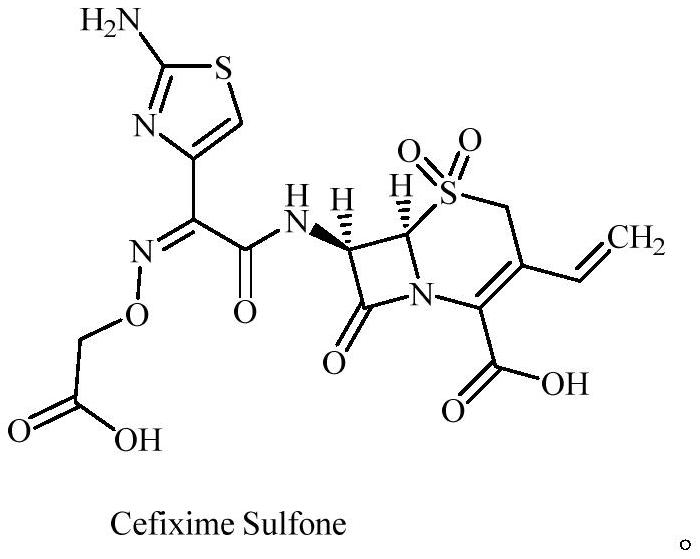
Cefixime impurity and preparation method thereof
A technology for cefixime and impurities, applied in the field of cefixime impurities and its preparation, to achieve the effects of reasonable route design, convenient purification, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of Compound A: Suspend 30g of cefixime in 300mL of tert-butyl propionate, add 16.4mL of boron trifluoride diethyl ether in an ice bath, and react at 40°C for 8 hours. After the reaction is complete, the reaction solution is added to water, extracted three times with ethyl acetate, Dried over anhydrous sodium sulfate, filtered, and spin-dried, the crude product was purified by column to obtain 27.8 g of white solid A with a yield of 74.3%.
[0033]
[0034] Preparation of Compound B: Dissolve 15.2g of Compound A in 304mL of acetonitrile, then add 18.3g of 25% hydrogen peroxide and 0.1g of sodium tungstate, react at 10°C for 12 hours, monitor the end of the reaction by TLC, dilute with 600mL of ethyl acetate, wash three times with water, Dried over anhydrous sodium sulfate, filtered, spin-dried, and purified by column to obtain 10.2 g of white solid B with a yield of 63.5%.
