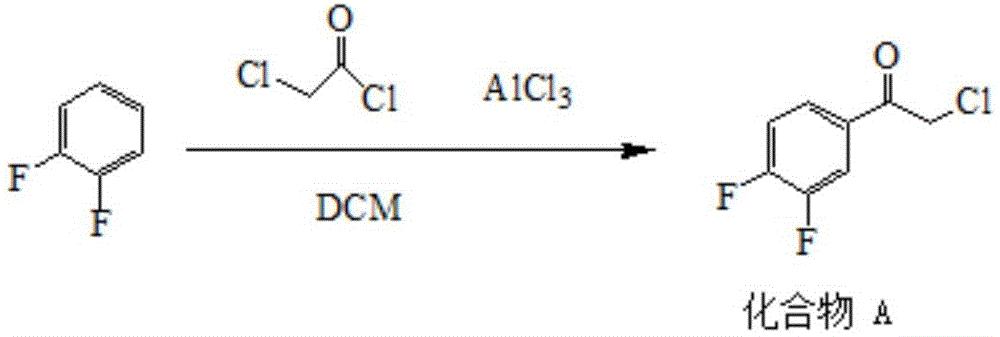
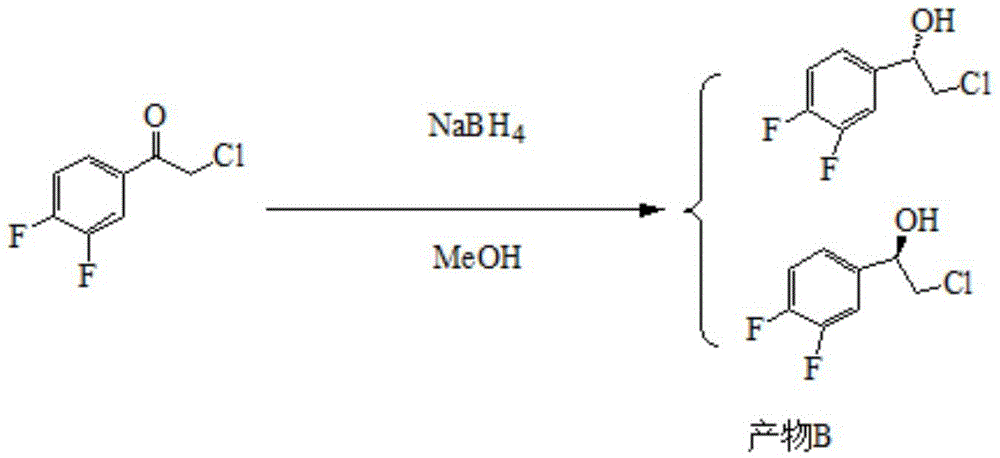
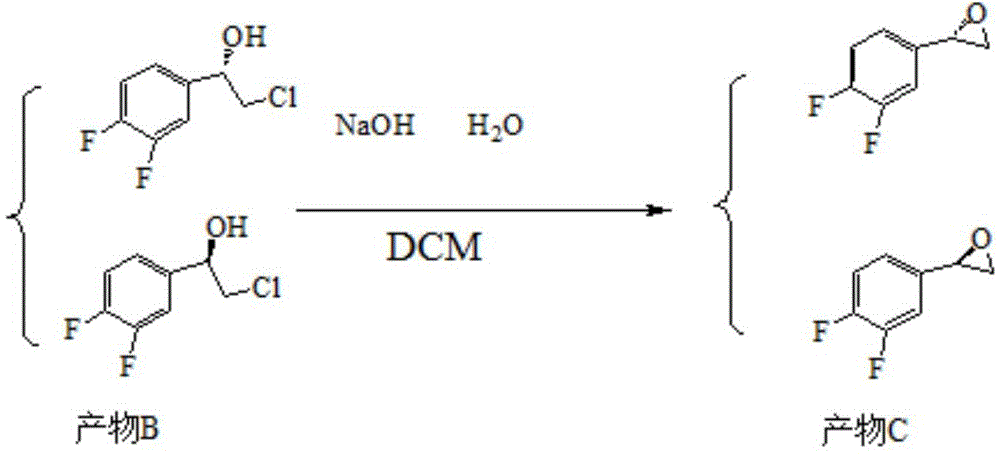
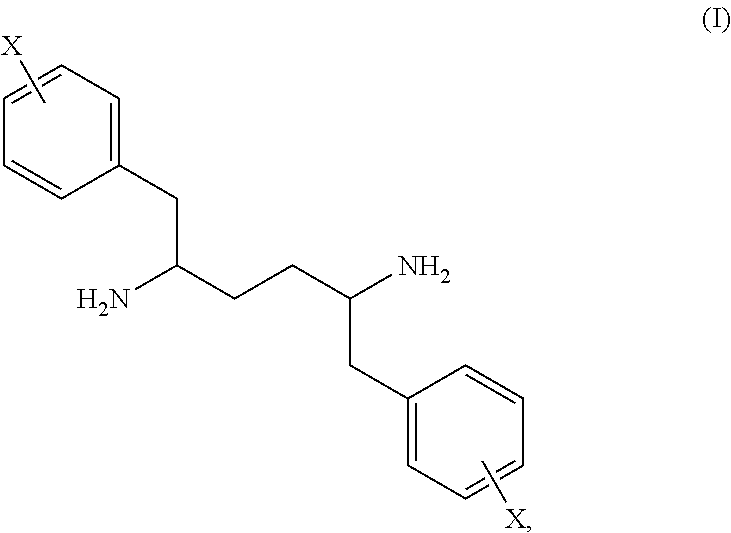
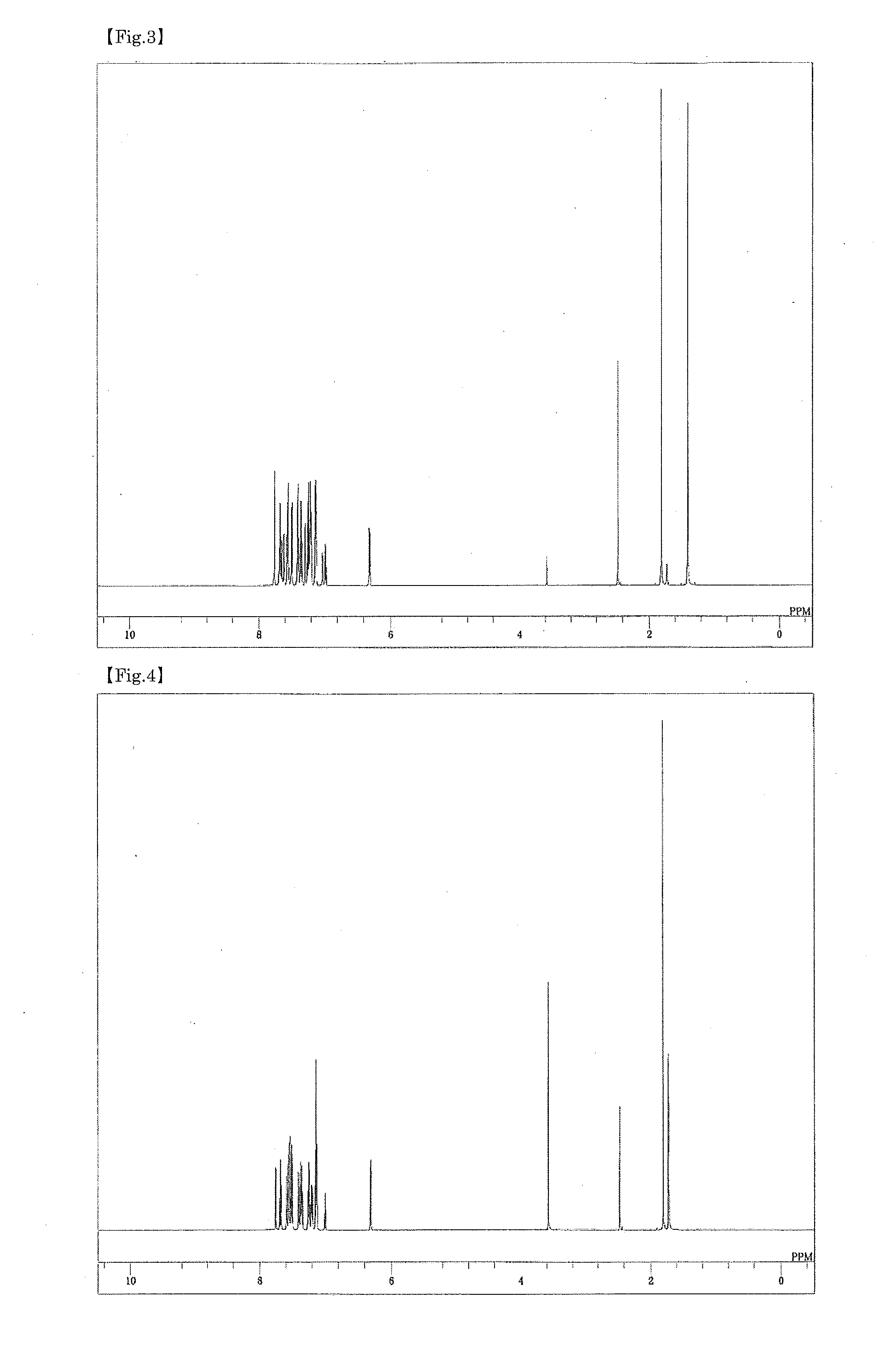
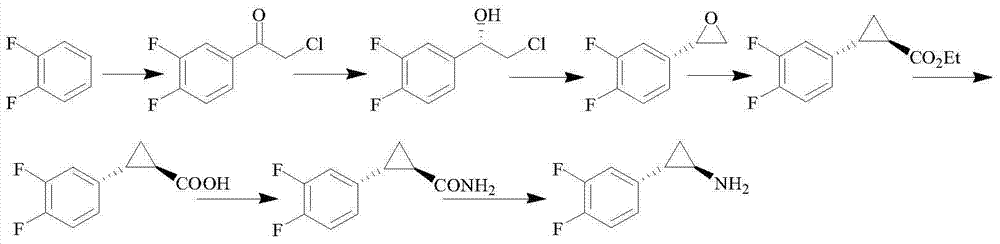
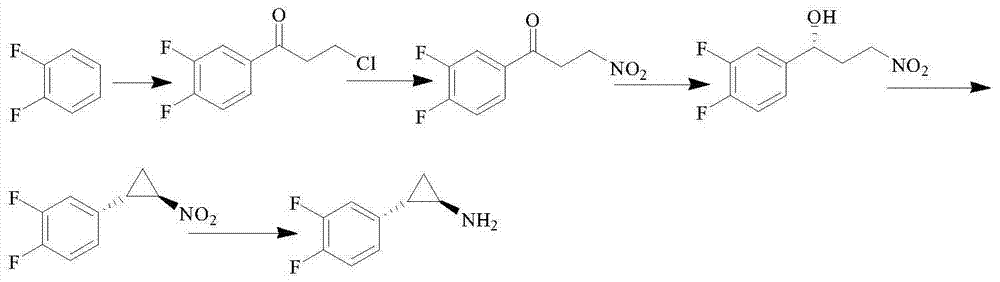
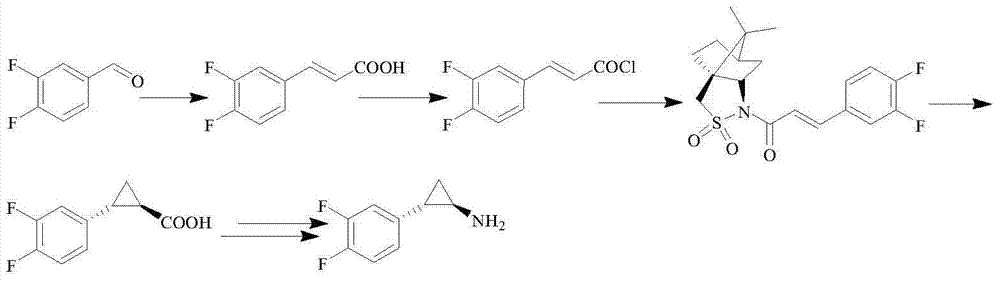
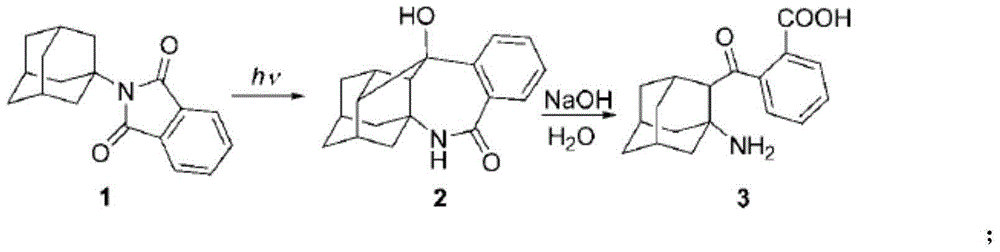
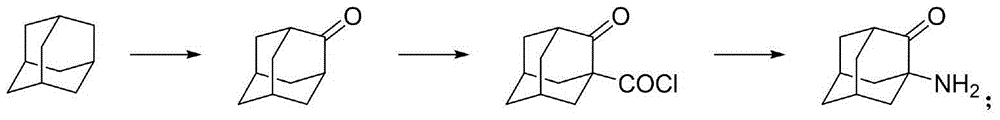
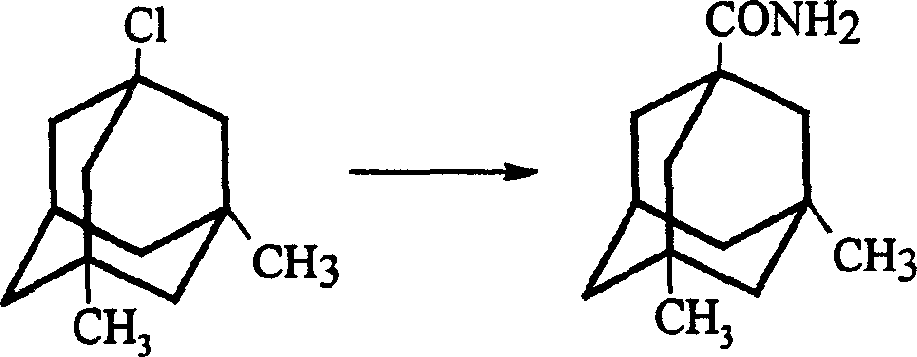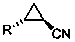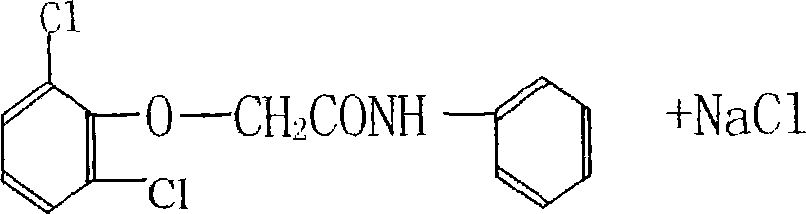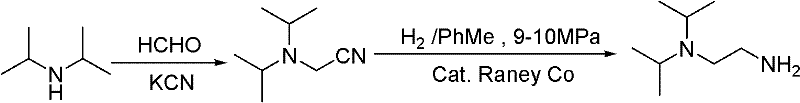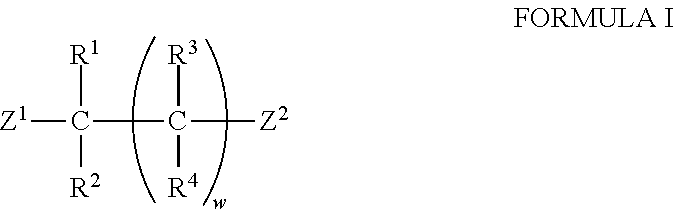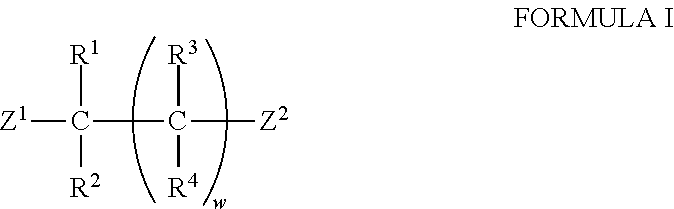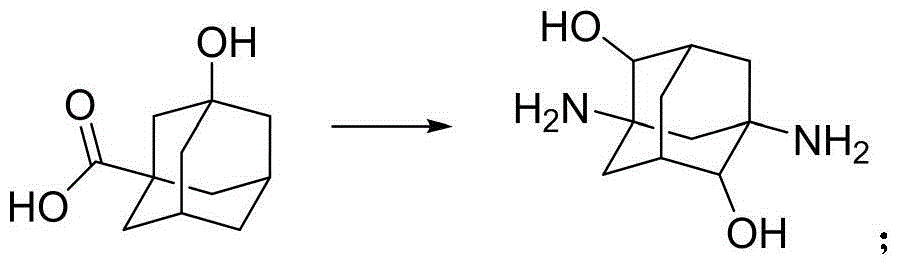Patents
Literature
155results about "Preparation by rearrangement reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
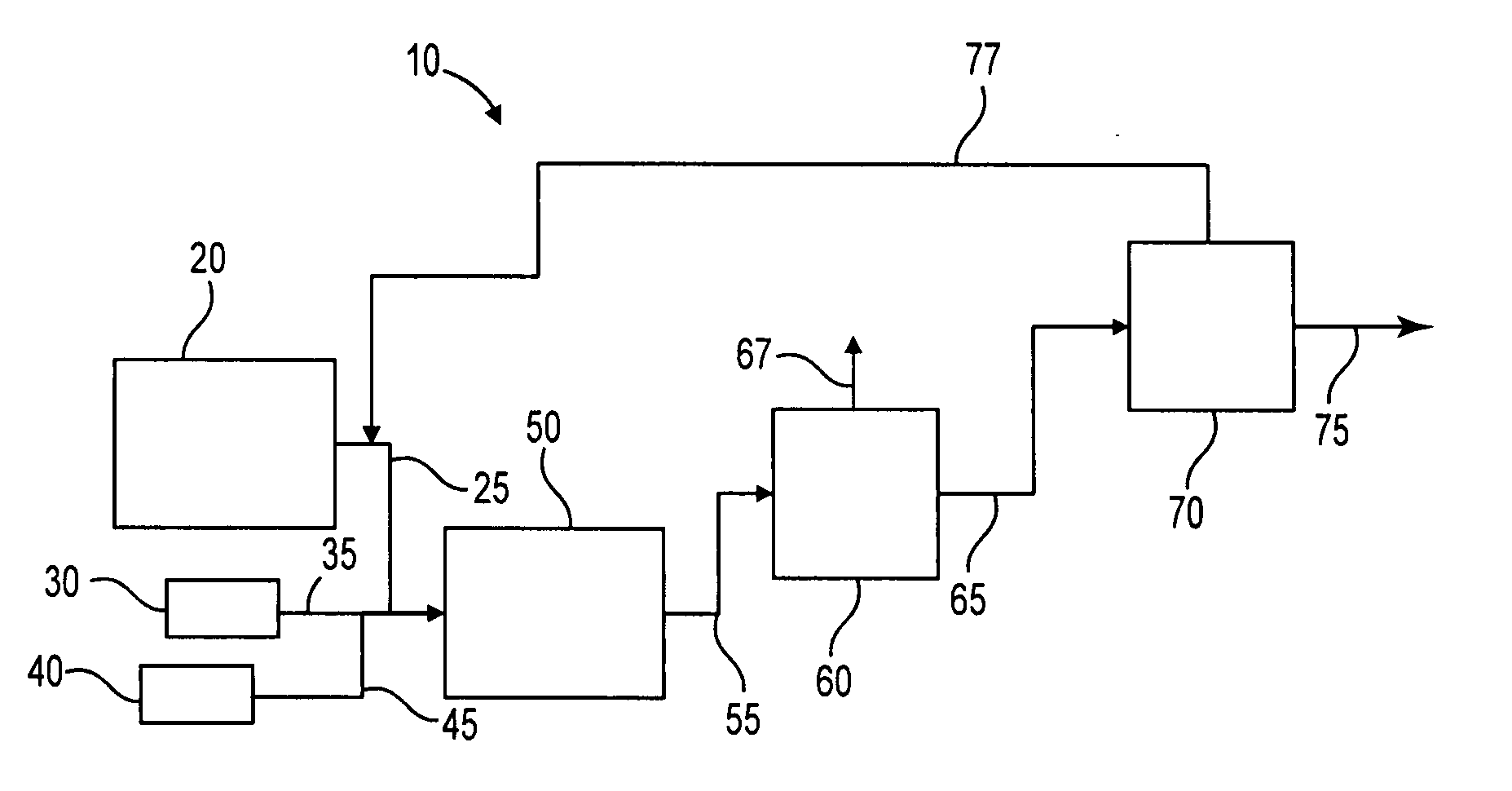
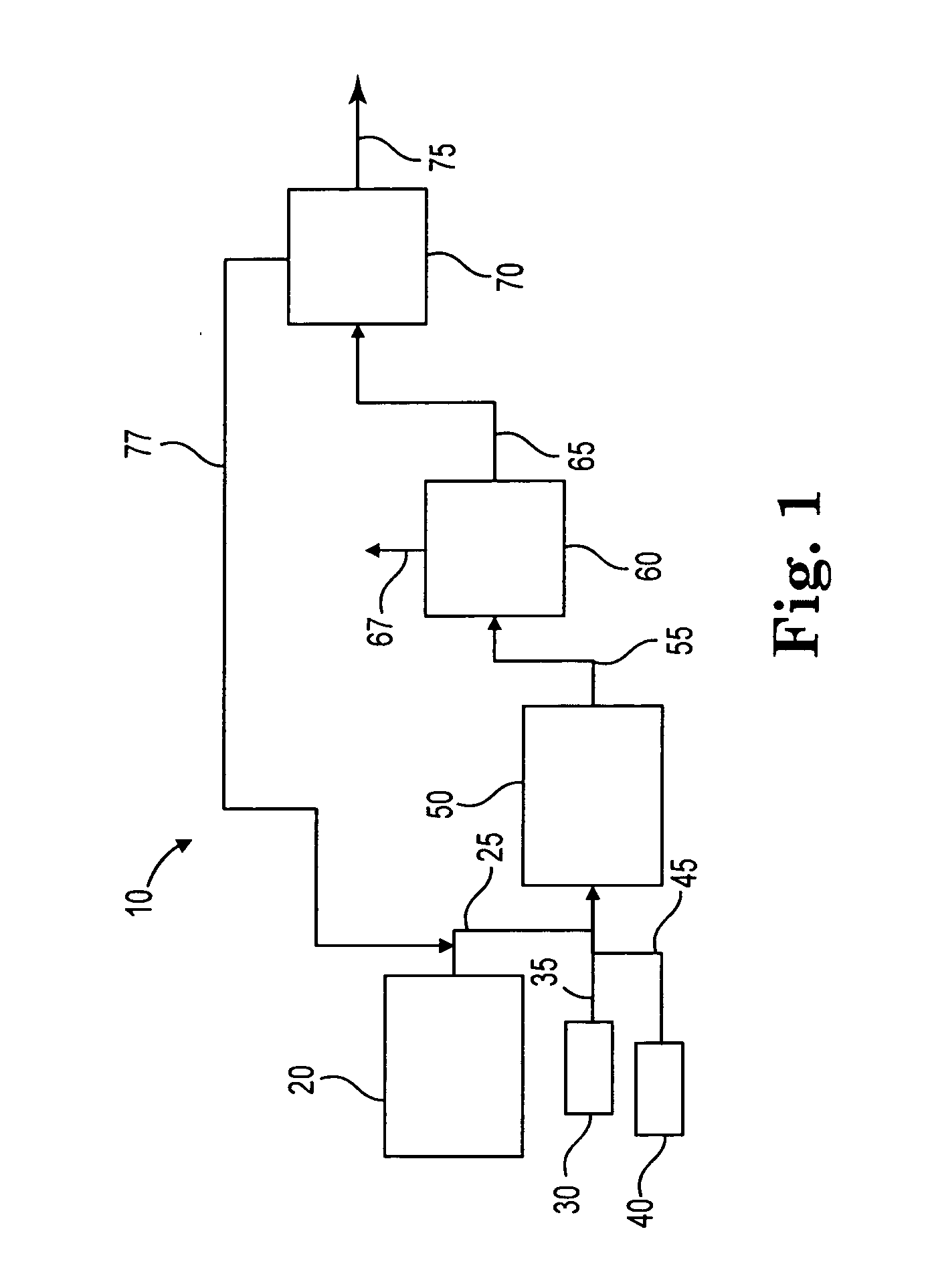
Process to selectively manufacture diethylenetriamine (DETA) or other desirable ethylenamines via continuous transmination of ethylenediamine (EDA), and other ethyleneamines over a heterogeneous catalyst system
ActiveUS20100087683A1Quantity maximizationQuantity minimizationOrganic compound preparationAmino compound preparation by disproportionationEthylenediamineDiethylenetriamine
