Mirabegron metabolite synthesis method
A synthetic method and metabolite technology, applied in the field of drug metabolism, can solve the problems of Mirabegron’s short time to market and achieve the effects of large application research value, reasonable process design, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
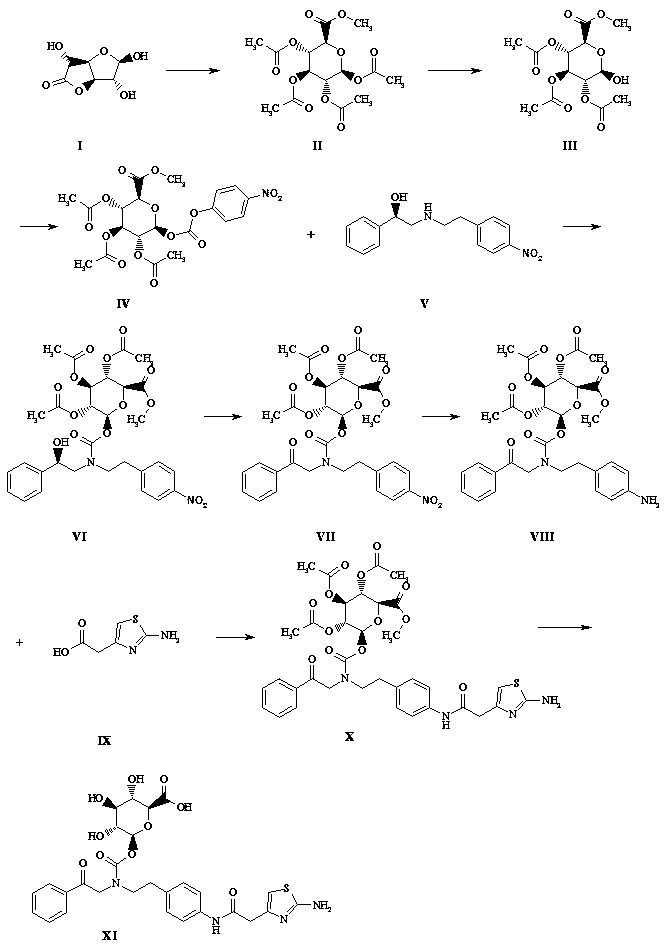
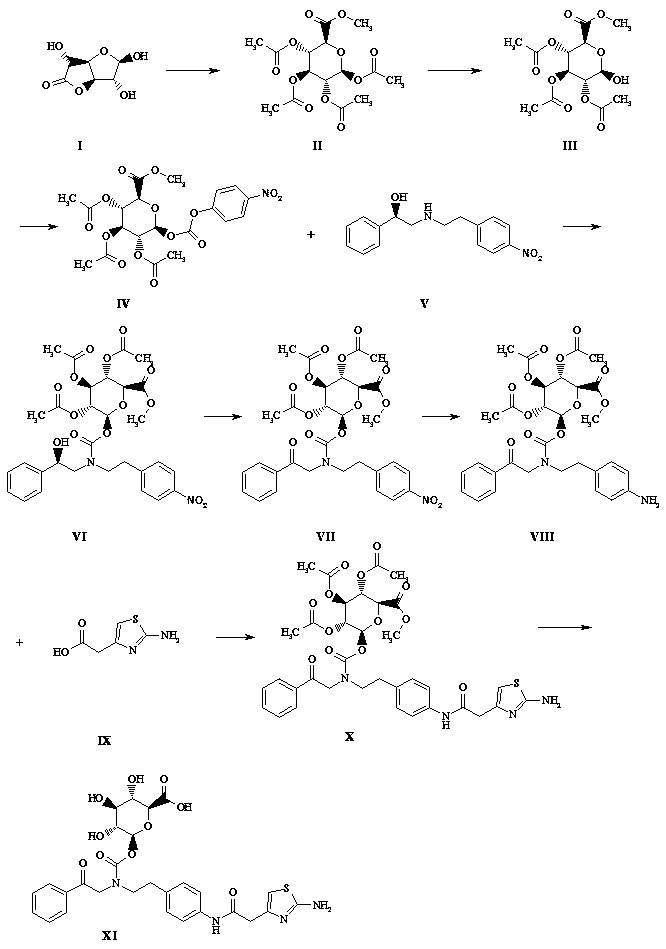
[0027] Such as figure 1 Shown, a kind of synthetic method of mirabegron metabolite comprises the following steps:
[0028] (1) Take a 1-liter flask and add 750 ml of methanol, add 5 g of solid sodium hydroxide, add 100 g of glucuronolactone in batches while stirring at 0°C, react at 28°C for 2 hours, TLC (sampling, DCM dilution spot plate) , the raw material reaction is complete, the reaction solution is concentrated to dryness by rotary evaporation at 20°C, and the foam is pulled dry with an oil pump, and 250 ml of triethylamine is added to stand for 10 minutes, and 380 ml of acetic anhydride is slowly added in an ice bath, and it is left to stand for 15 minutes after the addition is completed. The reaction solution turned black, stirred and reacted overnight in an ice bath, a large amount of solids precipitated in the reaction mixture the next day, filtered with suction, rinsed with n-hexane: DCM volume ratio 4:1, rinsed with 100 ml each time for 3 times, and transferred the...
Embodiment 2
[0040] Such as figure 1 Shown, a kind of synthetic method of mirabegron metabolite comprises the following steps:
[0041] (1) Take a 1-liter flask, add 600 ml of methanol, add 3 g of solid sodium hydroxide, stir at 0°C in batches (after dissolving, add 100 g of glucuronolactone, react at 28°C for 3 hours, TLC (sampling, DCM dilution point plate), the raw materials reacted completely, the reaction solution was evaporated and concentrated to dryness at 20 ° C, and the oil pump was used to dry the foam, and 250 ml of triethylamine was added to stand for 10 minutes, and 380 ml of acetic anhydride was slowly added in an ice bath. Let it stand for 15 minutes after the addition, the reaction solution turns black, stir the reaction overnight in an ice bath, a large amount of solids precipitate out of the reaction mixture the next day, filter with suction, rinse with n-hexane:DCM volume ratio 4:1 (100 ml x 3), and transfer the solids Diethyl ether was dissolved in DCM to recrystalliz...
Embodiment 3
[0053] Such as figure 1 Shown, a kind of synthetic method of mirabegron metabolite comprises the following steps:
[0054](1) Take a 1-liter flask and add 750ml of methanol (newly opened), add 5g of lithium hydroxide solid, stir at 0°C in batches (after dissolving, add another batch), add 100g of glucuronolactone, and react at 28°C 2 hours, TLC (sampling, DCM dilution spot plate), the reaction of the raw materials is complete, the reaction solution is concentrated to dryness by rotary evaporation at 20 ° C, and then dried with an oil pump to foam, add 250 ml of triethylamine and let it stand for 10 minutes, slowly add 380 After the addition, let it stand for 15 minutes. The reaction solution turned black. Stir the reaction overnight in an ice bath. A large amount of solids precipitated in the reaction mixture the next day. Suction filtration, n-hexane: DCM volume ratio 4:1 rinse (100 ml x3 ), the solid was transferred to DCM to dissolve ether for recrystallization, suction fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com