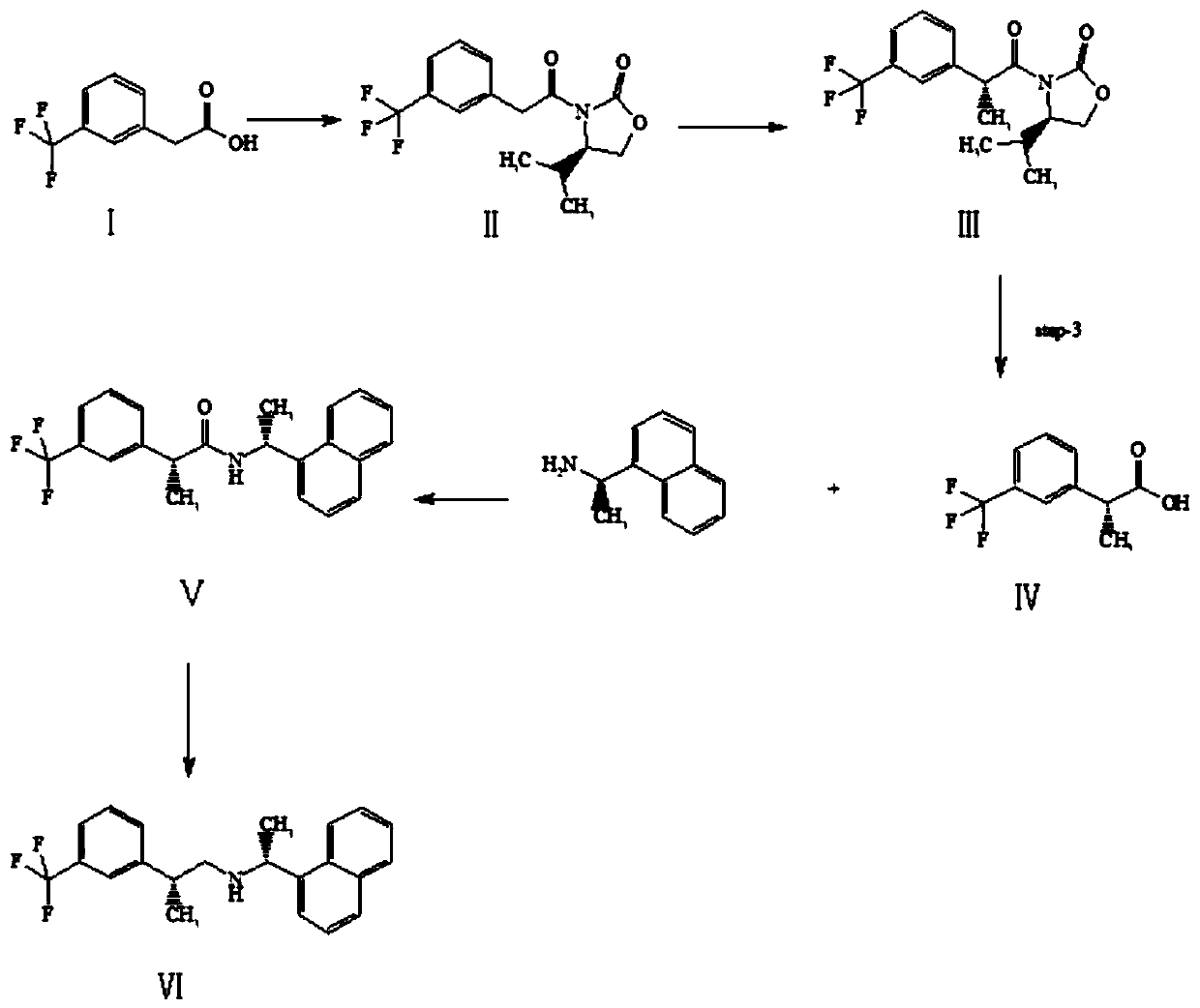
Preparation method of cinacalcet impurity
A technology of cinacalcet and impurities, applied in the field of compound preparation, to achieve the effect of simple operation method, high purity and reasonable process design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
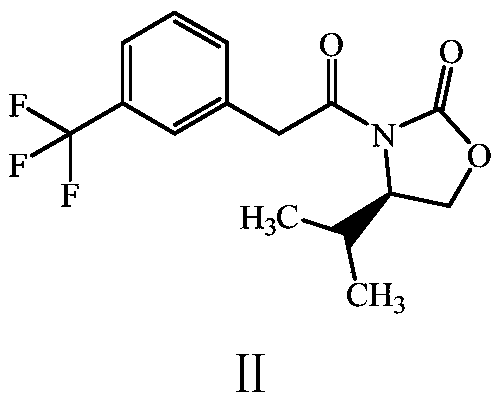
[0028] Preparation of Compound II: Dissolve 50g of m-trifluoromethylphenylacetic acid in dichloromethane, add 58.28g of thionyl chloride, and react at 60°C for 2 hours. When the reaction is complete, take it with 200mL of tetrahydrofuran for later use. Dissolve 31.63g of (R)-(+)-4-isopropyl-2-oxazolinone in 474mL of tetrahydrofuran, add 5g of sodium hydride, stir at 20°C for 20 minutes, dissolve the prepared acid chloride in 50mL of tetrahydrofuran, Add it dropwise to the reaction solution, stir at room temperature for 1.5 hours, after the reaction is over, add 100mL of water under ice bath, spin off most of the tetrahydrofuran, extract three times with 100mL of ethyl acetate, combine the organic layers, dry with anhydrous sodium sulfate, and filter , spin-dried to obtain 79g of crude product, separated by column chromatography to obtain 72g of yellow oil II, the yield was 93.2%.
[0029]
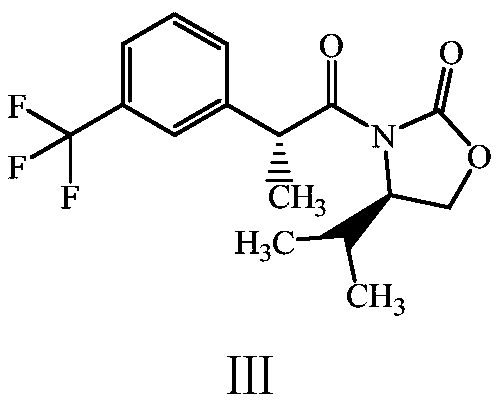
[0030] Preparation of Compound III: Dissolve 31g of Compound II in 620mL of tetrahyd...
Embodiment 2
[0039] Preparation of Compound II: Dissolve 40 g of m-trifluoromethylphenylacetic acid in dichloromethane, add 93.24 g of thionyl chloride, and react at 60°C for 2 hours. When the reaction is complete, take it with 200 mL of tetrahydrofuran for later use. Dissolve 25.31g of (R)-(+)-4-isopropyl-2-oxazolinone in 430mL of tetrahydrofuran, add 4g of sodium hydride, stir at 20°C for 20 minutes, dissolve the prepared acid chloride in 40mL of tetrahydrofuran, Add it dropwise into the reaction solution, stir at room temperature for 10 hours, after the reaction is over, add 100mL of water under ice bath, spin off most of the tetrahydrofuran, extract three times with 100mL of ethyl acetate, combine the organic layers, dry with anhydrous sodium sulfate, and filter , spin-dried to obtain 56g of crude product, separated by column chromatography to obtain 45g of yellow oil II, and the yield was 72.84%.
[0040]
[0041] Preparation of compound III: Dissolve 40 g of compound II in 800 mL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com