The present invention reacts ethylenediamine with one or more additional ethyleneamines in the presence of a transamination catalyst to provide a different, preferably more desirable product mix of one or more ethyleneamines.
Owner:UNION CARBIDE CORP
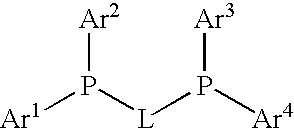
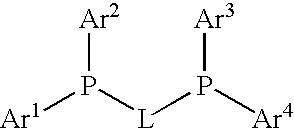
Process for preparing arylamines
InactiveUS20080039633A1Organic compound preparationPreparation by rearrangement reactionsArylHydrogen
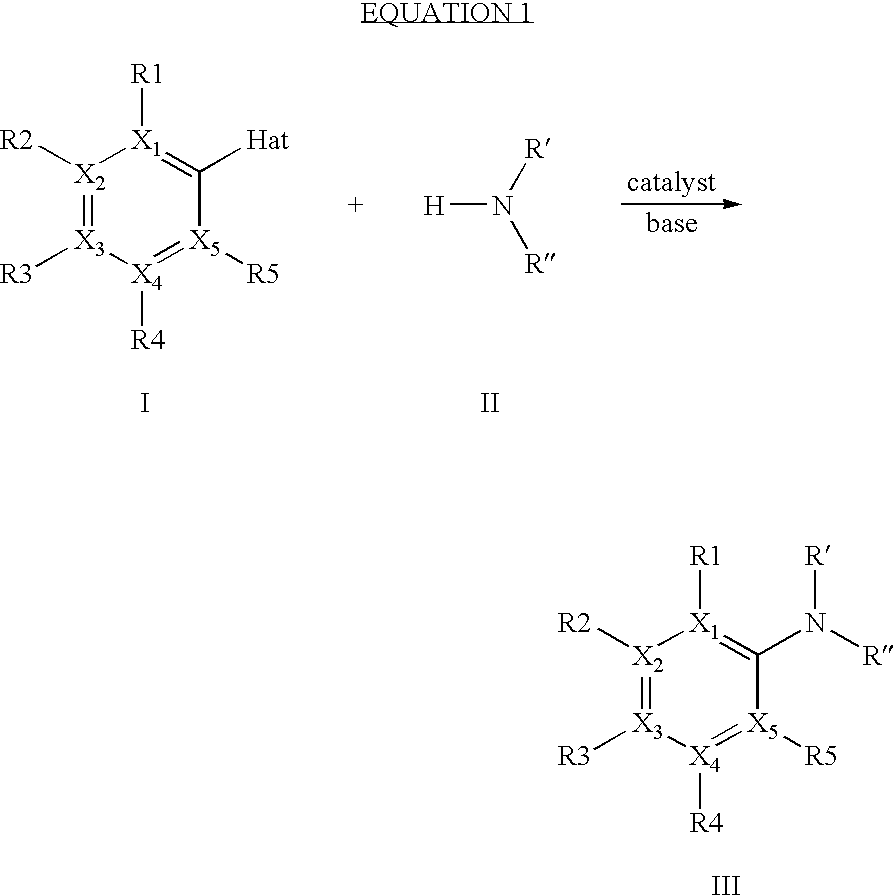
The invention relates to a process for preparing arylamines or heteroarylamines or arylamides or heteroarylamides by cross-coupling of primary or secondary amines or amides with substituted aryl or heteroaryl compounds in the presence of a Brønsted base and a catalyst or precatalyst, wherein the catalyst comprisesa) a transition metal, a complex, a salt or a compound of this transition metal selected from the group consisting of Ni, Pd andb) at least one ligand selected from the group consisting of bidentate bis(phosphino)alkanediyls having the following formula in a solvent or solvent mixture,where the radicals Ar1-4 are each, independently of one another, an aryl or heteroaryl substituent selected from the group consisting of phenyl, naphthyl, pyridyl and biphenyl or Ar1-4 is hydrogen, C1-, C2-alkyl, straight-chain, branched or cyclic C3-C8-alkyl, andL is an alkanediyl bridge which has from 1 to 20 carbon atoms.
