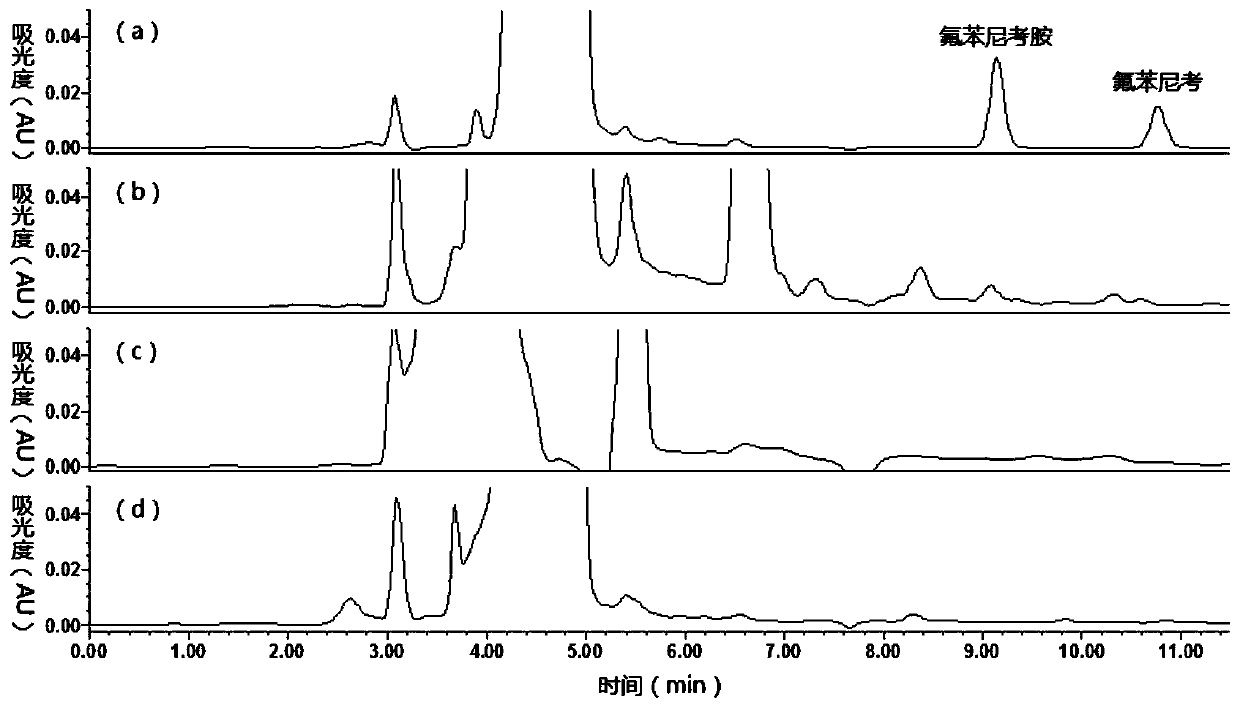
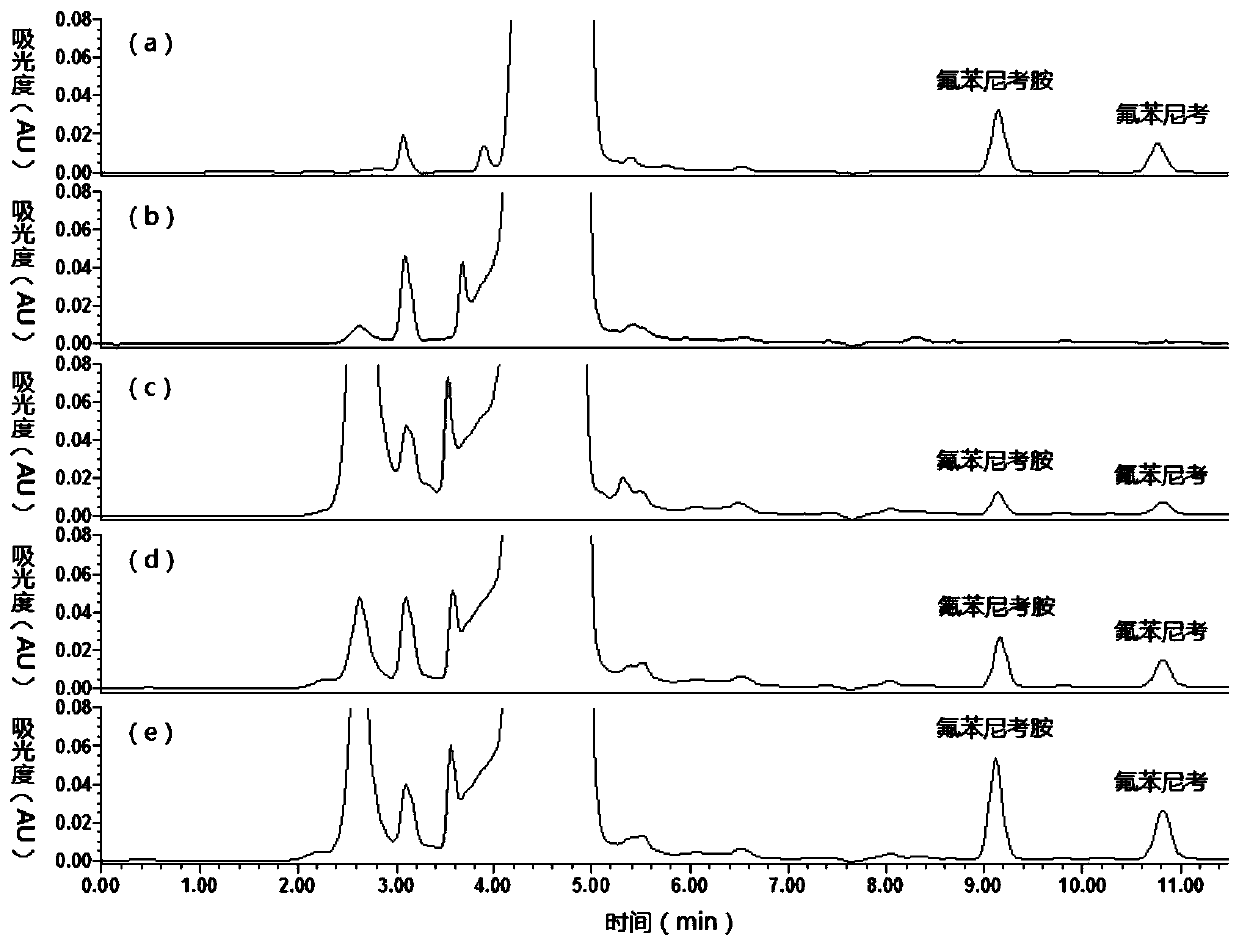
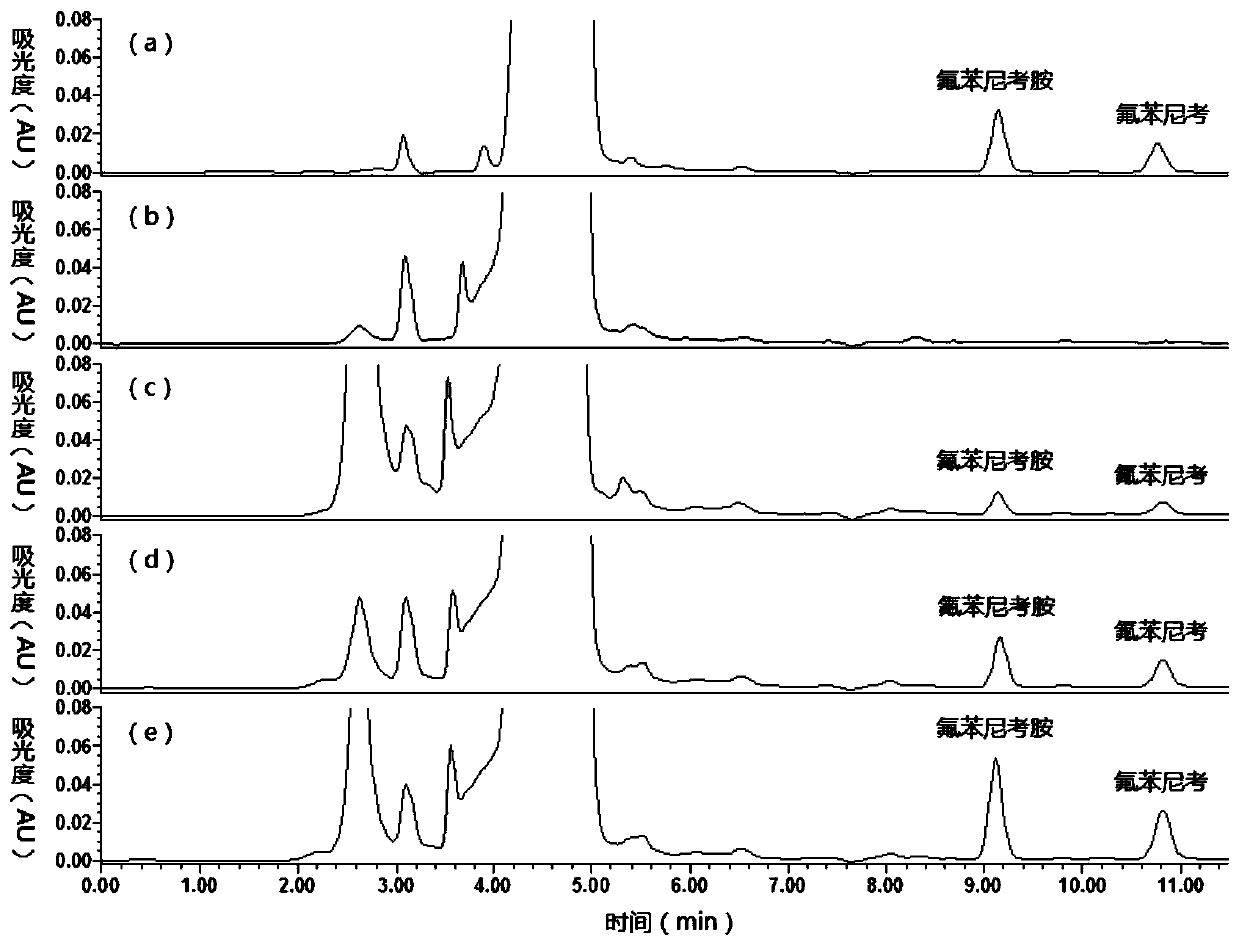
Method for detecting florfenicol and metabolite florfenicol amine in pig urine
The invention discloses a method for detecting florfenicol and metabolite florfenicol amine in pig urine, and belongs to the field of clinical pharmacokinetics of veterinary antibacterial drugs. The method includes the following steps: adding proper amount of a sodium hydroxide solution in pig urine to adjust pH to alkalinity; performing extraction by ethyl acetate; performing condensation on an extract product, and taking a mixed liquid with a ratio of dichloromethane : acetone : ammonia water being (1-6) : (4-9) : (0.05-0.5) as a developing solution to perform thin layer chromatography purifying; and performing detection by adopting high performance liquid chromatography. The method effectively removes endogenous substances contained in the urine extract product by using thin layer chromatography, so that the interference on chromatographic analysis can be avoided, detection costs can be reduced, and the using amount of an organic solvent can be decreased; and the method can combinethe thin layer chromatography and high-performance liquid chromatography, so that deficiency of a detector matching a high performance liquid chromatograph on specificity can be made up, and applications of the high-performance liquid chromatography in clinical pharmacokinetics can be widened.