[0035]
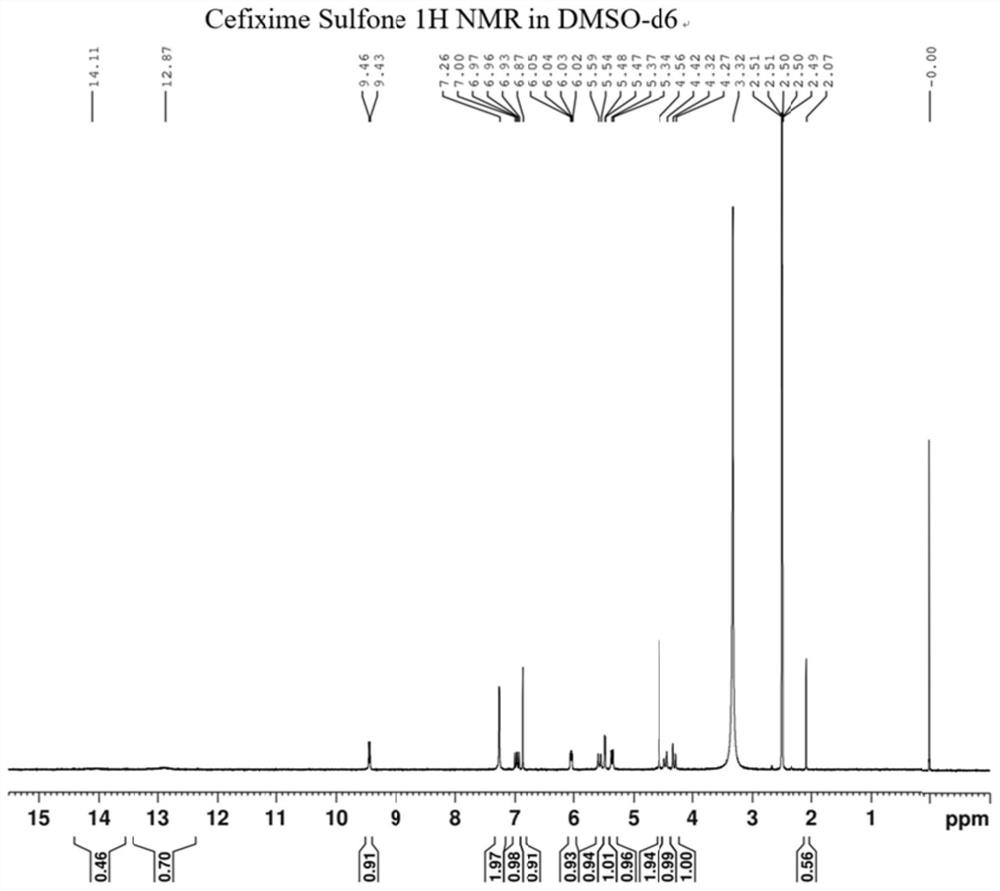
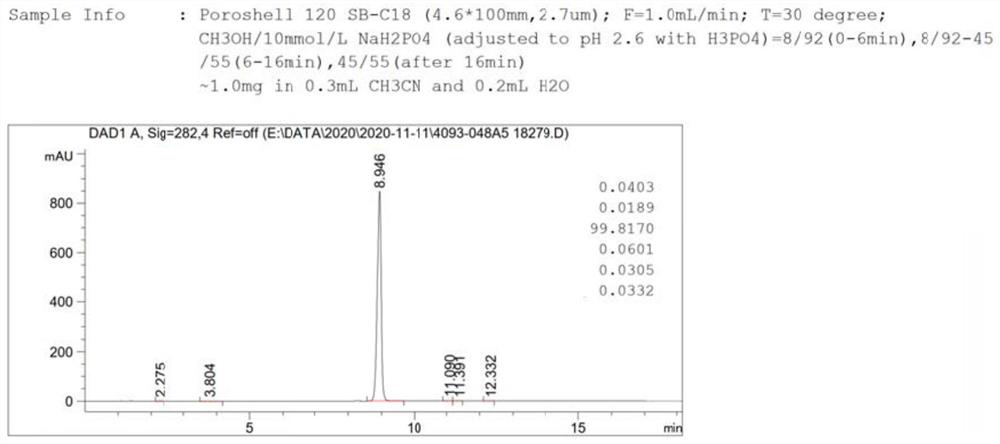
[0036] Preparation of compound Cefixime Sulfone: Dissolve 10g of compo...
Embodiment 2
[0039] The preparation of compound A: 30g cefixime was suspended in 600mL tert-butyl propionate, 19.8mL concentrated sulfuric acid was slowly added dropwise in an ice bath, and reacted for 6 hours at 25 degrees Celsius. After the reaction was completed, the reaction solution was added to water, extracted three times with ethyl acetate, and no Dried over sodium sulfate, filtered, and spin-dried, the crude product was purified by column to obtain 29.5 g of white solid A with a yield of 78.8%.
[0040]
[0041] The preparation of compound B: get 15.5g compound A and dissolve in 310mL acetonitrile, then add 16.7g peroxyacetic acid, react 8 hours at 30 degrees centigrade, TLC monitoring reaction finishes, add 100mL saturated sodium thiosulfate aqueous solution, quench excessive oxidant, The organic phase was washed three times with water, dried over anhydrous sodium sulfate, filtered, spin-dried, and purified by column to obtain 8.3 g of white solid B with a yield of 50.7%.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com