Owner:ARCHIMICA GMBH
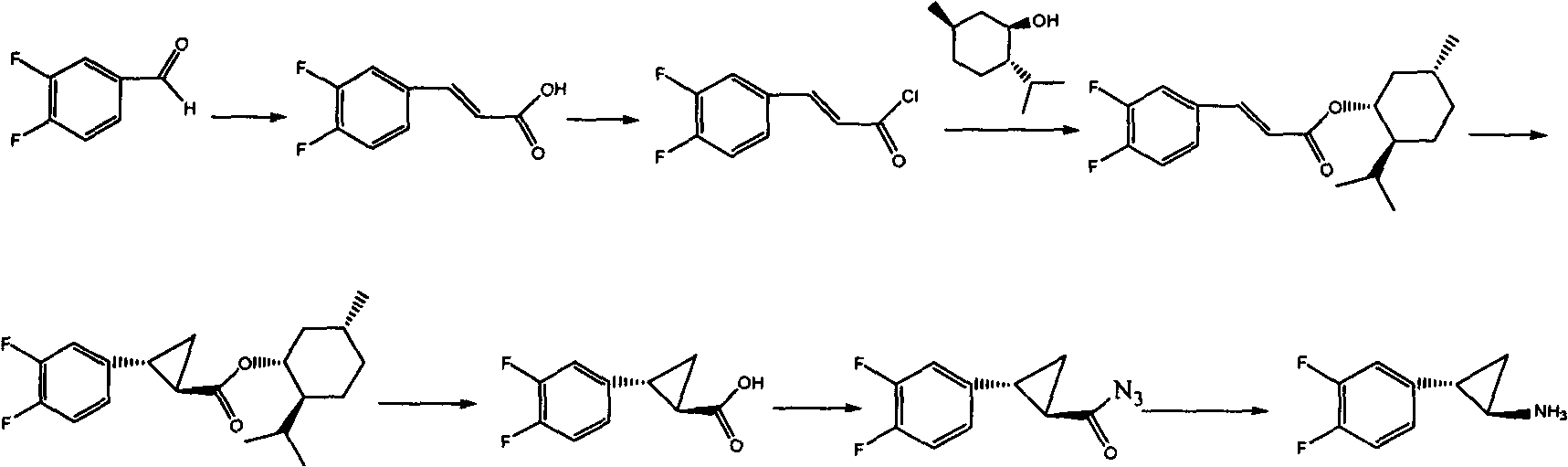
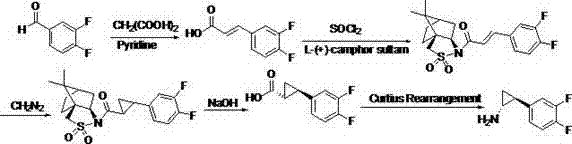
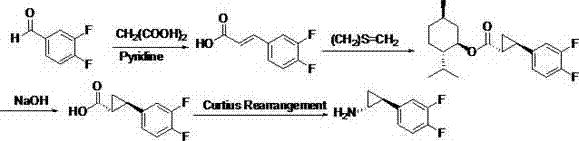
Preparation method of Ticagrelor intermediate
ActiveCN102796007AEasy to prepareEasy to operatePreparation by rearrangement reactionsChemical synthesisTicagrelor
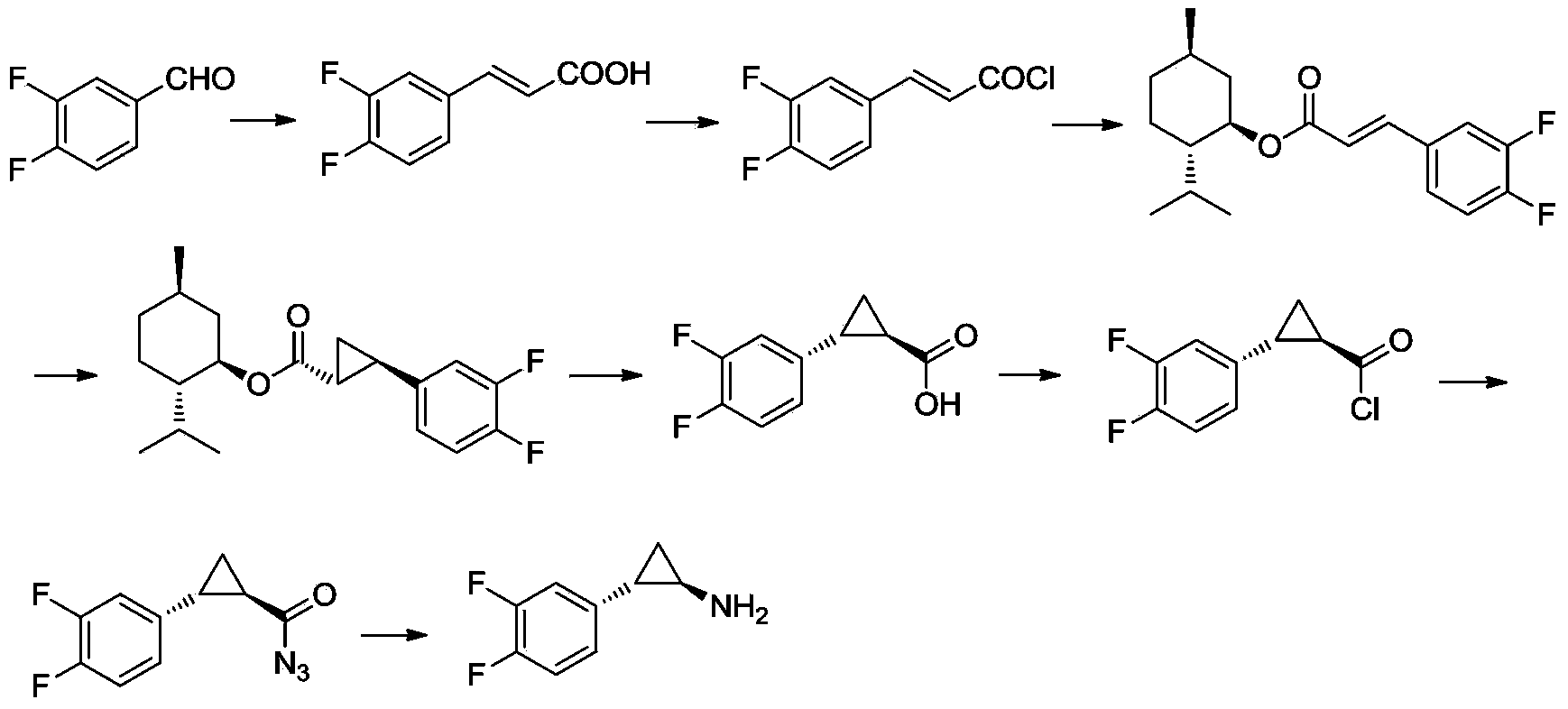
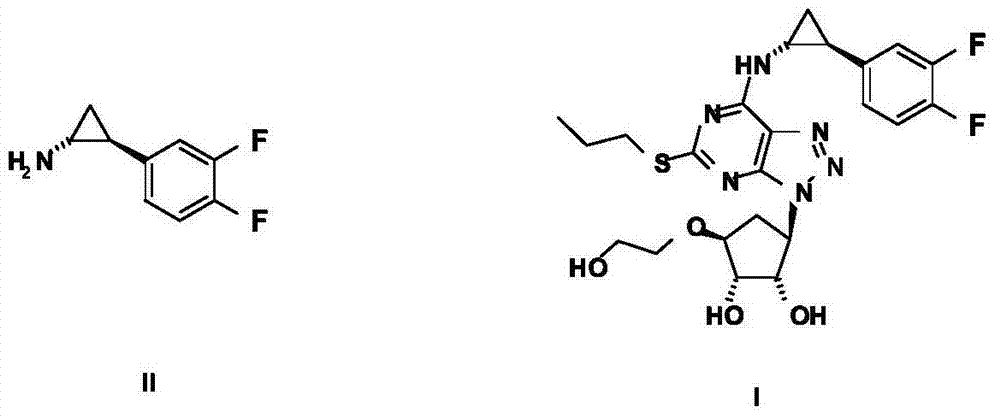
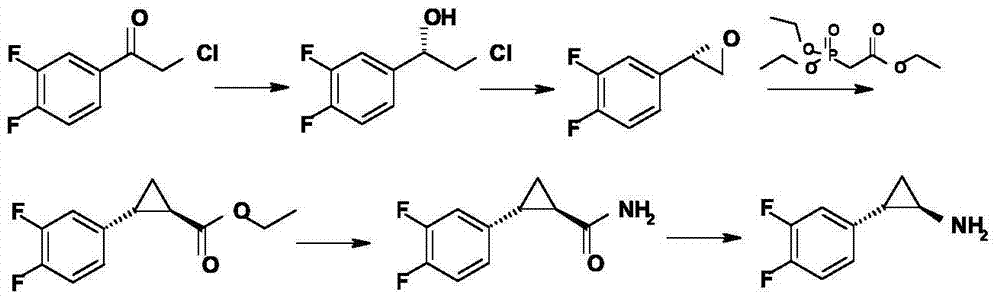
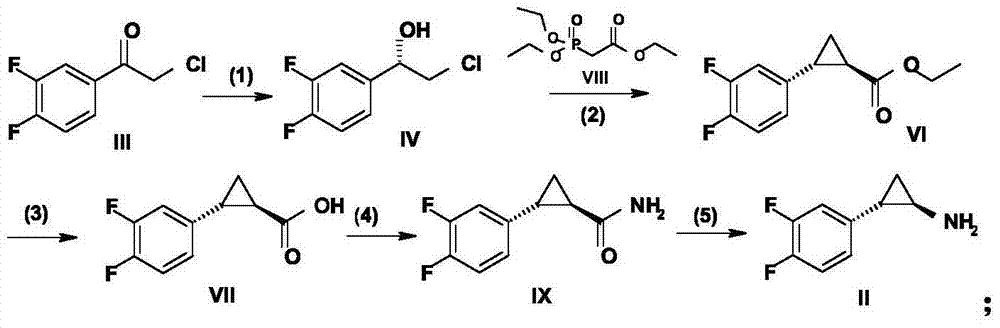
The invention relates to the medicine chemical synthesis field, and especially discloses a preparation method of a Ticagrelor intermediate. The preparation method comprises the following steps: 1) taking 3,4-difluorobenzaldehyde (I) as an initial raw material, reacting with a phosphorus ylide material liquid to obtain (E)-3-(3,4-difluorophenyl)-2-acrylic acid ester (II); 2) performing a Simons-Smith asymmetric cyproteronethe reaction on the (E)-3-(3,4-difluorophenyl)-2-acrylic acid ester (II) to obtain trans-(1R,2R)-2-(3,4-difluorophenyl) cyclopropanecarboxylic acid ester (III); 3) performing aminolysis on the trans-(1R,2R)-2-(3,4-difluorophenyl) cyclopropanecarboxylic acid ester to obtain trans-(1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxamide (IV); and 4) performing a Huffman rearrangement reaction on the trans-(1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxamide (IV) to obatain the Ticagrelor intermediate (V). The method of the invention has the advantages of simple process, convenient operation, mild reaction condition and easy control, low cost and easy acquisition of raw material, high product yield and product purity, and is adapted to large scale industrial production.