Owner:WUHAN INST OF BIOENG
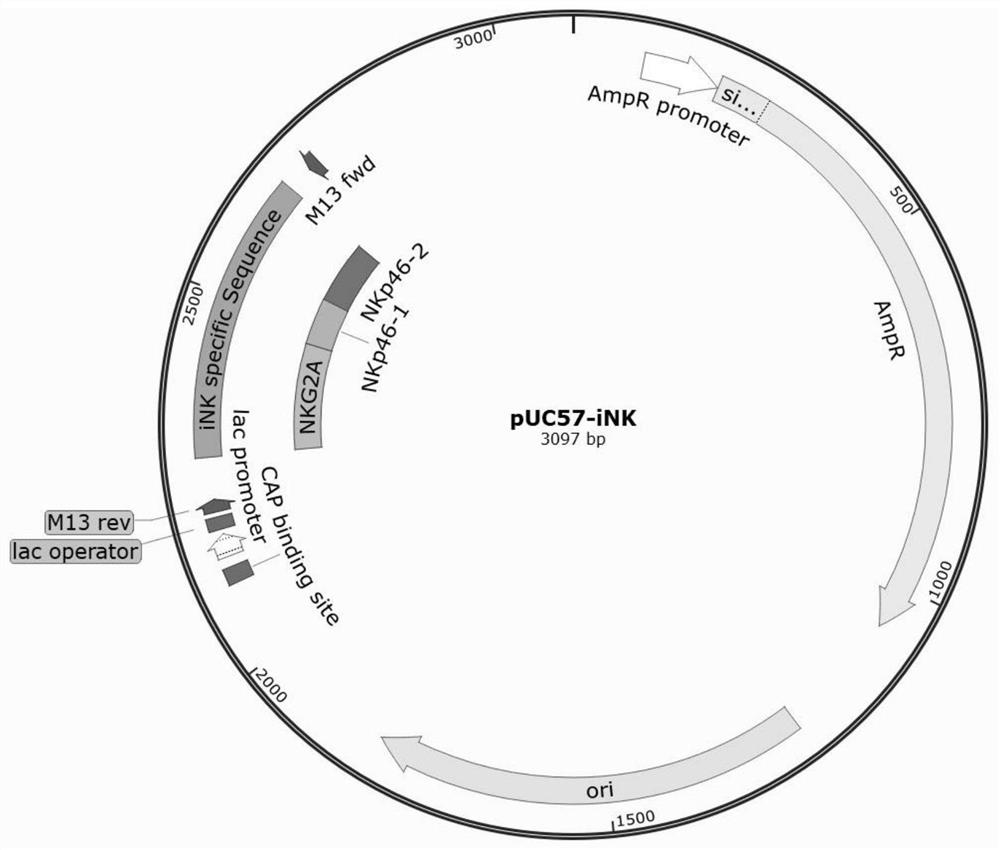
Application of NKG2A in detection of human NK cells in mouse humanized tumor model
PendingCN114214430AReduce uncertaintyImprove detection efficiencyMicrobiological testing/measurementBiological material analysisHuman tumorSide effect
The invention provides application of NKG2A in detection of human NK cells in a mouse humanized tumor model, and belongs to the technical field of biology. The invention relates to an application of NKG2A in detection of human NK cells in a mouse humanized tumor model. The NKG2A fragment is adopted as a detection target, human NK cells and human tumor cells can be distinguished in a mouse tumor-bearing tumor model, and the NKG2A fragment has the characteristics of short detection time, high detection efficiency and high detection sensitivity, is beneficial to research on non-clinical pharmacokinetics, particularly animal experiments, exploration of in-vivo dynamic and prediction of a treatment scheme in the cell treatment research and development process, and has a wide application prospect. The method is very helpful for reducing the uncertainty of cell therapy, increasing the curative effect and reducing the toxic and side effects, and has important clinical guiding significance.
Owner:安徽中盛溯源生物科技有限公司
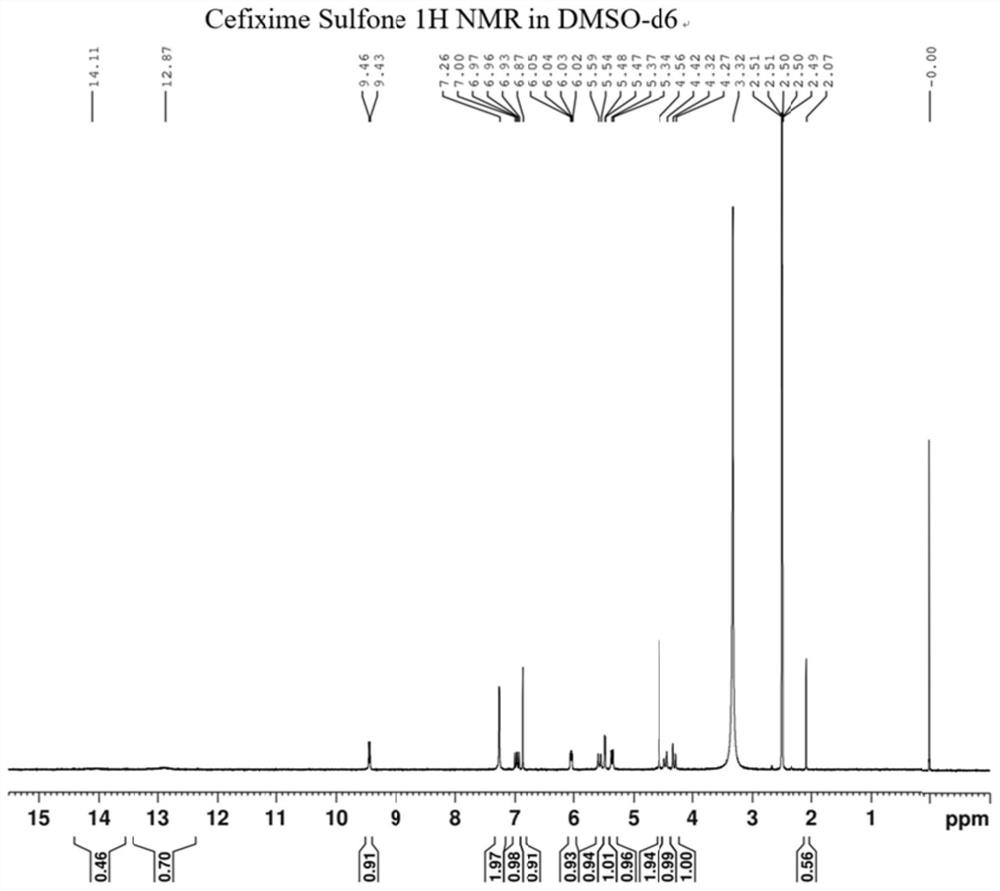
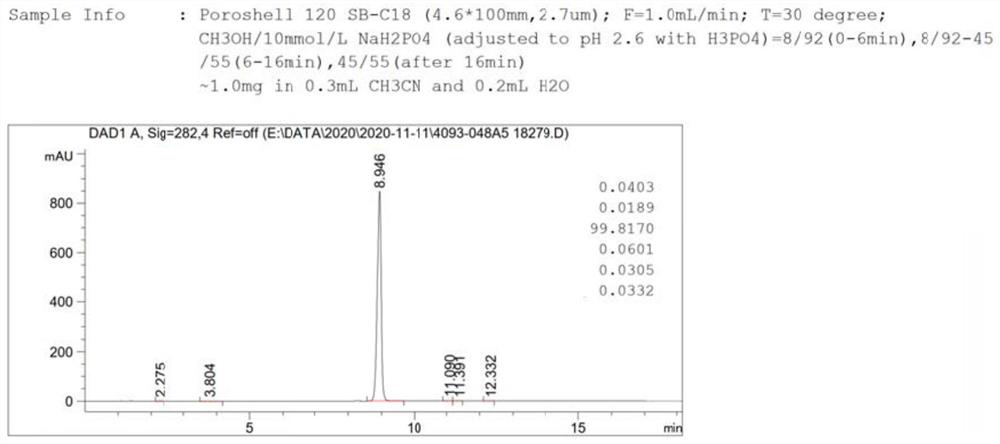
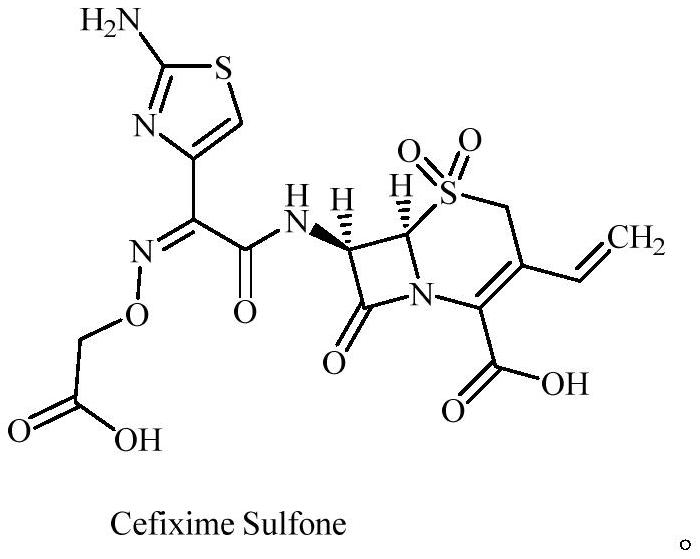
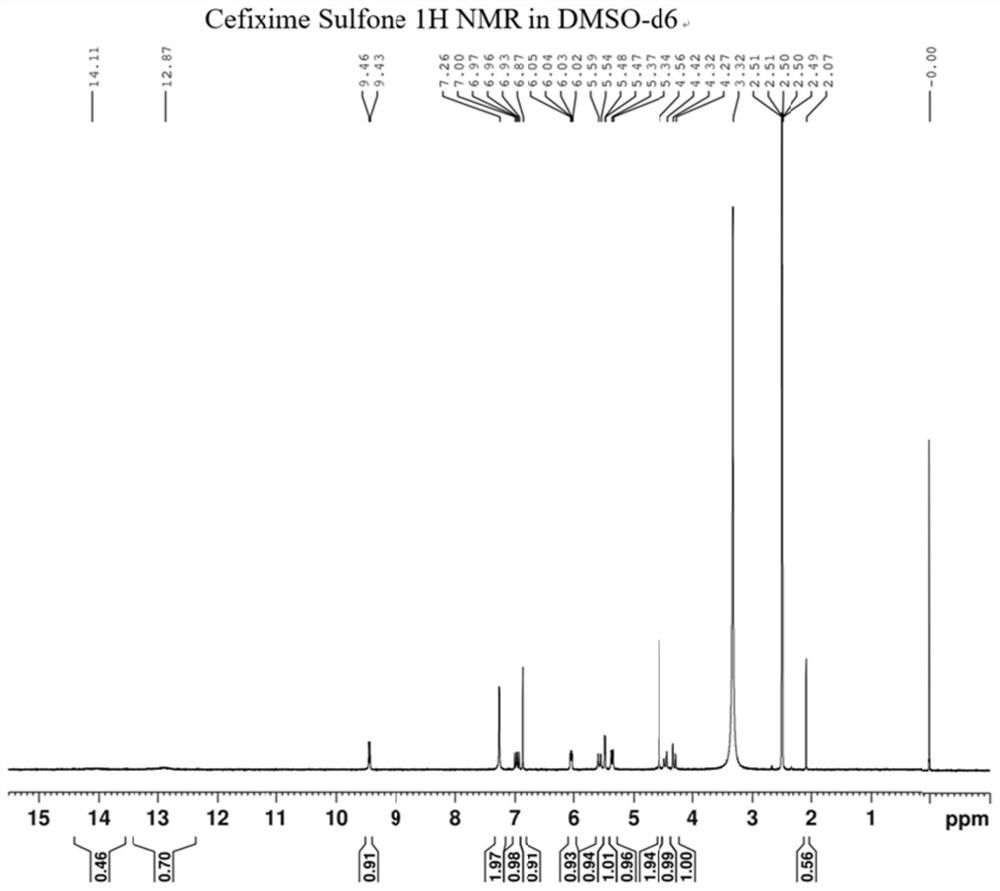
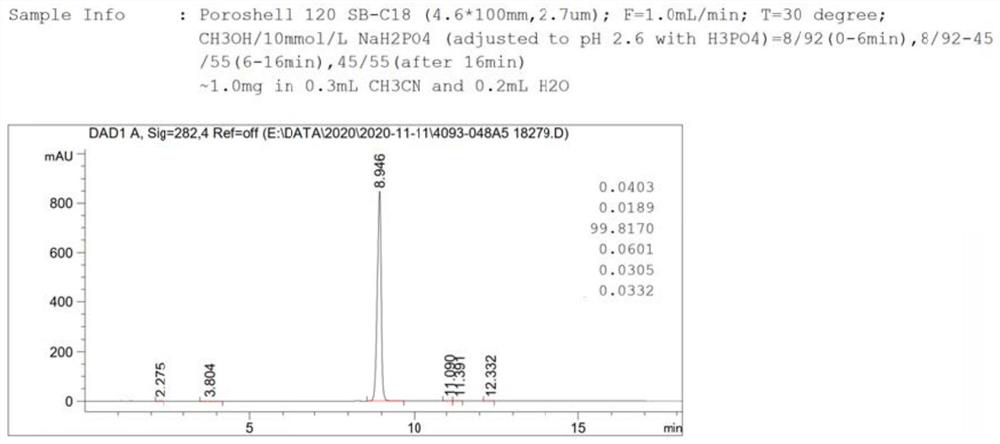
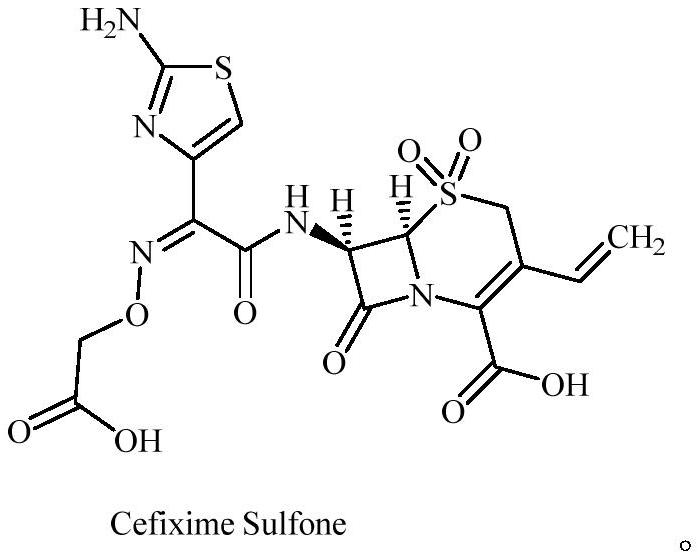
Cefixime impurity and preparation method thereof
ActiveCN113135939AReasonable route designRaw materials are easy to getOrganic chemistryReference sampleAcid catalysis
The invention discloses a cefixime impurity and a preparation method thereof, and belongs to the field of medicine synthesis. According to the method, cefixime serves as a starting raw material, CefiximeSulfone is obtained through acid catalysis esterification, oxidation and hydrolysis, the route design is reasonable, the raw materials are easy to obtain, operability is high, purification is convenient, aftertreatment is simple, the purity of the prepared target product can reach 99.5% or above, a reference sample is provided for cefixime research, a reference substance for analysis and research can be provided for clinical, pharmacological and pharmacokinetics of cefixime, and the method has important research value in clinical pharmacokinetics research.
