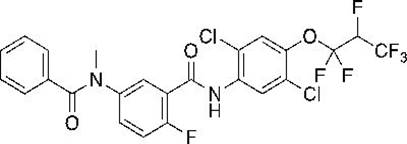
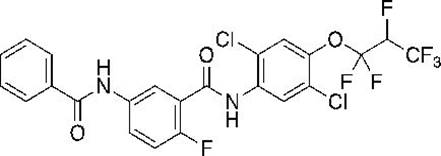
Synthesis method of aromatic amine carboxylic acid derivative
A technique for the synthesis of aromatic amine carboxylic acid and its synthesis method, which is applied in the field of synthesis of aromatic amine carboxylic acid derivatives, can solve problems such as insufficient variety, and achieve the effects of reasonable route design, high yield, and excellent pest control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
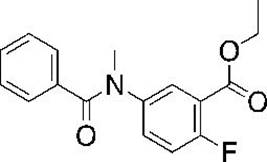
[0032] This example uses X 1 =F,R 2 =Phenyl is taken as an example for description, but the present invention is not limited to this embodiment.
[0033] Synthesis of ethyl 5-benzamido-2-fluoro-benzoate:
[0034]
[0035] Dissolve 5-amino-2-fluoro-benzoic acid ethyl ester (400 mg, 2.184 mmol, 1 equiv) in dichloromethane, and add benzoyl chloride (306.95 mg, 2.184 mmol, 1 equiv) successively at 0°C , pyridine (345.5 mg, 4.368 mmol, 2 equiv), after the addition was completed, the reaction was stirred at room temperature for 12 h, and TLC (PE / EA=3:1, Rf=0.5) was detected. After the reaction was completed, 20ml of 1N hydrochloric acid aqueous solution was added to wash the organic phase , extracted with ethyl acetate, combined the organic phases, and washed with water again, the organic phase was dried over anhydrous sodium sulfate, the solvent was distilled off under negative pressure, and the crude product was purified by silica gel column (PE / EA=3:1) to obtain 5-benzyl Am...
Embodiment 2
[0039] This example uses X 1 =F,R 2 = phenyl, R 1 =Me as an example for description, but the present invention is not limited to this embodiment.
[0040] Synthesis of ethyl 2-fluoro-5-(N-methylbenzamido)benzoate:
[0041]
[0042] Dissolve ethyl 5-benzamido-2-fluoro-benzoate (1 g, 3.481 mmol, 1 equiv) in tetrahydrofuran (10 ml), under the protection of nitrogen, cool the liquid nitrogen to -78 °C, and start adding LiHMDS dropwise (0.87 g, 5.221 mmol, 1.5 equiv). After the dropwise addition, methyl iodide (0.74 g, 5.221 mmol, 1.5 equiv) was added dropwise for 10 min. After the addition was completed, the reaction was stirred at room temperature for 1 h, TLC (PE / EA =4:1, Rf=0.5) After the completion of the detection reaction, add 2ml of acetic acid at 0°C to quench the reaction, extract with ethyl acetate, combine the organic phases, wash with water again, dry the organic phases over anhydrous sodium sulfate, and steam under negative pressure The solvent was removed, and...
Embodiment 3
[0046] This example uses X 1 =F,R 2 = phenyl, R 1 =Me as an example for description, but the present invention is not limited to this embodiment.
[0047] Synthesis of 2-fluoro-5-(N-methylbenzamido)benzoic acid
[0048]
[0049] Ethyl 2-fluoro-5-(N-methylbenzamido)benzoate (750 mg, 2.489 mmol, 1 equiv), sodium hydroxide (199.11 mg, 4.978 mmol, 2 equiv) was added to water (10ml ), at 60°C, stirred for 2 hours, TLC (PE / EA=1:1, Rf=0.4) detected the reaction, cooled down to room temperature, added 1N hydrochloric acid aqueous solution to adjust to PH=2, extracted with ethyl acetate, The organic phase was washed with water, dried over anhydrous sodium sulfate, and the solvent was distilled off under negative pressure to obtain 2-fluoro-5-(N-methylbenzamido)benzoic acid (620 mg, 91%) as a yellow oily liquid.
[0050] 1 H NMR (400 MHz, Chloroform- d ) δ 7.79 (m, 1H), 7.34-7.27 (m, 3H), 7.25-7.16 (m, 3H), 7.01 (m, 1H), 3.51 (s, 3H).
[0051] LCMS: (ESI, m / z): [M+1] + = 274....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com