Owner:JINAN RUIFENG PHARMA +2
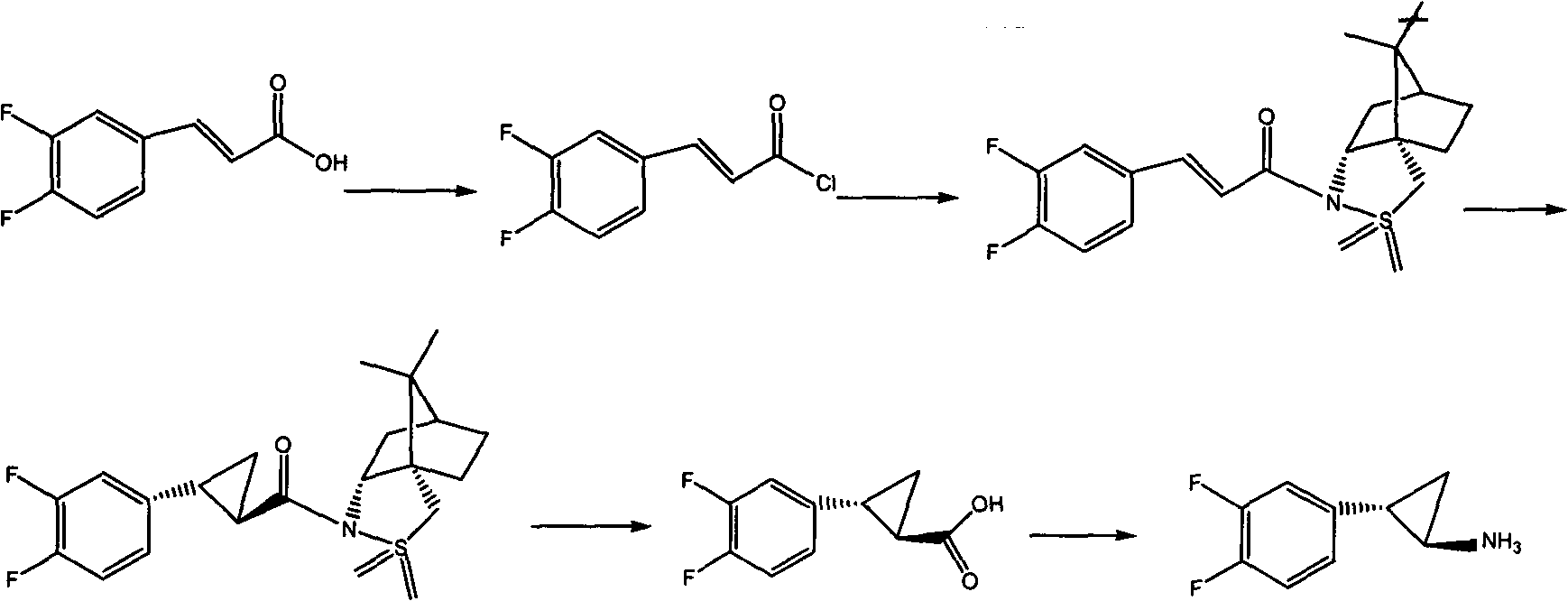
Method for preparing ticagrelor key intermediate and racemate thereof and special intermediate for implementing method
ActiveCN103508899ALow priceEasy to getCarboxylic acid nitrile preparationOrganic compound preparationAfter treatmentTicagrelor
The invention discloses a method for preparing a ticagrelor key intermediate VII and a racemate thereof. The method comprises the following steps: by using a compound V or a racemate thereof as a raw material, performing acidic hydrolysis to obtain a compound VI or a racemate thereof; and performing Curtis rearrangement to obtain a compound VII or a racemate thereof. According to the ticagrelor key intermediate and the racemate thereof prepared by the method, the adopted initial raw materials are low in price and readily available, the requirements of reaction conditions on solvents are low, the operation is safe, simple and convenient, and the method is environment-friendly; moreover, when the ticagrelor key intermediate and the racemate thereof are prepared by adopting a special intermediate, the after-treatment is simple and convenient, and the large-scale production is more easily realized.
Owner:KAIYUAN HENGTAI PHARMA
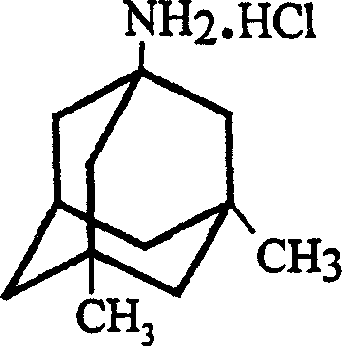
Method for preparing memantine hydrochloride
InactiveCN1488622AGood crystal formHigh purityNervous disorderPreparation by rearrangement reactionsMemantine HydrochlorideTert-Butyl chloride
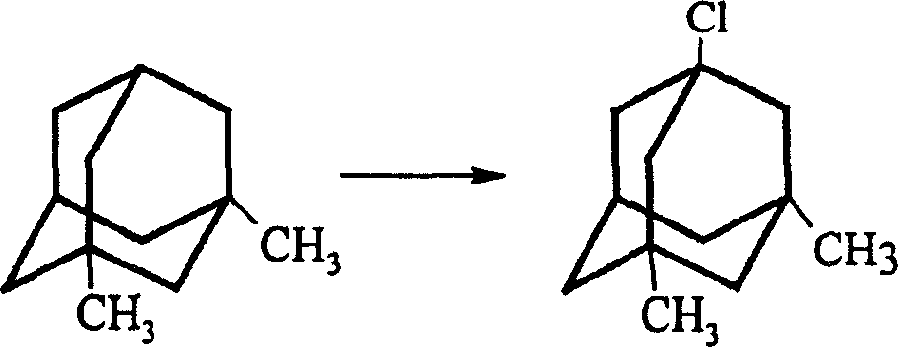
The invention is a manufacturing method of diamante amine hydrochlorate. The invention adopts 1, 3-dimethyl adamantine to reacts with tert-butylchlorine and gets 1-chlorine-3, 5-dimethyl adamantine; then reacts with acetamide directly, the educt from water reacts with sodium hydroxide in ethanediol or glycerine solvent, extracts by acetic ester, condenses, and blow in dry hydrochloride gas and gets the coarse product. There gets the pure product through alcohol-acetic ester recrystallization.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Chemical method used for preparing aromatic cyclopropanecarbonitrile and cyclopropylamine
The invention relates to a method for preparing trans-aryl cyclopropanecarbonitrile with a structure shown in a formula (IV) in the specification through reaction between aryl substituted ethylene oxide and cyan substituted phosphate and further relates to a method for preparing cyclopropylamine from trans-aryl cyclopropanecarbonitrile. Trans-aryl cyclopropanecarbonitrile and cyclopropylamine are used for preparing drugs, especially ticagrelor.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
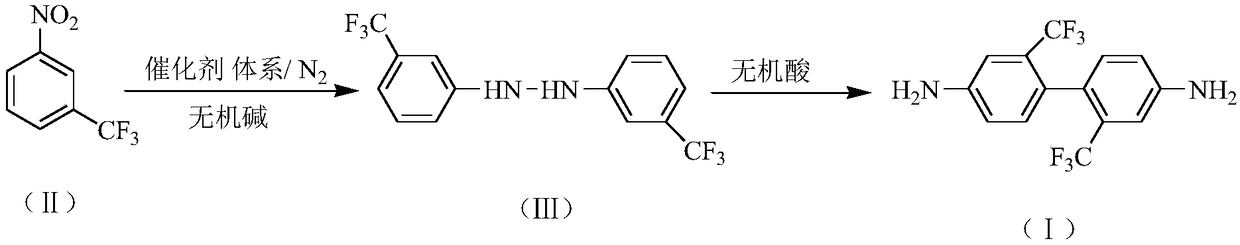
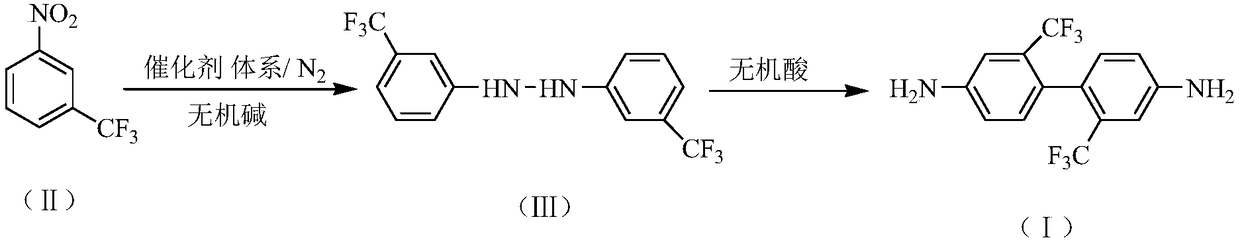
Preparation method of 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl
InactiveCN109232273AReduce generationHigh purityHydrazine preparationPreparation by rearrangement reactionsHydroxyanthraquinoneDodecylsulfonic acid
The invention relates to a preparation method of 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl. The preparation method comprises the following steps: synthesizing 3,3'-bis(trifluoromethyl)hydrazo-benzene in an inorganic alkaline aqueous solution by adopting nitrobenzotrifluoride as a raw material, adopting a phase transfer catalyst, a co-catalyst and Pd / C as a catalytic system and adopting aromatic hydrocarbon as a solvent, and performing the re-arrangement reaction on the 3,3'-bis(trifluoromethyl)hydrazo-benzene in an inorganic acid aqueous solution, thus obtaining 2,2'-bis(trifluoromethyl)-4,4'-diaminodiphenyl, wherein the phase transfer catalyst is one or a mixture of more of sodium dodecyl benzene sulfonate, sodium dodecyl sulfate and cetyl trimethyl ammonium bromide, and the co-catalyst is one or a mixture of more of 2,3-dichloro-1,4-naphthoquinone, 2-hydroxyanthraquinone and 2,6-dioxyanthraquinone. The preparation method has the advantages of mild reaction condition, simple process, high product quality and yield, low production cost, environmental friendliness, suitability for continuous production and the like.
Owner:烟台海川化学制品有限公司
Method and device for preparing diphenylmethane series diamine and polyamine with low N-methyl impurity content and catalyst
ActiveCN107827756AReduce contentHigh selectivityMetal/metal-oxides/metal-hydroxide catalystsPreparation by rearrangement reactionsDiphenylmethaneFixed bed
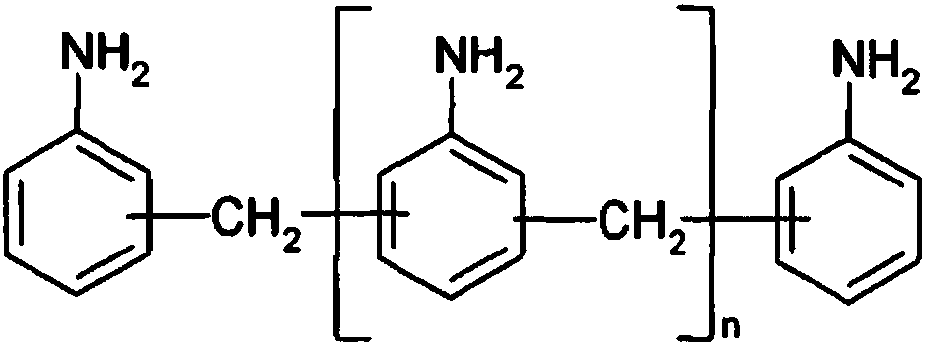
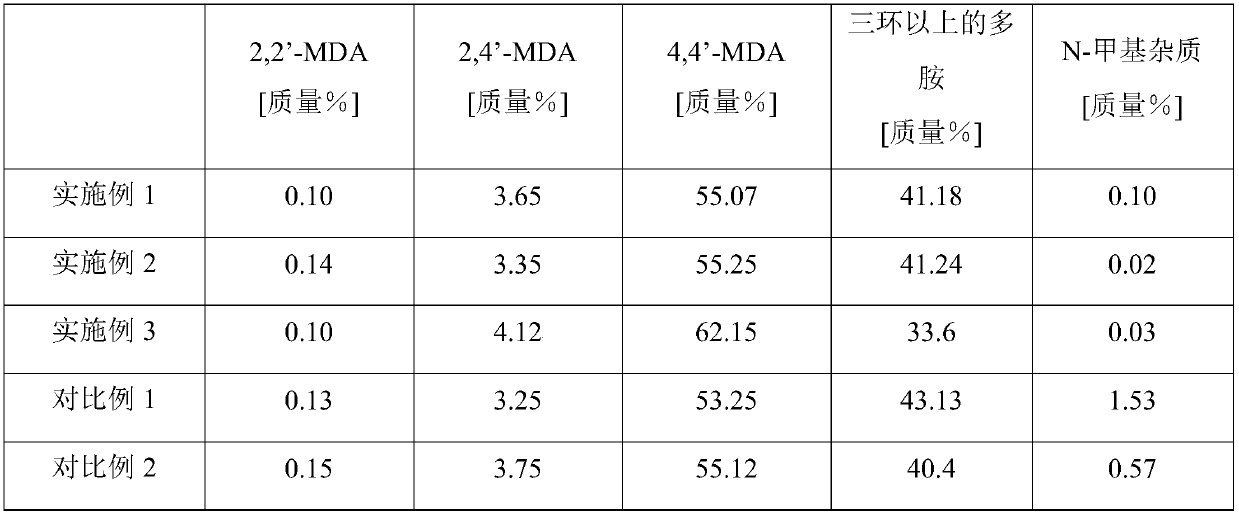
The invention relates to a method and a device for preparing diphenylmethane series diamine and polyamine with low N-methyl impurity content and a catalyst. The method comprises the steps as follows:a) phenylamine and formaldehyde are subjected to a condensation reaction in the presence of an acid catalyst, and a reaction mixture containing polyamino benzylaniline salt is obtained; b) the reaction mixture from a) enters a fixed bed reactor loaded with the catalyst for a transposition rearrangement reaction, a mixture containing diphenylmethane series diamine salt and polyamine salt is obtained after the reaction, and active components of the transposition rearrangement reaction catalyst comprise one or more of vanadium phosphate oxide, a Nb2O5-La2O3 solid solution and a Pr2O3-Ce2O3 solidsolution. The reaction selectivity at the transposition rearrangement stage can be improved, so that the content of N-methyl MDA impurities in the product can be decreased, the product quality can besubstantially improved, and the N-methyl MDA content can be decreased to 0.01% or below.