Owner:TLC NANJING PHARMA RANDD CO LTD
Method for determining hydroxytyrosol in Beagle dog plasma
The invention relates to a method for determining hydroxytyrosol in Beagle dog plasma, and belongs to the field of biological detection. According to the method, the concentration of hydroxytyrosol ina Beagle dog plasma sample is detected by adopting high performance liquid chromatography-electrospray ionization tandem mass spectrometry after butylurea tosylate is adopted as an internal standardand acetonitrile is adopted for protein precipitation treatment. The accuracy, precision, specificity, stability, extraction recovery rate, matrix effect and the like of the method all meet the requirements of Chemical Drug Non-Clinical Pharmacokinetic Research Technical Guidance principles issued in 2015 edition of Chinese Pharmacopoeia and NMPA2014 on biological sample analysis.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Method for measuring aescin A, B, C and D in human plasma through utilization of LC-MSMS method and application thereof
PendingCN109975460AStable recoveryReduce processing timeComponent separationLower limitCentrifugation
The invention discloses a method for measuring aescin A, B, C and D in human plasma through utilization of an LC-MSMS method. The method comprises the following steps of carrying out acidizing; carrying out high speed centrifugation; carrying out drying through utilization of a termovap sample concentrator; and carrying out redissolving and swirling. According to the method, a sample is processedthrough precipitation of protein by methanol, and after nitrogen drying and redissolving are carried out, the sample is injected. Compared with solid phase extraction, the method has the advantages that a recovery rate is stable, processing time is greatly reduced, cost is clearly reduced, four compositions are measured, quantitation lower limits are clearly separated, the quantitation lower limits of aescin A, B, C and D are 20pg / mL, 20pg / mL, 40pg / mL and 40pg / mL, and an oral pharmacokinetic sample measurement requirement is satisfied. Plasma is added to acidizing stabilizer. ISR analysis qualification rates of the four compositions are more than 95%. The method is applied to aescin oral preparation clinical pharmacokinetic study. A pharmacokinetic behavior can be drawn completely.
