Preparation method of sparsenatan for stabilizing isotope labeling
A diphenylsulfonamide and isotope labeling technology, which is applied in the field of medicine, can solve the problems of no stable preparation methods of diphenylsulfonamide drugs, and achieve the effects of large application research value, reasonable process design and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
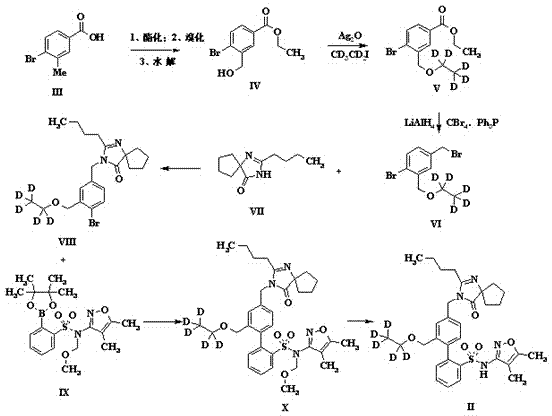
[0030] Example 1 The preparation of a stable isotope-labeled diphenylsulfonamide drug (Sparsenatan) is as follows: figure 1 Shown is the reaction flow diagram of the present invention:
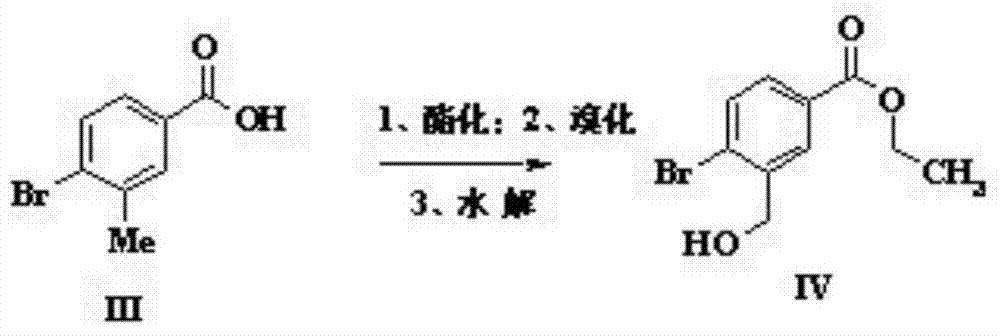
[0031] (1) Dissolve 3-methyl-4-bromobenzoic acid (0.093mol) in a mixed solvent of ethanol and toluene (1:1, 560ml), add a catalytic amount of concentrated sulfuric acid and then reflux for overnight reaction. After the reaction, use Sodium bicarbonate was neutralized and added to water to extract the organic phase obtained with dichloromethane. After drying and concentrating, it was dissolved in carbon tetrachloride (200ml) and N-bromosuccinimide (6.5g) was added for reflux reaction for three days. After cooling and filtration, the filtrate was concentrated and spin-dried to obtain 11g of bromide, which was dissolved in methanol (200ml), added sodium formate (8.87g) and refluxed for 6 hours, then concentrated and purified by column chromatography to obtain 8.5g of intermediate IV;
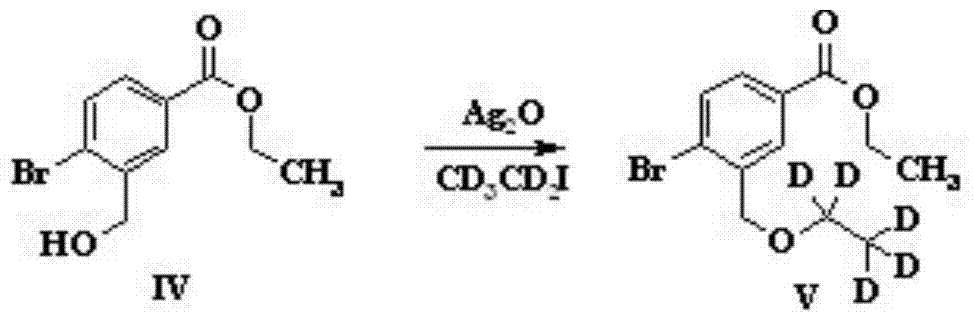
[0032] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com