Owner:WANHUA CHEM GRP CO LTD +1
Preparation method for ticagrelor intermediate and mandelate thereof
PendingCN106906249AWide variety of sourcesHigh yieldOrganic compound preparationCarboxylic acid esters preparationTicagrelorWastewater
The invention discloses a synthetic method for an intermediate compound (I) of ticagrelor and a mandelate compound(VI) thereof. Asymmetric reduction is carried out by the enzymic method; the synthetic method has the advantages of simple operation, mild reaction condition, little pollution, high yield of the product, good optical purity, thus the synthetic method is suitable for large-scale production; the energy consumption and the discharge of organic waste-water are greatly reduced, and requirements of large-scale industrial production are met well.
Owner:LIAONING TIANHUA CHEM
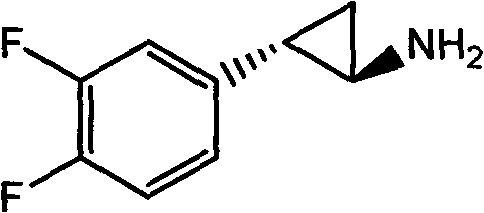
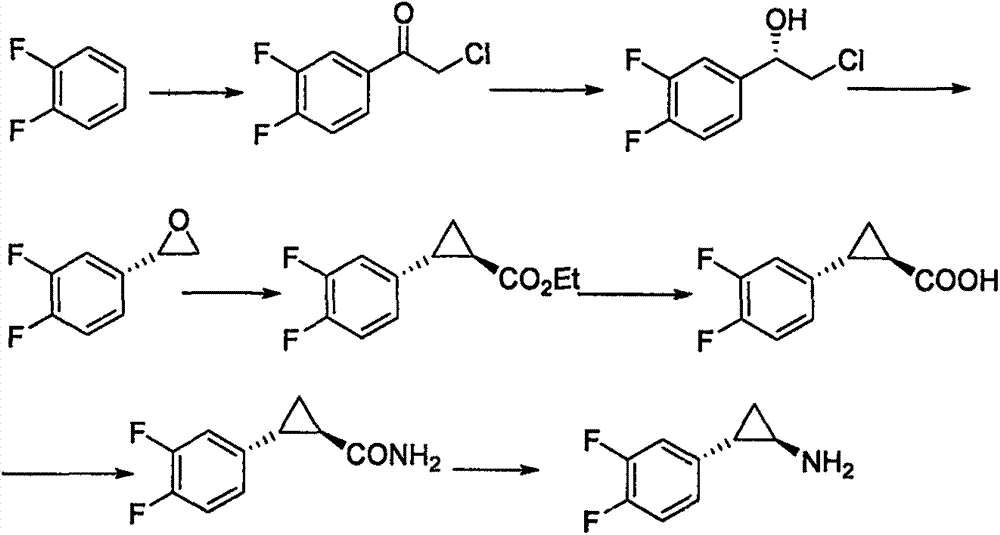
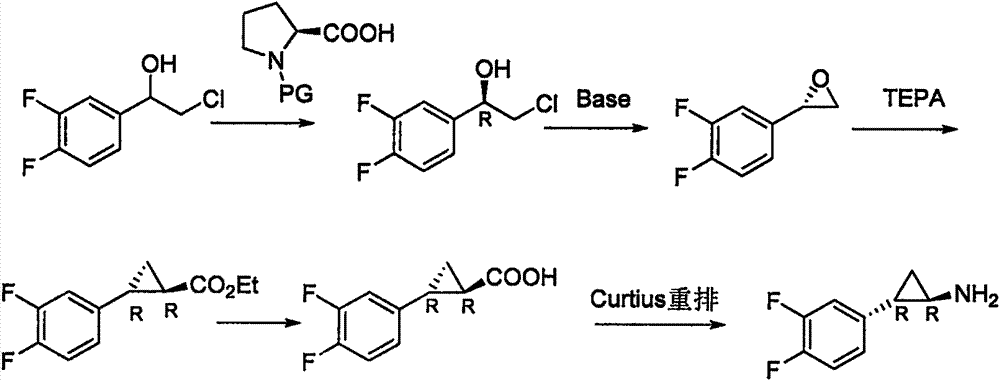
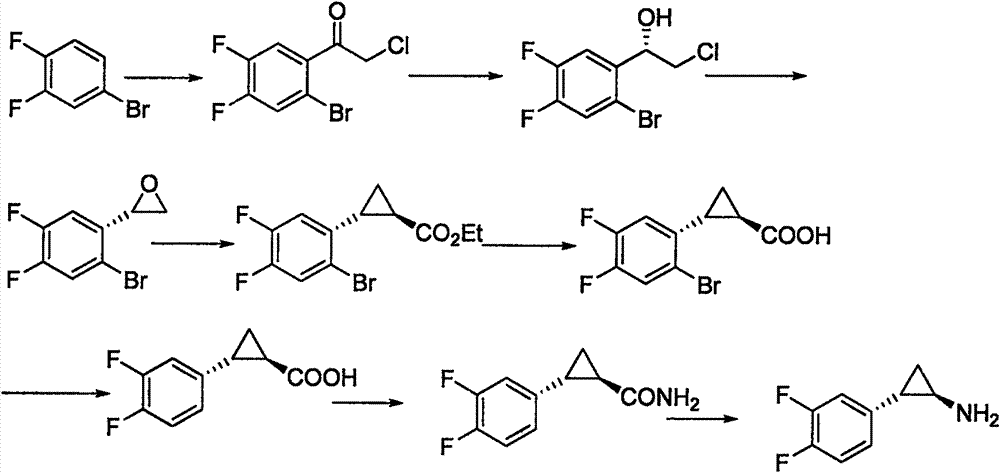
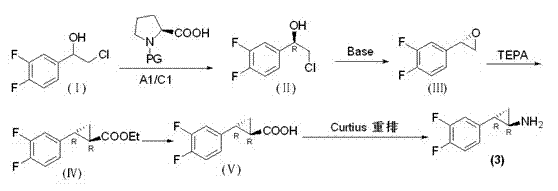
Preparation method of trans-(1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine
InactiveCN102775314AFew stepsEasy to operateHydroxy compound separation/purificationPreparation by rearrangement reactionsEpoxyPtru catalyst
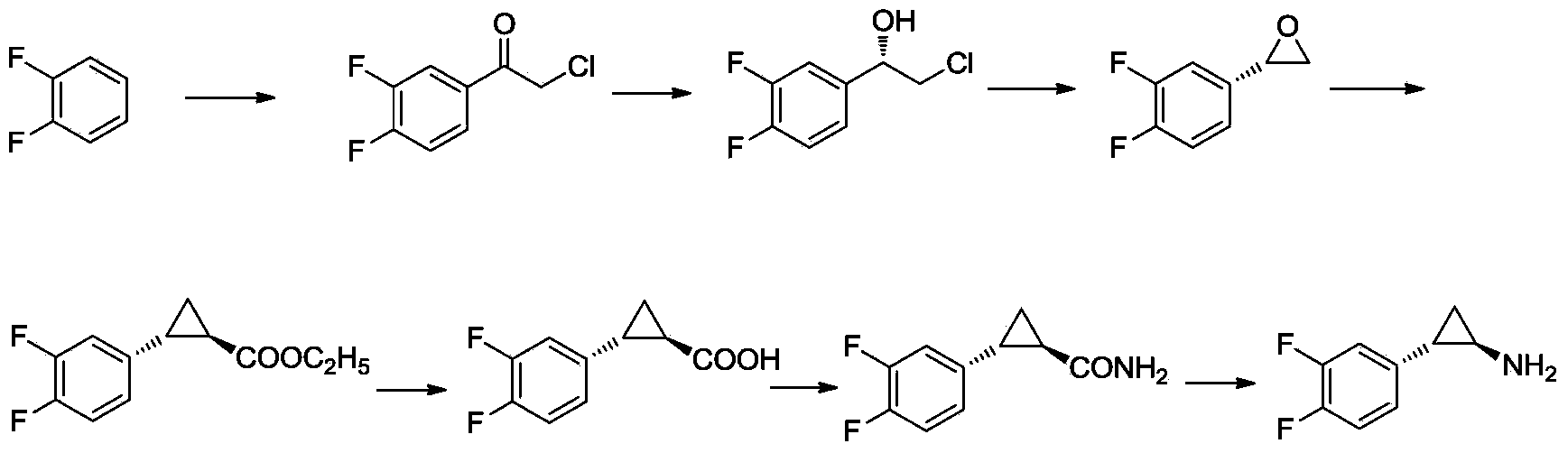
The invention provides a preparation method of trans-(1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine. The preparation method comprises the following steps: enabling racemic chloro phenethyl alcohol (I) and N-protection proline to undergo a reaction under the effects of a condensing agent A1 and a catalyst C1, and obtaining chiral chlorohydrin (II); enabling the chiral chlorohydrin (II) to generate an epoxy compound (III) under the conditions of alkalinity; enabling the epoxy compound (III) and TEPA to react and generate cyclopropyl ethyl formate (IV) under the conditions of alkalinity; removing ester from cyclopropyl ethyl formate (IV) under the conditions of alkalinity, and generating cyclopropanecarboxylic acid (V); and enabling cyclopropanecarboxylic acid (V) and azide to generate a target compound (3) by Curtius rearrangement. The preparation method has the advantages that the steps of a used synthetic process route are few, the operation is simple, and industrial production is achieved easily; a kinetic resolution method is utilized to synthesize chiral chlorohydrin (II) and is simple in reaction conditions and easy to operate; and a TEPA method is utilized synthesize the cyclopropyl ethyl formate, the product yield is high, cis-trans selectivity is good, and the purity is over 99%.
Owner:江苏富泽药业有限公司
Method for preparing (1R, 2R)-2-(3, 4-difluoro phenyl)cyclopropylamine
ActiveCN105712889AReduce usageEasy to operateGroup 4/14 element organic compoundsOrganic compound preparationHydrolysisRaw material
Owner:DAILY FAME TRADING LTD +1
Novel stereoisomeric mixtures, synthesis and uses thereof
InactiveUS20140051888A1Increase heightImprove responseAmino compound purification/separationOrganic compound preparationStereoisomerismDiamine
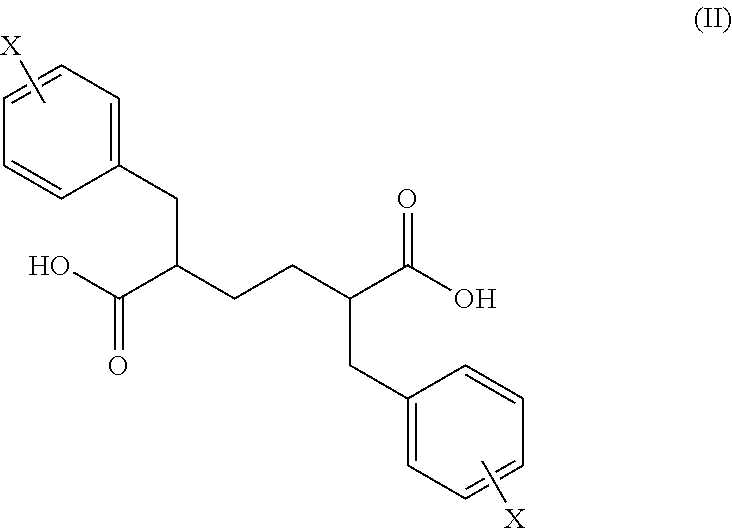
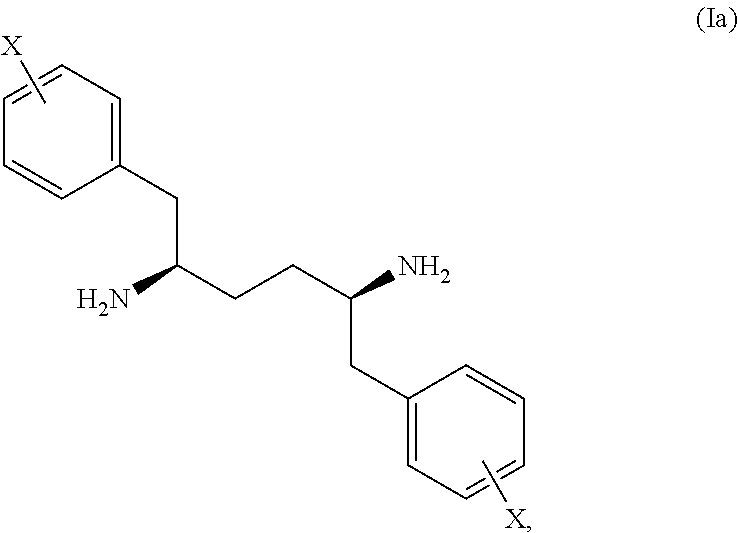
A novel stereochemical mixture of 1,6-diaryl-2,5-diaminohexanes, such as a mixture of stereoisomers of 1,6-diphenylhexane-2,5-diamine, is described. Also described are methods of preparing stereochemically pure 1,6-diaryl-2,5-diaminohexanes, and particularly stereochemically pure 1,6-diphenyl-2,5-diaminohexane. Also described is the use of both the mixture of stereoisomers and the individual stereoisomers.
Owner:AMPAC FINE CHEM
Novel technique for producing 2,6-dichloro diphenylamine
ActiveCN101230007ARelieve pressureSave moneyPreparation by rearrangement reactionsWastewaterAcetylation
The invention relates to a new process for producing 2, 6-dichloro diphenylamine, which includes a chloroacetylization step, an etherification step, rearrangement and post-processing steps; wherein, the reaction formula of the final product, 2, 6-dichloro diphenylamine, is shown as the right: The process of the invention causes no effluent, and the biproducts such as the sodium glycollate, the sodium chloride, etc during the reaction are recycled and transferred into reused resources. Moreover, the production technology is clean and environmentally friendly with no wastes and reduced total cost, and no catalyst and sodium carbonate are needed.