Owner:武汉伯瑞恒医药科技有限公司
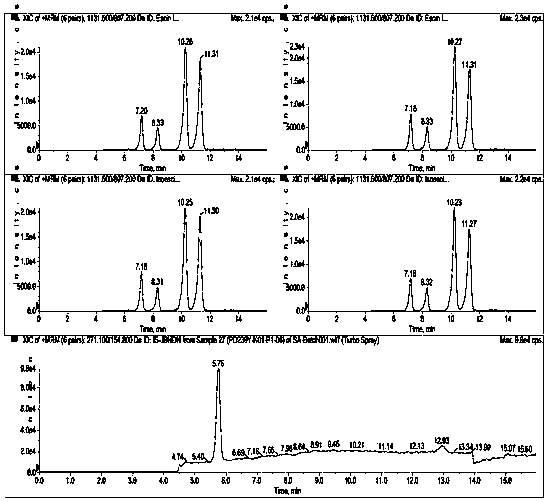
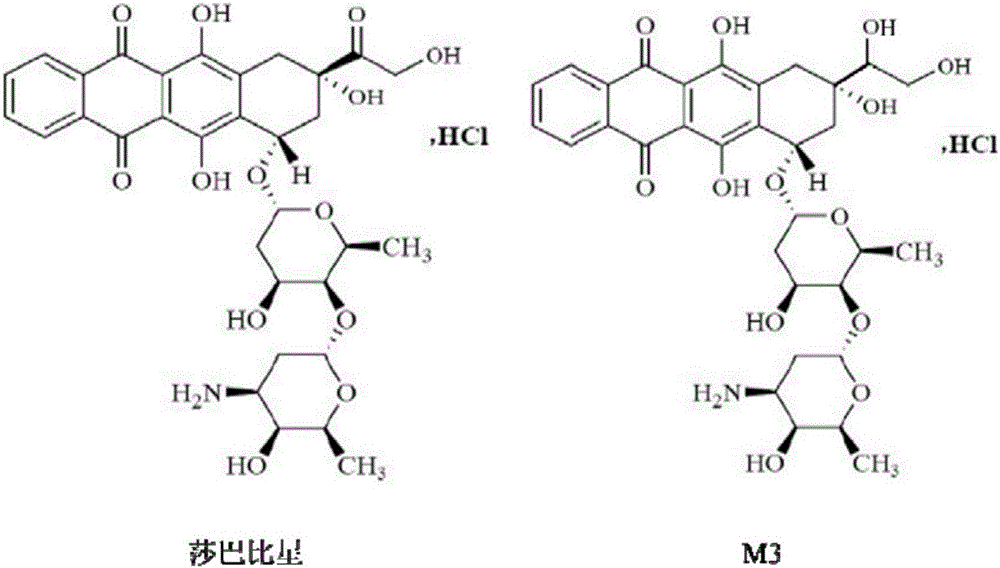
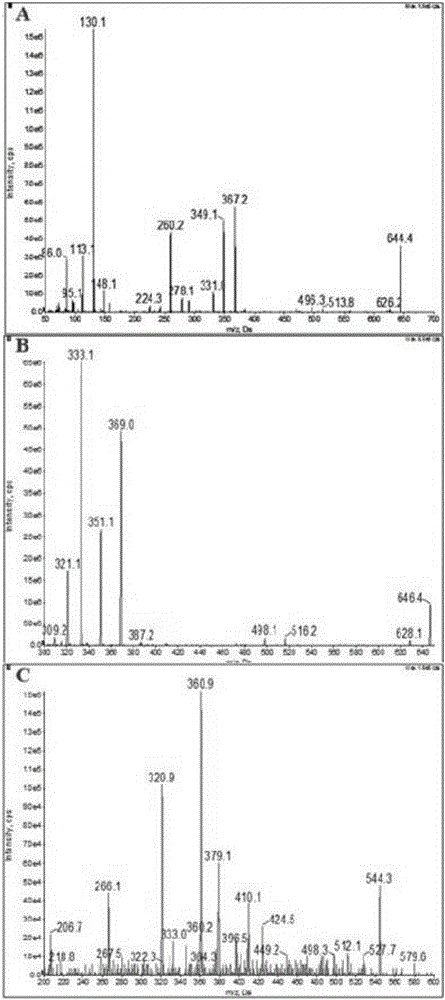
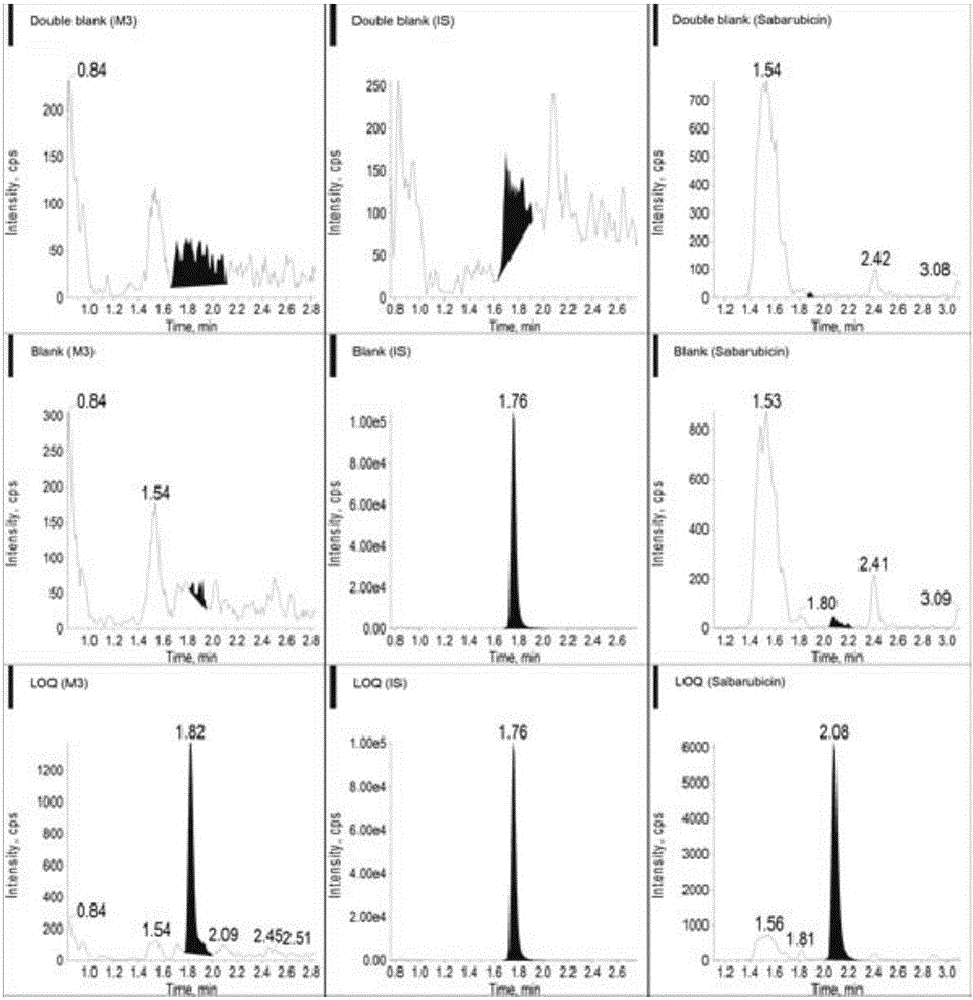
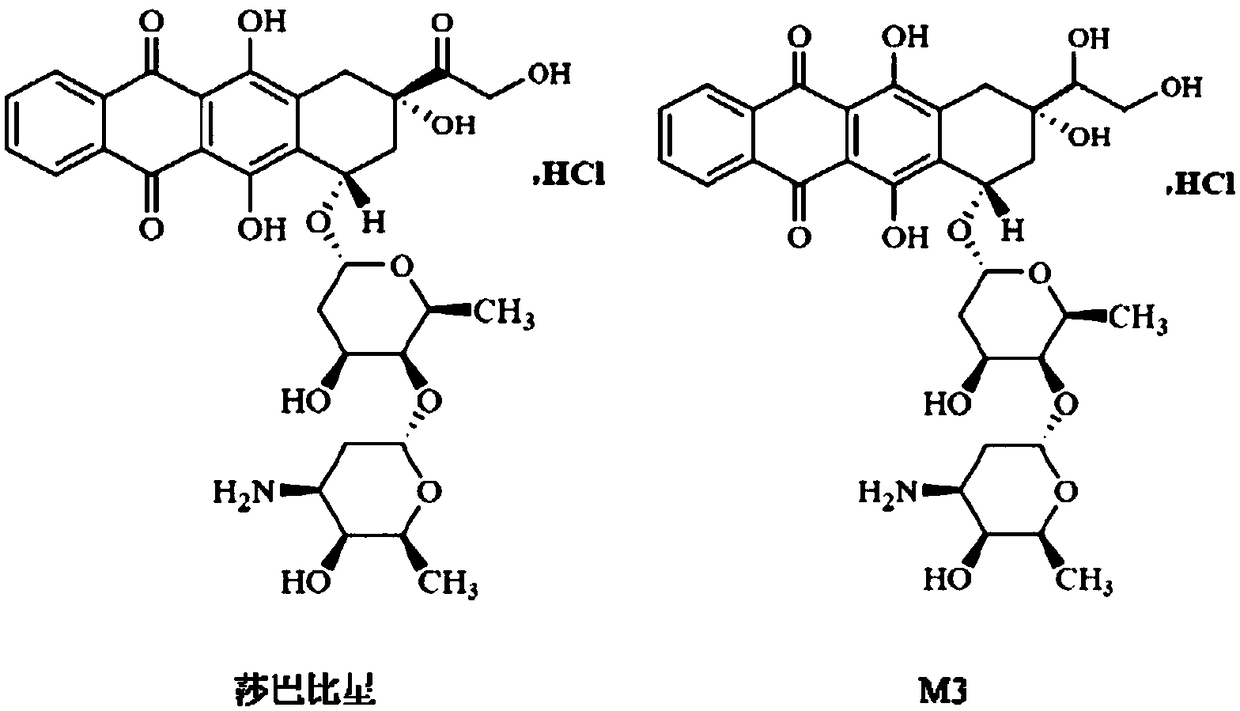
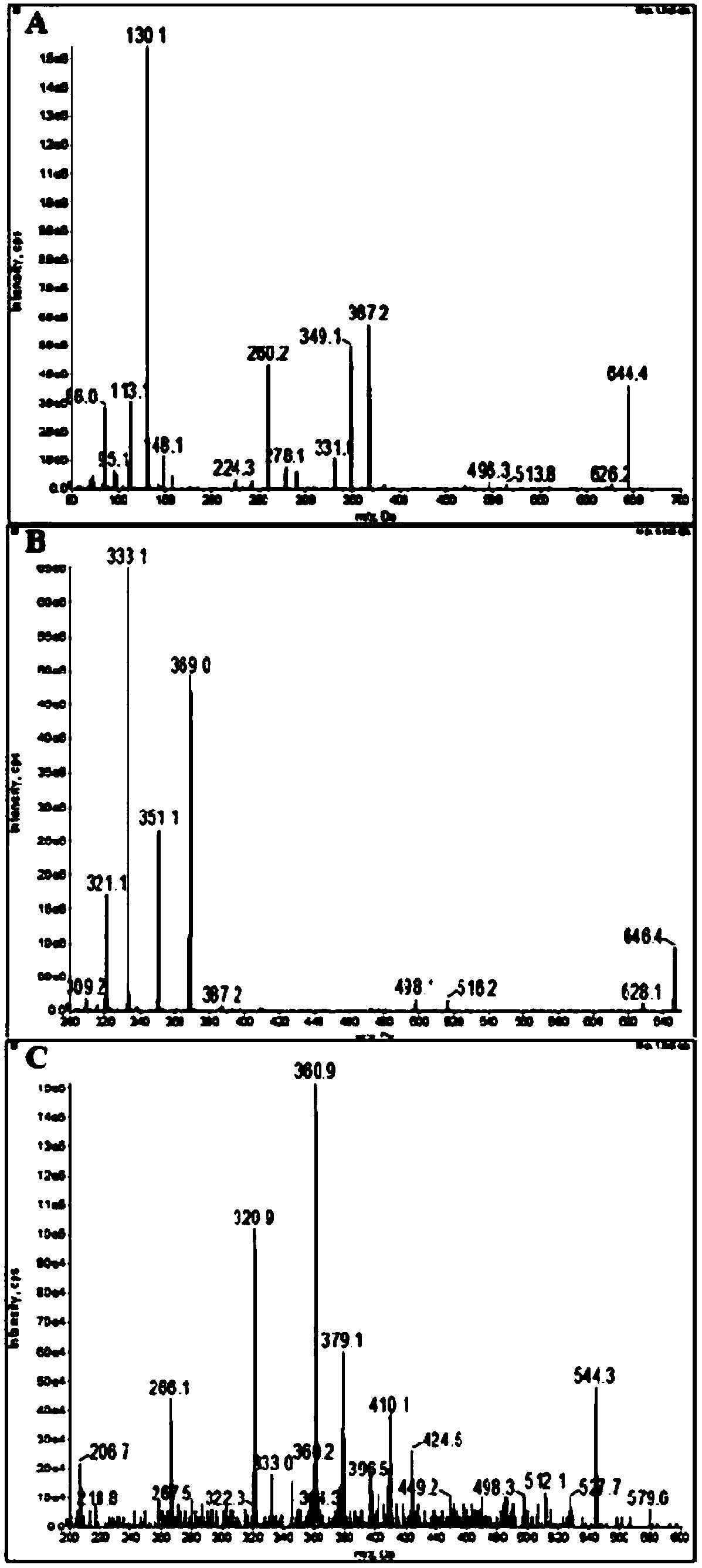
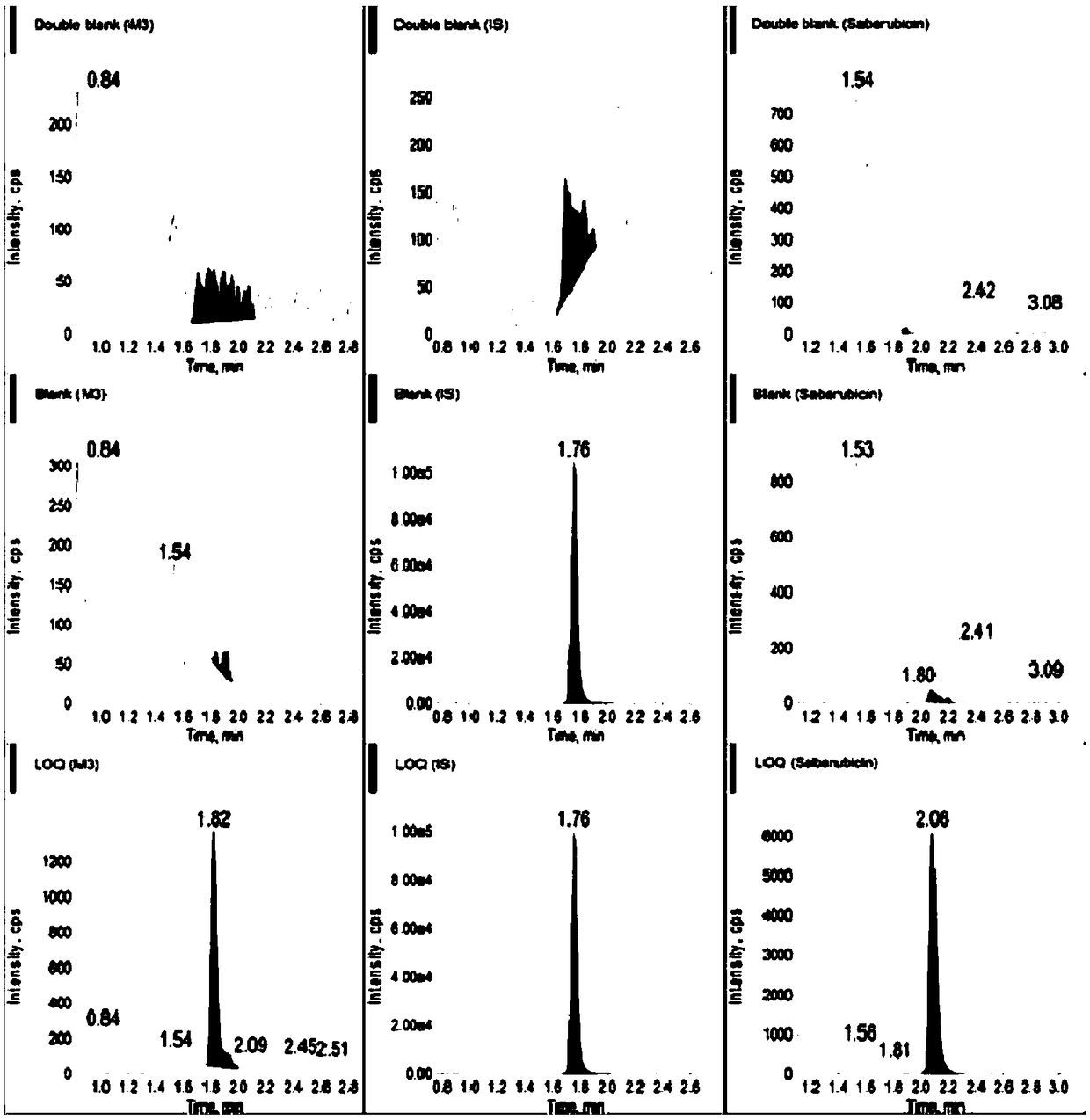
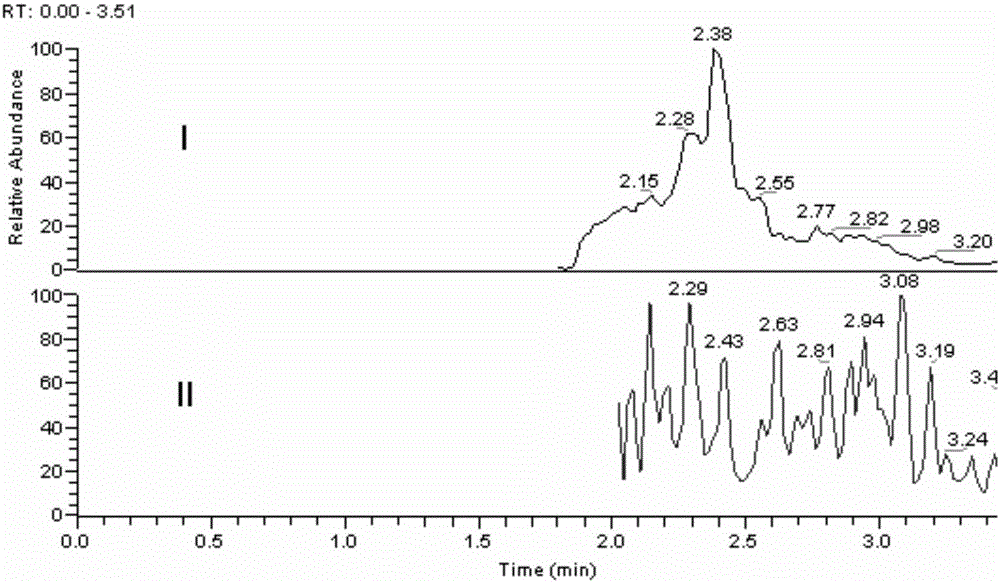
Ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method for determining plasma concentration of doxorubicin analogue and metabolite M3 in human plasma
The invention relates to an ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS / MS) method for determining plasma concentration of doxorubicin analogue and metabolite M3 in human plasma and provides a method for determining the concentration of doxorubicin analogue and C13 alcohol metabolite M3 in human plasma through UPLC-MS / MS. The method is carried out with formic acid water-acetonitrile as a mobile phase. The method for detecting the doxorubicin analogue and the metabolite M3 provided by the invention is high in sensitivity and good in repeatability, and can meet the requirements of quantitative analysis of clinical pharmacokinetic biological samples for treating malignant parenchymatous tumors by the doxorubicin analogue.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Sensitive and rapid method for analyzing concentration of sufentanil in plasma suitable for pharmacokinetic research
InactiveCN112782305AFast analysisIncrease throughputComponent separationBiotechnologyChromatographic separation
The invention relates to a sensitive and rapid method for analyzing the concentration of sufentanil in plasma suitable for pharmacokinetic research. The method comprises the following steps: 1, pre-treating a plasma sample; 2, performing chromatographic separation; and 3, performing mass spectrum detection. The detection method disclosed by the invention is high in sensitivity, simple in pretreatment operation, small in sample dosage, high in instrument analysis flux and suitable for clinical pharmacokinetic research of sufentanil patches.