Owner:HENAN DONGTAI PHARM
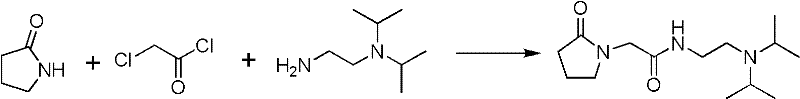
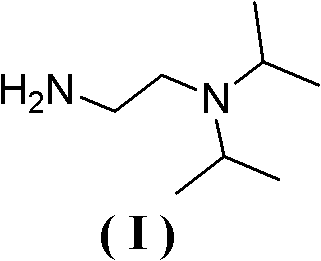
Synthesis method of N,N-diisopropyl quadrol
ActiveCN102249928ARaw materials are cheap and easy to getMild reaction conditionsPreparation by rearrangement reactionsSynthesis methodsDiisopropylamine
The invention discloses a synthesis method of N,N-diisopropyl quadrol. According to the synthesis method, N,N-diisopropyl quadrol is synthesized through Michael addition and Hoffmann degradation by taking acrylamide and diisopropylamine as starting raw materials. The synthesis method has the advantages of advanced process route, reasonable process condition, cheap and available raw materials, mild reaction conditions, high atom economy, low production cost and less three wastes, is simple and safe to operate, is suitable for industrial production, and has a large implementation value and large social and economic benefits.
Owner:ZHEJIANG UNIV OF TECH +1
Transamination of nitrogen-containing compounds to high molecular weight polyalkyleneamines
ActiveUS9000217B2Easy to customizeHigh molecular weightOrganic compound preparationAmino compound preparation by disproportionationDehydrogenationAlkyl amine
A process for preparing high molecular weight acyclic polyamines comprising providing a reaction mixture that includes at least a first component comprising a first organic, nitrogen-containing compound that contains at least two non-tertiary amine groups separated from one another by a ternary or higher carbon atom spacing that can be transaminated in the presence of a hydrogenation / dehydrogenation catalyst to form a mixture of higher molecular weight, acyclic polyamines while minimizing the formation of cyclic polyamines.
Owner:DOW GLOBAL TECH LLC
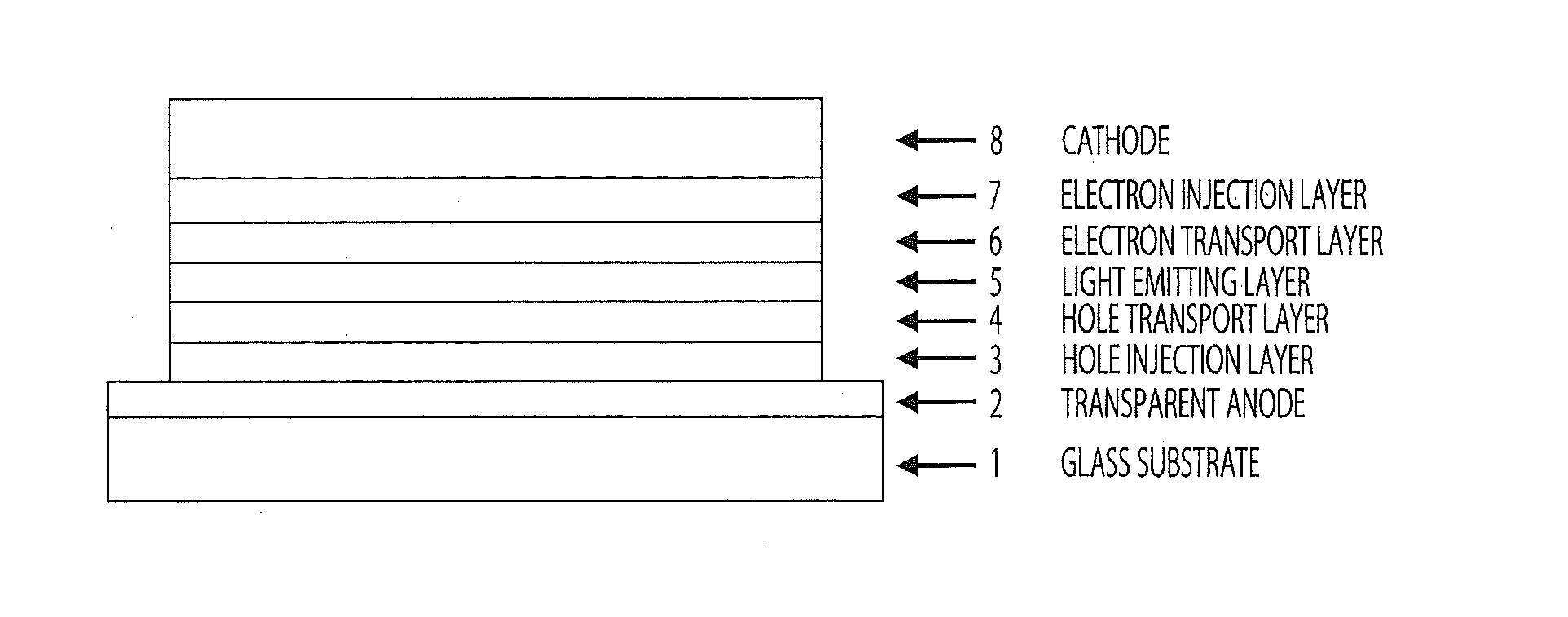
Compound having acridan ring structure, and organic electroluminescent device
ActiveUS20130075715A1Excellent electron blocking abilitySatisfactory amorphousnessOrganic compound preparationElectroluminescent light sourcesAcridineSimple Organic Compounds
An organic compound with characteristics excelling in hole-injecting / transporting performance and having an electron blocking ability, a highly stable thin-film state, and excellent heat resistance is provided as material for an organic electroluminescent device of high efficiency and high durability, and the organic electroluminescent device of high efficiency and high durability is provided using this compound. The compound of a general formula (Chemical Formula 1) having a substituted acridan ring structure is used as a constituent material of at least one organic layer in the organic electroluminescent device that includes a pair of electrodes and one or more organic layers sandwiched between the pair of electrodes.
Owner:HODOGOYA CHEMICAL CO LTD
Method for preparing ticagrelor midbody (1R,2S)-2-(2,3-difluorophenyl) cyclopropylamine
ActiveCN104326922AReduce dosageHigh yieldPreparation by rearrangement reactionsOrganic synthesisTicagrelor
The invention discloses a method for preparing ticagrelor midbody (1R,2S)-2-(2,3-difluorophenyl) cyclopropylamine, belonging to the technical fields of organic synthesis route design and preparation of raw material medicines and midbodies. The method comprises the following steps: performing alcoholysis on succinic anhydride, thereby obtaining mono-methyl succinate, performing acylating chlorination reaction on mono-methyl succinate, thereby obtaining a compound methyl 4-chloro-4-oxobutyrate, performing Fridel-Crafts reaction on methyl 4-chloro-4-oxobutyrate and o-difluorobenzene, thereby obtaining a compound methyl 4-ketone-4-(3,4-difluorophenyl) butyrate (IV), and further performing asymmetric reduction reaction, cyclization reaction and Hoffman degradation on the compound IV, thereby obtaining the compound (1R,2S)-2-(2,3-difluorophenyl) cyclopropylamine. Initial raw materials used in the method are low in cost and easy to obtain, the reaction condition is gentle, the operation is safe, simple and convenient, the environment pollution is small, and the key ticagrelor midbody prepared by using the method is simple and convenient in after treatment, and is beneficial to on-scale production.
Owner:CHENGDU BAIYU PHARMA CO LTD
Method for preparing (1R,2S)-2-(3,4-difluorophenyl) -cyclopropylamine
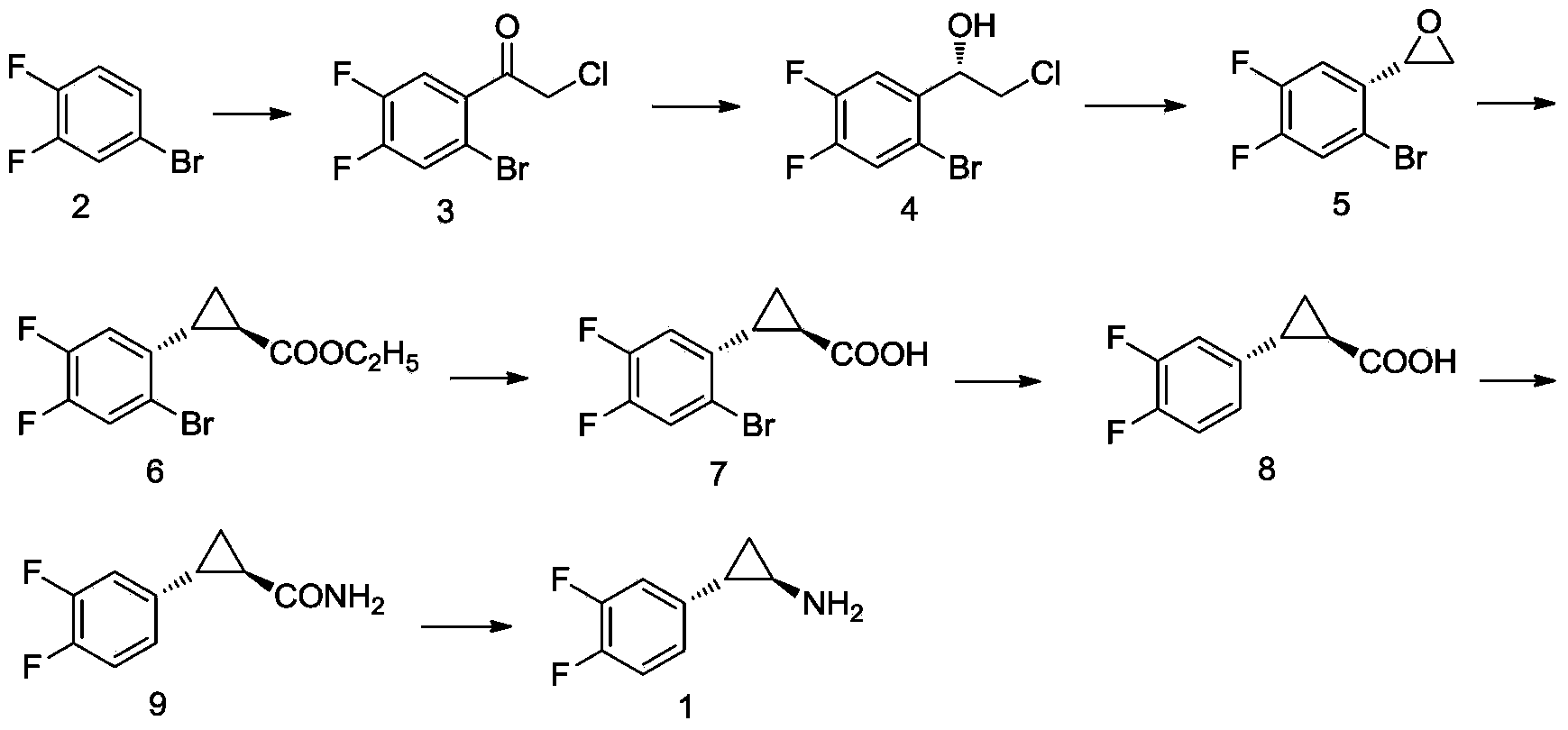
InactiveCN104341310ALow priceSimple stepsPreparation by rearrangement reactionsCombinatorial chemistryCyclopropanation
The invention discloses a method for preparing (1R,2S)-2-(3,4-difluorophenyl)-cyclopropylamine. The method comprises the following steps: carrying out a Fuke acyl reaction on 3,4-difluorobromobenzene (compound 2) which serves as a raw material and chloroacetyl chloride under conditions of lewis acid and certain temperature to obtain a compound 3; carrying out stereoselective reduction to obtain a compound 4; carrying out cyclization, olefination, hydrolyzation and debromination to obtain a compound 8; and preparing amide, and carrying out Hoffmann elimination to obtain a target compound 1. According to the method, the traditional preparation process is improved, the (1R,2S)-2-(3,4-difluorophenyl)-cyclopropylamine can be effectively, simply and conveniently prepared, the total yield is relatively high, the stereoselection is relatively high, and the intermediate in each step is easily purified and stored. The reaction formula in the method is as shown in the specification.
Owner:SHANGHAI SUN SAIL PHARMA SCI & TECH CO LTD
Preparation method of ticagrelor intermediate
ActiveCN104744266AReduce pollutionReduced Possibility of ContaminationOxygen-containing compound preparationOrganic compound preparationPotassium borohydrideTicagrelor
The inventiondiscloses a preparation method of ticagrelor. The method comprises the following steps: (1) reducing a compound shown in a formula III in the presence of a proton source provided by sodium borohydride or potassium borohydride and diethyl aniline hydrochloride to obtain a compound shown in a formula IV; (2) reacting the compound IV in the presence of alkali to generate a compound VI; (3) hydrolyzing the compound VI without purification to generate a compound VII; (4) reacting the compound VII to generate acyl chloride, reacting the acyl chloride to generate formamide, thus obtaining a compound shown in a formula IX; and (5) carrying out Hofmann rearrangement on the compound IX to obtain a compound shown in a formula II. Regents used in the method are nontoxic, harmless, environmentally friendly and low in price; the used key reagents can be recycled. Therefore, the method is applicable to industrial production.