Owner:苏州海科医药技术有限公司
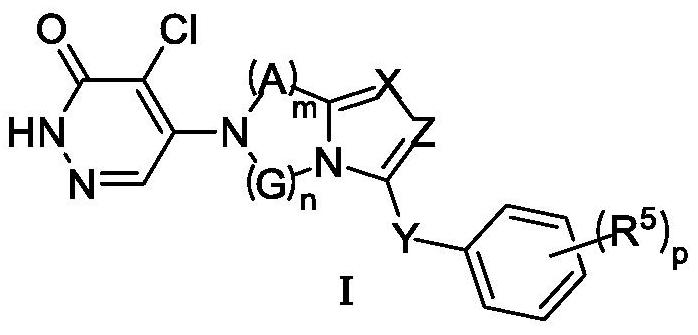
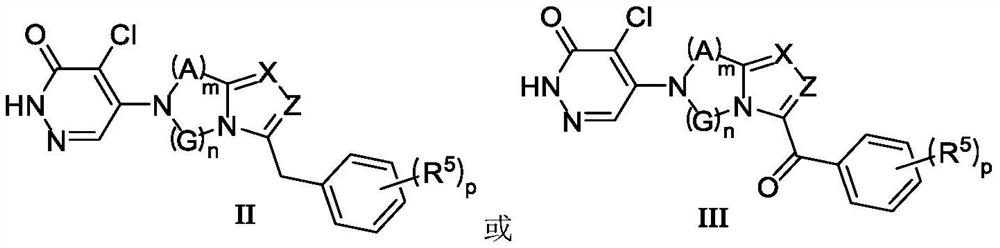
Heterocyclic compound and application thereof
ActiveCN113880843AStrong inhibitory activityGood metabolic stabilityOrganic active ingredientsNervous disorderMetabolic stabilityPharmaceutical medicine
The invention discloses a heterocyclic compound and application thereof. The invention specifically discloses a heterocyclic compound as shown in a formula I, and a tautomer or pharmaceutically acceptable salt thereof. The compound has better inhibitory activity on TRPC5, has better metabolic stability in liver microsome, and has better clinical pharmacokinetic properties.
Owner:WUHAN LL SCI & TECH DEV
A kind of cefixime impurity and preparation method thereof
ActiveCN113135939BReasonable route designRaw materials are easy to getOrganic chemistryReference sampleAcid catalysis
The invention discloses a cefixime impurity and a preparation method thereof, belonging to the field of pharmaceutical synthesis. The method of the present application uses cefixime as the starting material, and obtains CefiximeSulfone through acid-catalyzed esterification, oxidation, and then hydrolysis. The route design is reasonable, the raw materials are readily available, the operability is strong, the purification is convenient, the post-processing is simple, and the prepared target The purity of the product can reach more than 99.5%, which can provide a reference sample for the research of cefixime, and can provide the reference material for the analysis and research of the clinical, pharmacology and pharmacokinetics of cefixime, and has important research value in clinical pharmacokinetic research. .
Owner:TLC NANJING PHARMA RANDD CO LTD
A liquid chromatography-tandem mass spectrometry method for the detection of pitavastatin in human plasma
ActiveCN107144648BGood reproducibilityImprove accuracyComponent separationPretreatment methodEclipse
The invention relates to a liquid chromatogram-tandem mass spectrum method for detecting pitavastatin in human plasma, and application to clinical pharmacokinetic research. The invention provides a method for detecting pitavastatin concentration of plasma. By the method, the pitavastatin concentration of the plasma can be analyzed through LC-MS / MS. According to the method provided by the invention, a protein precipitate pretreatment method is preferably adopted, deuterated pitavastatin serves as internal standard, Eclipse Plus Phenyl-Hexyl column isocratic elution is adopted, and electrospray ionization (ESI) tandem mass spectrum detection is adopted. By adoption of the method provided by the invention, the extraction and recovery rate of the plasma sample is 93 percent or higher and is not influenced by matrix effect, the stability of the pitavastatin is inspected by counting the pitavastatin concentration RSD% before pretreatment, the accuracy of the measured data is guaranteed, the method is high in specificity and selectivity, high in sensitivity, rapid in detection and small in use amount, and simple, reliable, high-flux and condition-controllable clinical mass-batch sample analysis requirements are met. The specificity, the stability and the like of the method provided by the invention are verified, and the method can be used for evaluating the bioequivalence of various dosage forms of pitavastatin successfully.
Owner:苏州海科医药技术有限公司
Determination of plasma concentrations of sababicin and its metabolite m3 in human plasma by uplc-ms/ms
ActiveCN105806973BMeet the requirements of quantitative analysisHigh sensitivityComponent separationMetaboliteBlood plasma
The invention relates to an ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS / MS) method for determining plasma concentration of doxorubicin analogue and metabolite M3 in human plasma and provides a method for determining the concentration of doxorubicin analogue and C13 alcohol metabolite M3 in human plasma through UPLC-MS / MS. The method is carried out with formic acid water-acetonitrile as a mobile phase. The method for detecting the doxorubicin analogue and the metabolite M3 provided by the invention is high in sensitivity and good in repeatability, and can meet the requirements of quantitative analysis of clinical pharmacokinetic biological samples for treating malignant parenchymatous tumors by the doxorubicin analogue.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
A kind of determination method of strychnin biological sample
The invention relates to a method for determining loganin sapogenin in a biological sample and belongs to the technical field of medicine.The method includes that concentration of the loganin sapogenin in the biological sample is determined by ultra-performance liquid chromatography-electrospray ionization tandem mass spectrometry, wherein methyl alcohol in the biological sample serves a precipitation solvent; gradient elution is conducted, and a flow phase under a liquid phase condition consists of water with 0.1% formic acid and the methyl alcohol; cycloastragenol acts as an internal standard for determining the loganin sapogenin in the biological sample.The method for determining the loganin sapogenin in the biological sample has the advantage that the LC-MS-MS determining method satisfies the analysis requirements of Chinese Pharmacopoeia (2015 edition) and Technical Guidelines for Non-clinical Pharmacokinetic Studies on Chemical Drugs promulgated by SFDA (State Food and Drug Administration) in 2014 on biological samples in terms of accuracy, precision, specificity, stability, recovery rate, matrix effects and the like.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of preparation method of Apremilast impurity
ActiveCN113173878BReasonable process designSimple post-processingOrganic chemistryTest samplePhysical chemistry
The invention discloses a preparation method of apremilast impurity, which belongs to the field of medicine synthesis. The application screens out the optimal preparation steps and reaction conditions through experiments, and obtains the apremilast impurity through four-step reaction synthesis. The process design is reasonable, the operability is strong, and the purification is convenient. The prepared apremilast impurity has a purity of more than 98%, provides test samples for the research of apremilast, and has important research value in clinical pharmacokinetic research.
Owner:TLC NANJING PHARMA RANDD CO LTD
Detection method of ceftiofur and application thereof
The invention provides a method for detecting ceftiofur in plasma. The method for detecting the ceftiofur in the blood plasma comprises the following steps: extracting a ceftiofur metabolite, namely furanformyl ceftiofur (DFC), from a drug-containing blood plasma sample by virtue of an extracting solution-dithioerythritol (DTE) solution in a water bath, without derivatization of an iodoacetamide solution and solid-phase extraction column treatment, centrifuging the extracting solution to obtain a supernatant, and purifying the supernatant by virtue of a chromatographic column, so as to obtain the ceftiofur in the blood plasma. And filtering by using a filter membrane, and directly detecting by using a high performance liquid chromatograph. The method has the advantages that the sample pretreatment steps are simplified, the cost is reduced, the time is saved, the reagent usage amount is small, the toxicity is low, and the method is suitable for rapid detection of ceftiofur and metabolites thereof in a large batch of plasma samples in clinical pharmacokinetic tests.