Owner:SHANGYU JINGXIN PHARMA +1
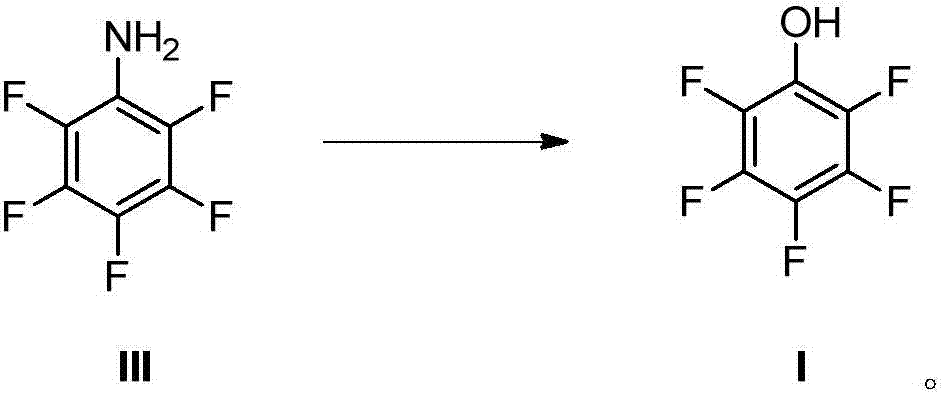
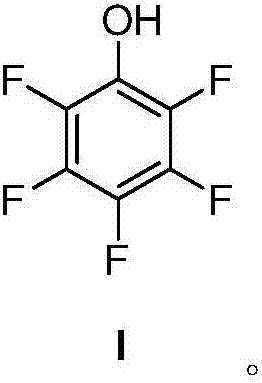
Preparation method for pentafluorophenol
ActiveCN106946659AOrganic compound preparationPreparation by rearrangement reactionsNitrosoOrganic synthesis
The invention relates to the field of organic synthesis and especially relates to a preparation method for pentafluorophenol. The preparation method comprises the steps of: 1) a Hoffmann rearrangement reaction: performing the rearrangement reaction to the compound (II) in the presence of alkali and a halogenation reagent to prepare the compound (III); 2) a diazo-hydrolysis reaction: performing a diazotization reaction to the compound (III) with a nitroso compound and performing a hydrolysis reaction in the presence of a catalyst to prepare the compound (I). The raw materials in the method are easy to obtain. The preparation method is short in synthesis route and is mild in reaction conditions, is simple in purification of the product, has high product purity and stable product quality, is low in cost of the whole synthesis route and is suitable for industrial large-scale promotion and application.
Owner:SHANGHAI CHEMSPEC CORP +1
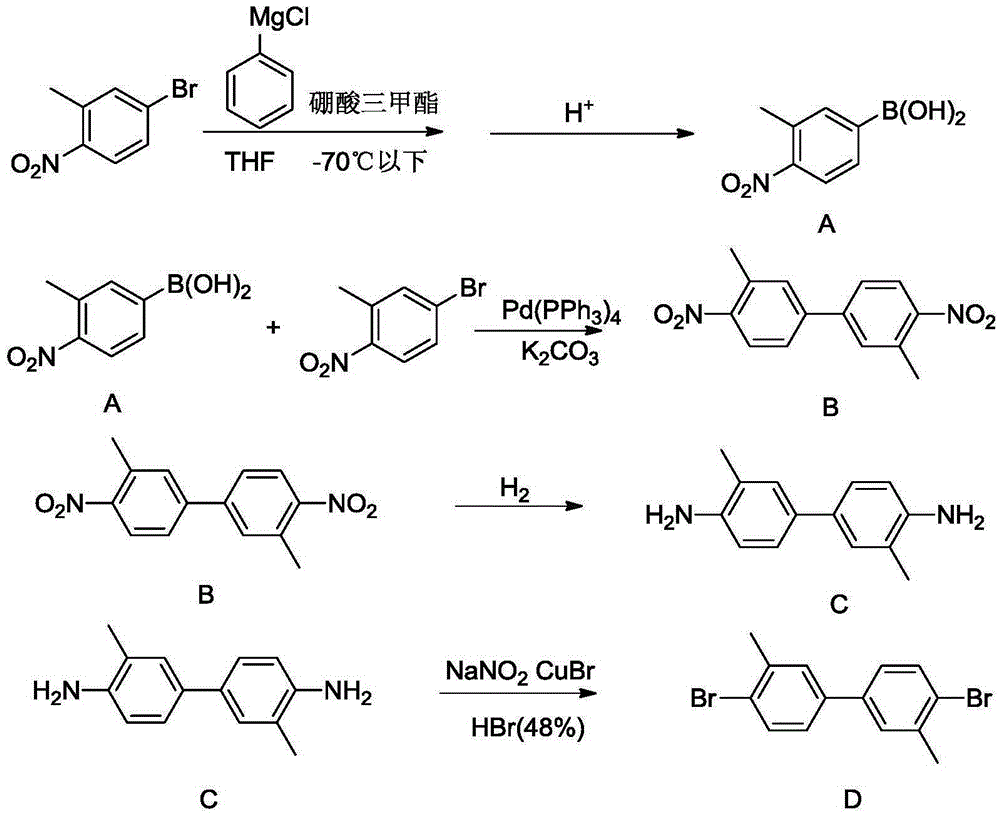
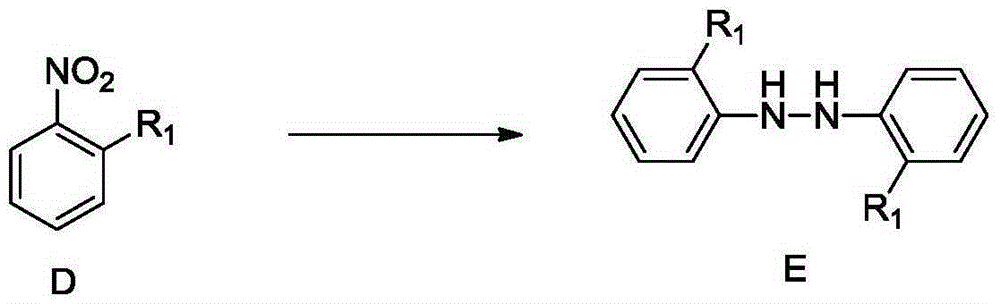
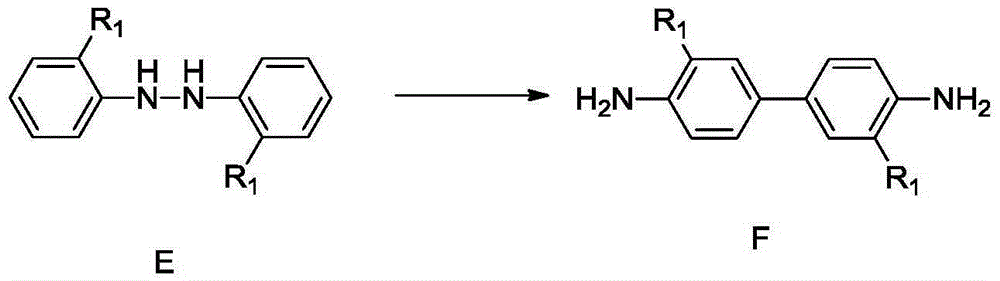
Synthetic method for 4,4'-dihalogenated-3,3'-dialkyl(alkoxyl) biphenyl compounds
InactiveCN105348038AMild reaction conditionsEasy to implementHydrazine preparationOrganic compound preparationBenzeneHydrazobenzene
The invention relates to a synthetic method for 4,4'-dihalogenated-3,3'-dialkyl(alkoxyl) biphenyl compounds. The method employs o-nitro alkyl (alkoxyl) benzene as an initial raw material, 2,2'-dialkyl(alkoxyl) hydrazobenzene is prepared through catalysis hydrogenation in an alkaline environment, hydrochloric acid acidifying rearrangement is carried out, 4,4'-diamino-3,3'-dialkyl(alkoxyl) biphenyl is prepared, finally, a diazotization reaction is carried out and 4,4'-dihalogenated-3,3'-dialkyl(alkoxyl) biphenyl compounds are prepared. The synthetic method is advantaged by cheap and easily available raw materials, mild reaction conditions, simple operation, high safety coefficient, high yield and low cost.
Owner:烟台九目化学股份有限公司
Preparation method of pentafluoroaniline
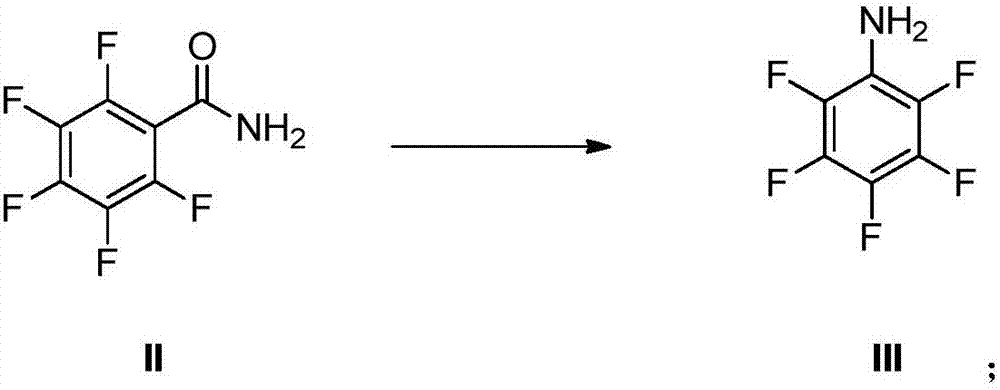
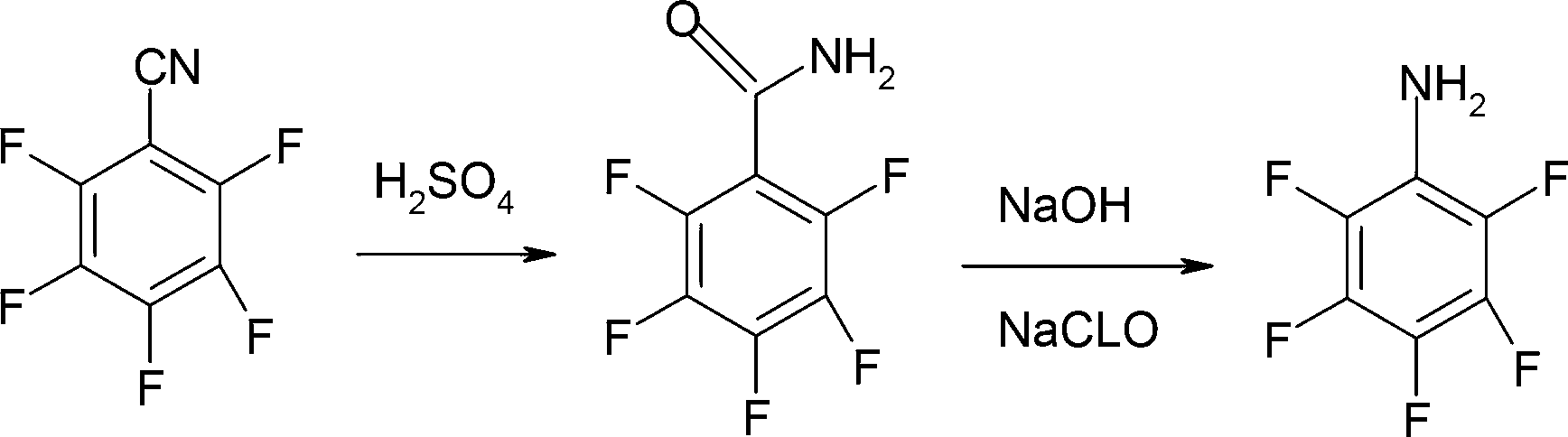
ActiveCN103012162AOvercoming not easy to getOvercome high pricesPreparation by rearrangement reactionsPentafluoroanilineSteam distillation
The invention provides a preparation method of pentafluoroaniline. The preparation method is characterized by comprising the following steps of: (1) adding pentafluorobenzonitrile into sulfuric acid with the concentration of 70-98% for hydrolysis, controlling the hydrolysis temperature to be 70-120 DEG C and the reaction time to be 3-5 hours, and then adding the product into water to separate out, and filtering to obtain pentafluorobenzamide; and (2) mixing the pentafluorobenzamide prepared by step (1) with alkali solution and halogen or hypohalite, preserving the heat for 4-6 hours at -10 to 20 DEG C and then heating up to 70-110 DEG C to carry out Hoffman degradation, controlling the reaction time to be 0.5-3 hours, and then performing steam distillation to obtain the pentafluoroaniline. The method provided by the invention has the advantages that the raw materials are easily available, the process is simple, the operation is convenient, the product purity is high (99% or higher), the yield is high (as high as over 80%), the cost is low, and the method is safe and efficient with energy conservation, consumption reduction and little discharge of three wastes, so the method provided by the invention is suitable for industrial production on a large scale.