Owner:TIANJIN RINGPU BIO TECH +1
UPLC-MS/MS (Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry) method for rapidly detecting fruquintinib in rat plasma
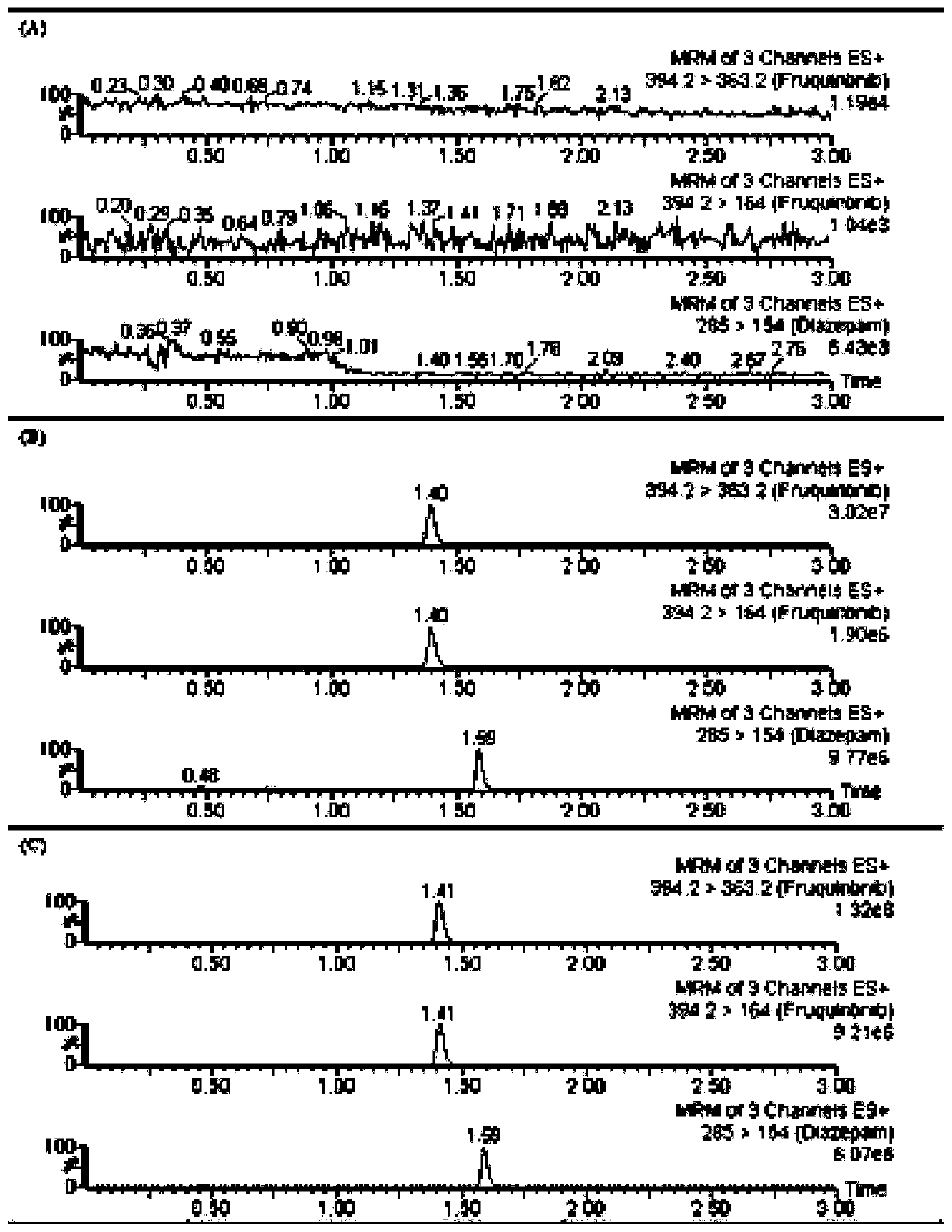
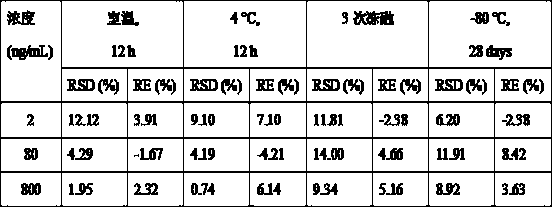
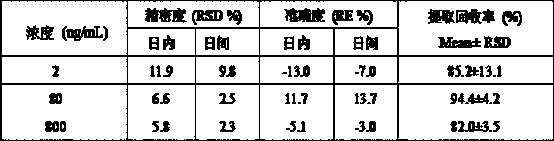
The invention discloses a UPLC-MS / MS (Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry) method for rapidly detecting fruquintinib in rat plasma, and belongs to the technical fieldof pharmacokinetics. The method comprises the following steps: 1) collecting a specimen; 2) treating a plasma sample; 3) performing mass spectrometric detection; and 4) reading and recording data. Through adoption of the UPLC-MS / MS method for rapidly detecting fruquintinib in rat plasma, the concentration of the fruquintinib in the rat plasma can be detected rapidly, easily and accurately. Through the method, the pharmacokinetics of the fruquintinib in the rat plasma can be investigated; the blood concentration and clinical pharmacokinetics of the fruquintinib in human plasma can be monitored; and the preclinical application and post-clinical application of the fruquintinib are facilitated.
Owner:THE FIRST AFFILIATED HOSPITAL OF WENZHOU MEDICAL UNIV
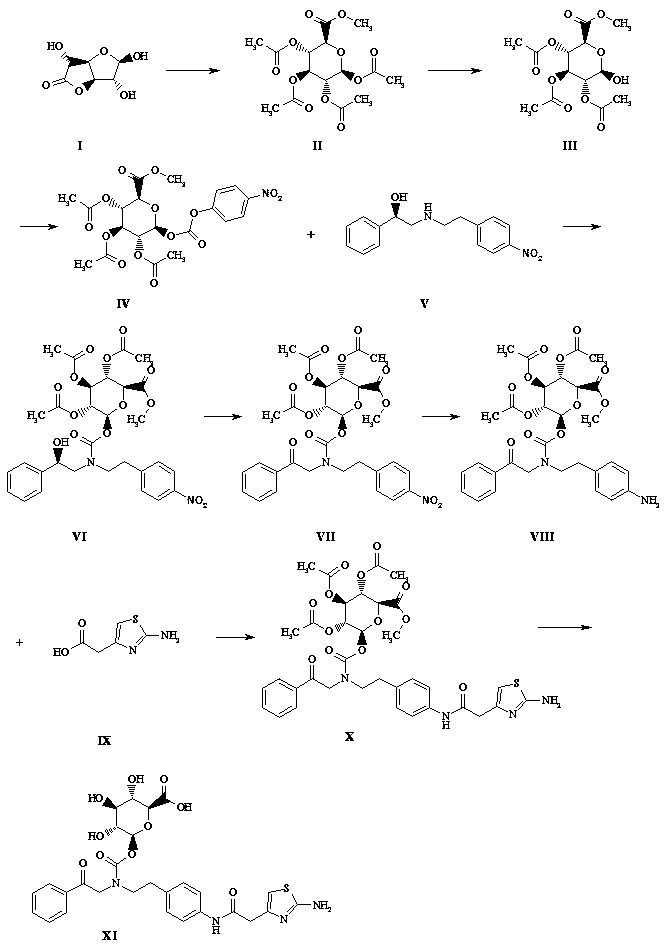
Mirabegron metabolite synthesis method
InactiveCN109912665ARealize industrial productionReasonable process designEsterified saccharide compoundsSugar derivativesMetaboliteSynthesis methods
The invention discloses a mirabegron metabolite synthesis method and belongs to the field of drug metabolism. The mirabegron metabolite synthesis method can prepare a mirabegron metabolite from glucurolactone (1R)-2-2[2-(4-nitrophenyl)ethylamino]-1-phenylethanol as starting materials through eight-step reaction. The mirabegron metabolite synthesis method has the advantages that the synthesis method is reasonable in process design and strong in operability on the basis of optimal preparation steps and reaction conditions screened by a great number of experiments; the synthesis method is high inyield and can reach high chemical purity above 99% and therefore can be applied to industrial production of mirabegron metabolite; the mirabegron metabolite can provide a standard substance for metabolic mechanism research of the mirabegron drug, can be used for exploring the metabolic process of the drugs in vivo, and has high application research value in clinical pharmacokinetic study.
Owner:TLC NANJING PHARMA RANDD CO LTD
Preparation method of perindopril impurity K
PendingCN114394969AReasonable process designEasy to operateOrganic chemistryTest samplePhysical chemistry
The invention relates to a preparation method of a perindopril impurity K. The invention finds that an intramolecular ring-closed compound IV can be generated when a compound III is subjected to hydrogenation debenzylation, and the ratio of converting the compound III into the compound IV is relatively high under the condition of relatively high temperature, so that the compound IV can be directly prepared from the compound III by utilizing the phenomenon; the method is short in route, simple in process and easy to operate, and the prepared perindopril impurity K can provide a test sample for perindopril research and has important research value in drug process impurity research and clinical pharmacokinetic research.
Owner:SHENZHEN SUNGENING BIO-MEDICAL CO LTD
A preparation method of stable isotope-labeled diphenylsulfonamide drugs
The invention discloses a preparation method of sparsenatan for stabilizing isotope labeling. According to the method, 3-methyl-4-bromobenzoic acid is used as a raw material, iodoethane for deuterium labeling serves as a deuterium labeling initiator, and the sparsenatan is obtained through six-step reaction. Optimal preparation steps and reaction conditions are screened out through a large number of experiments, and the whole preparation method is reasonable in design and good in operability. The purity of the sparsenatan prepared by means of the method and used for stabilizing the isotope labeling can be higher than 99%, and the isotope abundance is higher than 99%. The sparsenatan prepared by means of the method and used for stabilizing the isotope labeling is a standard product for research on the metabolic mechanism of the sparsenatan, can be used for tracing the metabolic process of the sparsenatan in a living body and has great application and research value on clinic pharmacokinetic study.
Owner:TLC NANJING PHARMA RANDD CO LTD
Synthesis method of nitrendipine metabolite
InactiveCN108314643AReasonable process designEasy to operateOrganic chemistryCardiovascular disorderMetaboliteSynthesis methods
The invention discloses a synthesis method of nitrendipine metabolite. The method takes 4-(benzyloxy)-3-ethyl oxo-butyrate and 3-hydroxypropionitrile as starting raw materials to synthesize the nitrendipine metabolite through 6-step reaction. A lot of experiments are carried out to screen optimal preparation steps and reaction conditions; a whole technology is reasonably designed and strong in operability; the chemical purity of the nitrendipine metabolite prepared by the synthesis method can reach 99 percent or more and the yield is high. The nitrendipine metabolite prepared by the synthesismethod provides a standard product for metabolic mechanism researches of a nitrendipine medicine and can be used for exploring a metabolic process of the medicine in organisms; the nitrendipine metabolite has a relatively good potential hypertension-resisting effect and has extremely application and research value in clinical pharmacokinetics researches.
Owner:TLC NANJING PHARMA RANDD CO LTD
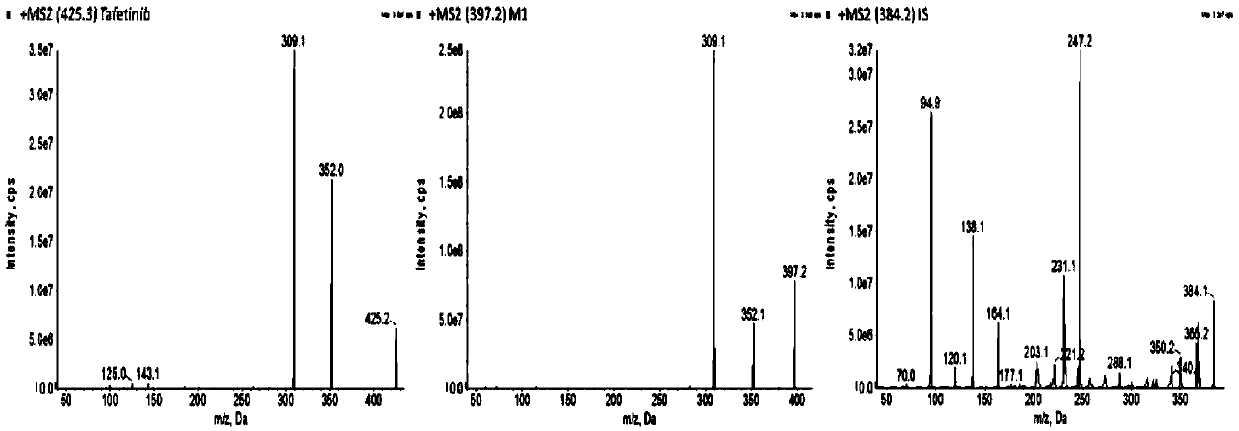
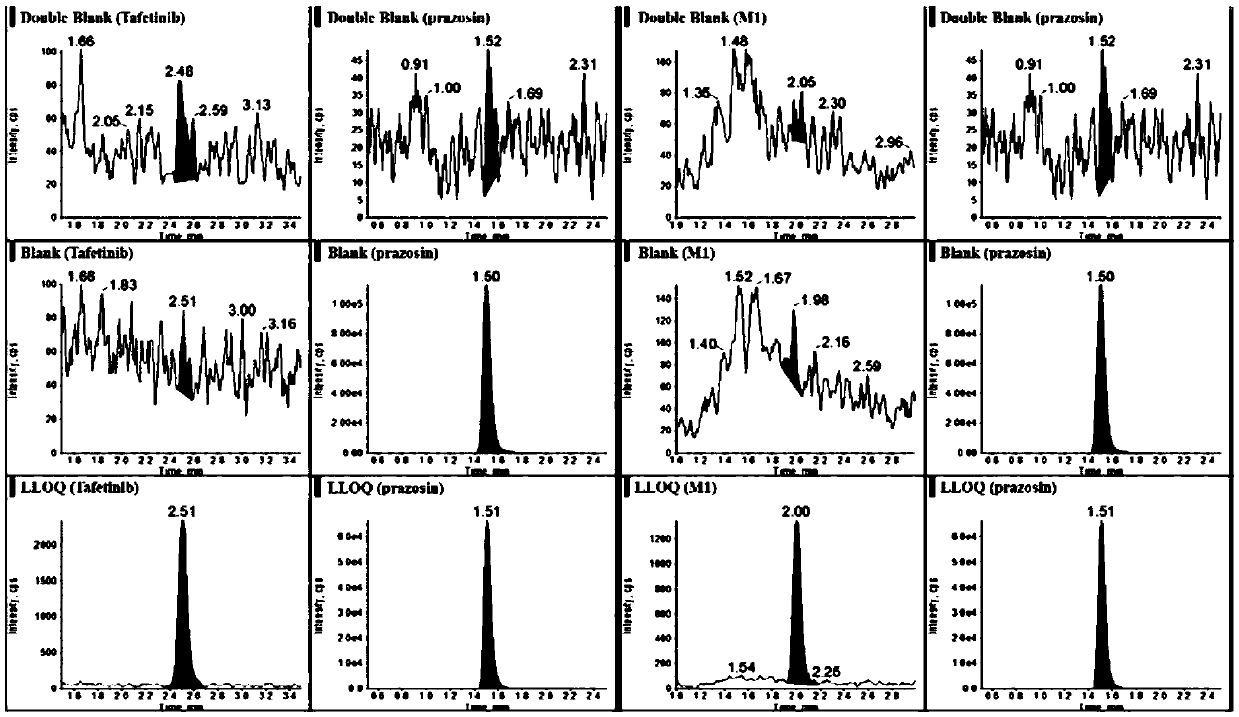
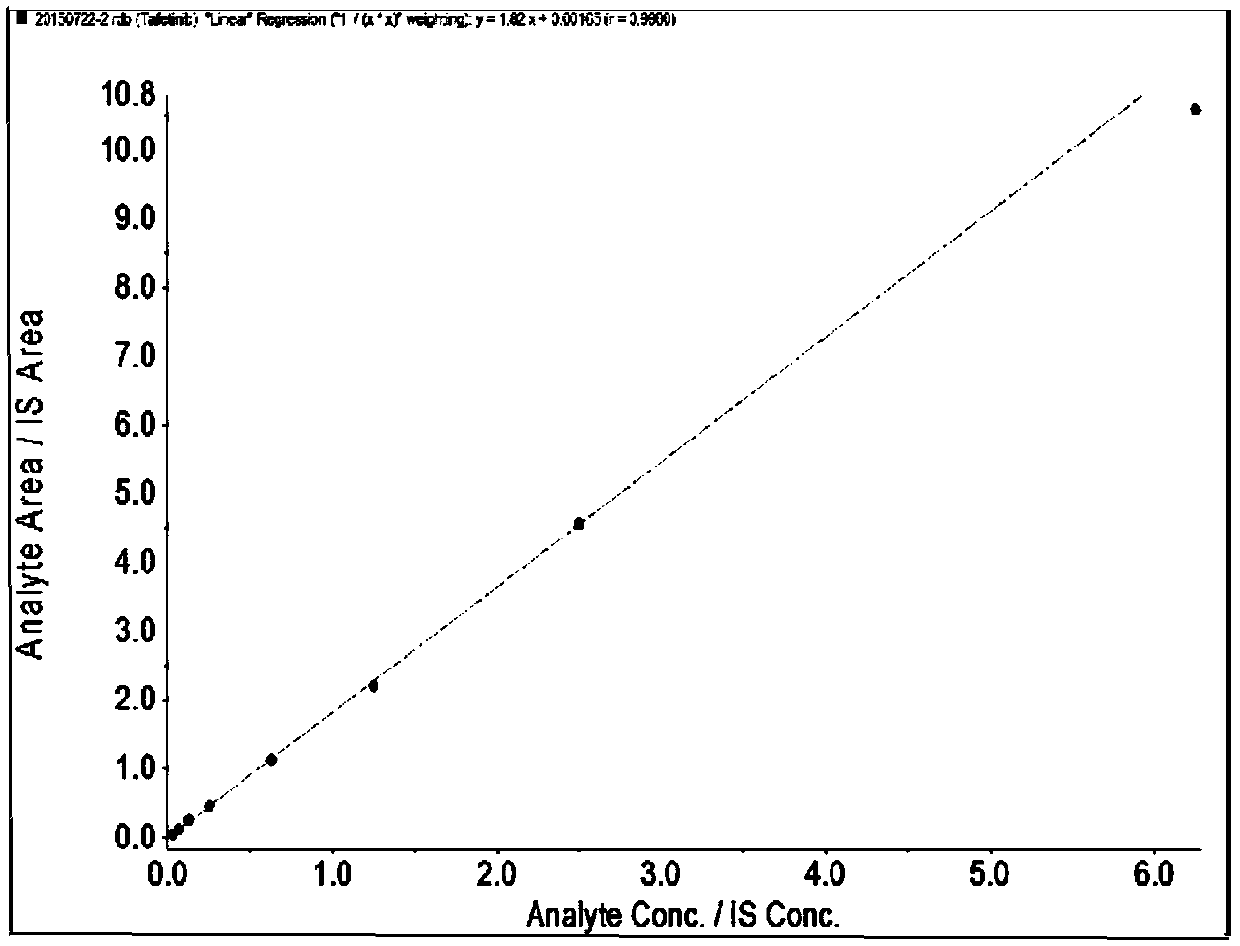
UPLC-MS/MS method for detecting concentrations of tafetinib and active metabolite SCR868 in human plasma
The invention relates to a UPLC-MS / MS method for detecting the concentrations of tafetinib and active metabolite SCR868 in human plasma. The invention provides the method for detecting the concentrations of the tafetinib and the active metabolite SCR868 in the human plasma through ultra-high performance liquid chromatography tandem mass spectrometry (UPLC-MS / MS), wherein the method is implemented by adopting ammonium formate water-acetonitrile containing formic acid as a mobile phase. The method provided by the invention is good in specificity and high in sensitivity, the linear relation between the tafetinib and the SCR868 is good within the range of 0.2 to 50ng / mL of plasma, and the method is successfully applied to the detection of clinical pharmacokinetic samples.
Owner:南京力博维制药有限公司
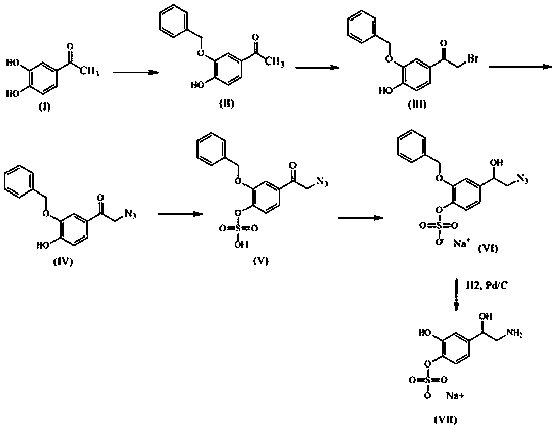
Method for synthesizing dl-norepinephrine 4-sulfate
InactiveCN108299252AReasonable process designEasy to operateOrganic compound preparationSulfuric acid esters preparationSulfateDrugs synthesis
The invention discloses a method for synthesizing dl-norepinephrine 4-sulfate, belonging to the field of drug synthesis. In the method, the dl-norepinephrine 4-sulfate is obtained through six-step reactive synthesis by taking 3,4-dihydroxyacetophenone as a starting material. According to the method, optimal preparation steps and reaction conditions are selected through a large number of experiments, the whole process design is reasonable, and the operability is strong. The chemical purity of the prepared product can reach 98.5% or above, and the overall yield is relatively high. The prepared dl-norepinephrine 4-sulfate provides a standard for the study of the metabolic mechanism of adrenaline and norepinephrine medicines, and can be used to explore the metabolic process of the medicines invivo. Dl-norepinephrine 4-sulfate has great application research value in clinical pharmacokinetic research.
Owner:TLC NANJING PHARMA RANDD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com