Owner:FUXIN RUIGUANG FLUORINE CHEM
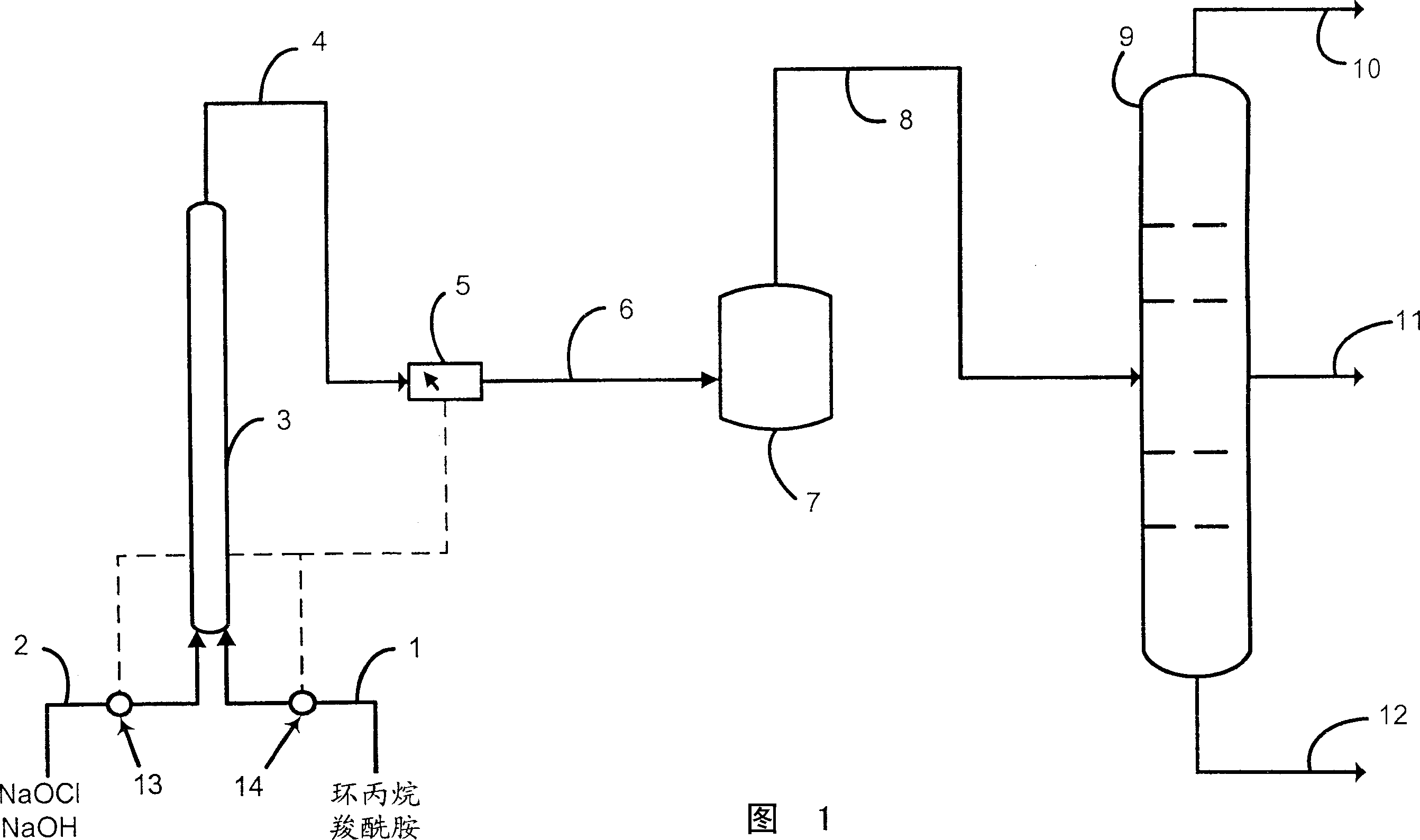
Continuous prepn. of amine
InactiveCN1498884AHigh yieldHigh selectivityOrganic compound preparationPreparation by rearrangement reactionsDistillationReaction zone
Disclosed is a process for the preparation of amines by continuously feeding a carboxamide, aqueous alkaline hypohalite, and aqueous alkaline hydroxide to a first reaction zone to form a N-halocarboxamide, measuring the concentration of at least one reaction component in the effluent from the first reaction zone, and using the result of that measurement to control the feed rate of at least one of the feed components of to achieve at least 90% conversion of the carboxamide in the first reaction zone. The effluent from the first reaction zone is fed continuously to a second reaction zone where it further reacts to form an aqueous solution of an amine. The effluent from the second reaction zone may be fed continuously to a distillation column. The process is particularly useful for the preparation of cyclopropylamine.
Owner:EASTMAN CHEM CO
Transamination of nitrogen-containing compounds to high molecular weight polyalkyleneamines
ActiveUS20130225864A1High molecular weightEasy to customizeOrganic compound preparationAmino compound preparation by disproportionationHigh carbonNitrogen
A process for preparing high molecular weight acyclic polyamines comprising providing a reaction mixture that includes at least a first component comprising a first organic, nitrogen-containing compound that contains at least two non-tertiary amine groups separated from one another by a ternary or higher carbon atom spacing that can be transaminated in the presence of a hydrogenation / dehydrogenation catalyst to form a mixture of higher molecular weight, acyclic polyamines while minimizing the formation of cyclic polyamines.
Owner:DOW GLOBAL TECH LLC
Method for preparing phenylaniline
ActiveCN102070465AGuaranteed catalytic efficiencyLow costPreparation by rearrangement reactionsNickel catalystHydrogen
The invention provides a method for preparing phenylaniline, which comprises the following steps: mixing cyanophenyl shown in the formula II, first alkaline compounds, nickel catalysts and borophenylic acid shown in the formula III or borophenylic esters shown in the formula VI to carry out suzuki coupling reaction for obtaining cyanobiphenyl shown in the formula IV; mixing the cyanobiphenyl, second alkaline compounds and oxyful, and hydrolyzing the cyanobiphenyl to obtain amido biphenyl shown in the formula V; and mixing the amido biphenyl, third alkaline compounds and sodium hypohalite to generate Hofmann degradation reaction for obtaining phenylaniline shown in the formula I, wherein R1 is chlorine, bromine, iodine, sulphonic acid ester radicals, carbonic ether radicals or alkyl ester radicals; R2 is alkyl, alkoxy, cyano, amido or hydrogen; R3 is chlorine, bromine or iodine; and R4 is alkyl, alkoxy, cyano, amido or hydrogen. The preparing method provided by the invention has the advantages of mild condition, low cost, cleanness and environment protection.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Preparation method of p-phenylenediamine
ActiveCN102993026AReduce consumptionSolve environmental problemsPreparation by rearrangement reactionsPolyesterSolvent
The invention discloses a preparation method of p-phenylenediamine, which comprises the following steps of: 1) ammonolysis: crushing waste polyester drink bottles, and mixing the crushed polyester drink bottle with ethylene glycol and liquid ammonia and stirring for reaction to obtain terephthalamide; 2) chlorination: reacting terephthalamide to react with chlorine to obtain a chloride; and 3) hofmann degradation: heating the water solution of chloride product NaOH or KOH, and carrying out the hofmann degradation to obtain p-phenylenediamine. In the ammonolysis and chlorination, mother liquor is applied, so that the yield is increased, and the consumption of raw material is reduced. In the degradation process, brown waste water contains more p-phenylenediamine. The p-phenylenediamine is extracted from the waste water by a solvent, so that the yield is increased, and the pollution control cost is reduced. The p-phenylenediamine is prepared in a way of waste ester regeneration, so that the problem of waste treatment in a environment-friendly manner is solved; furthermore, the content of isomer in the polyester is very low, so that the produced p-phenylenediamine contains little isomer, and the product is high in quality.
Owner:江苏万盛大伟化学有限公司
Process for producing antiaging agent, vulcanization accelerator or modified natural rubber by means of microorganism or plant
InactiveUS20090306431A1Process environmental protectionDecrease of petroleum resourcesBacteriaOrganic compound preparationBenzoic acidVulcanization
An object of the present invention is to provide processes for producing an antiaging agent, a vulcanization accelerator and a modified natural rubber, which are environmentally friendly and capable of making provision against a decrease of petroleum resources in the future. An antiaging agent, a vulcanization accelerator or a modified natural rubber is produced by a method comprising: converting glucose into benzoic acid or a benzoic acid derivative by a microorganism or extracting benzoic acid or a benzoic acid derivative from a plant; and converting the obtained benzoic acid or benzoic acid derivative into aniline or an aniline derivative.
Owner:RES INST OF INNOVATIVE TECH FOR THE EARTH +1
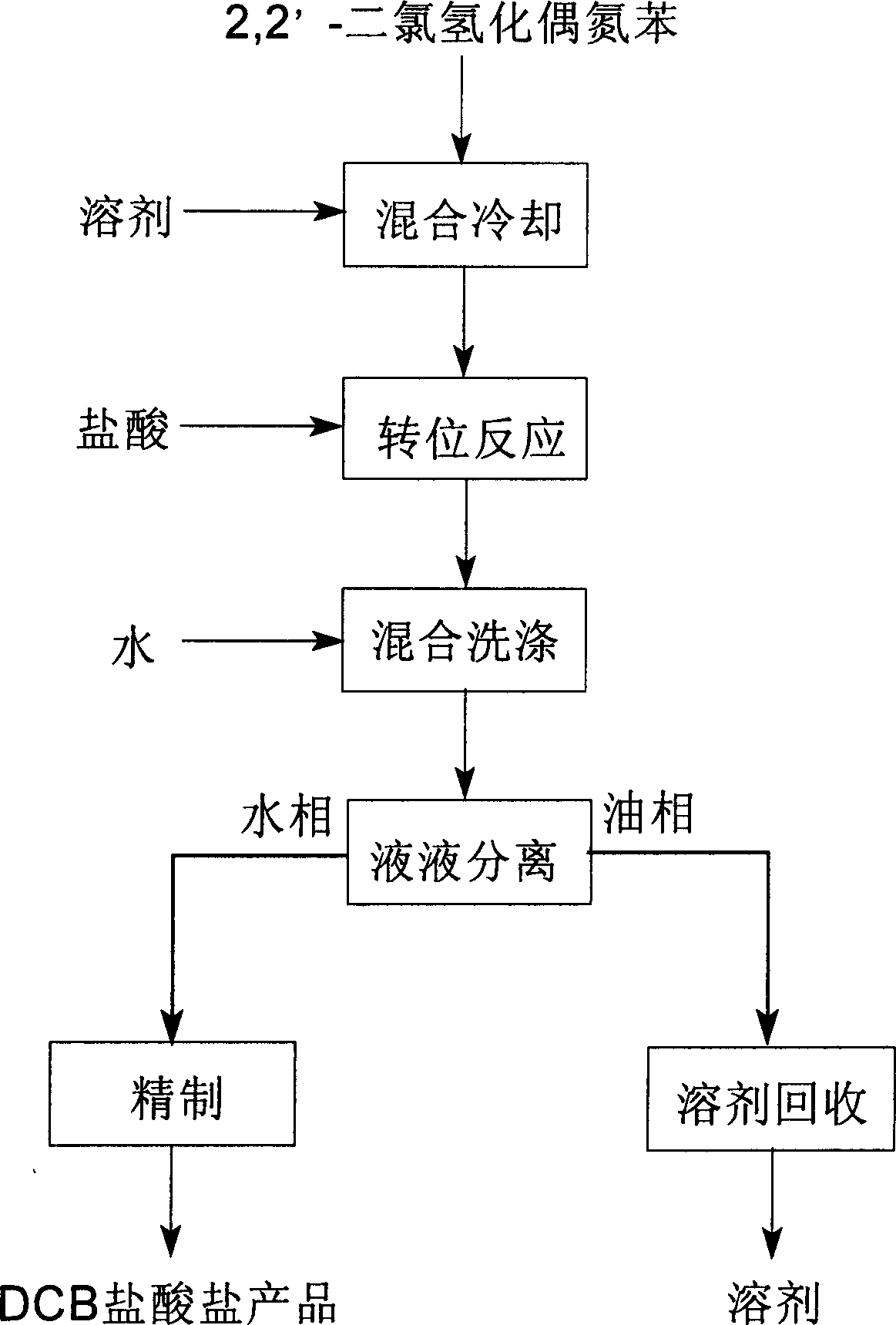
Tech. of preparing 3,3-dichlorobenzidine hydrochloride
InactiveCN1861568ALow viscosityIncrease productivityPreparation by rearrangement reactionsOil phaseSolvent
A process for preparing 3,3'-dichlorobiphenylamine hydrochloride includes such steps as mixing 2,2'-azobenzene dichlorohydride with arylhydrocarbon solvent, cooling, adding the solution to cooled solution of hydrochloric acid, translocation reaction, mixing the resultant with water or dilute solution of hydrochloric acid, washing, liquid-liquid separation to obtain lower-layer water phase 3,3'-dichlorobiphenylamine hydrochloride and upper-layer oil phase arylhydrocarbon solution, separating said water phase and refining.
Owner:CHANGZHOU JIASEN CHEM +1
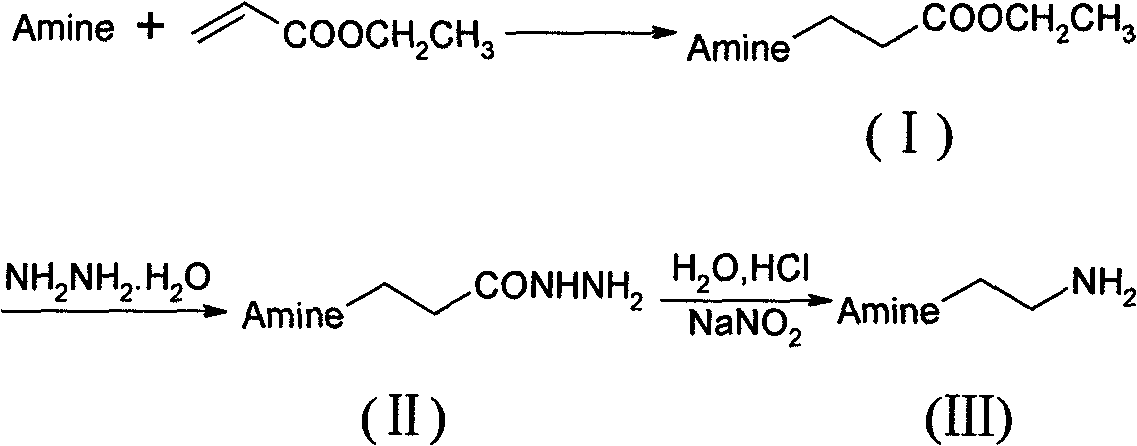
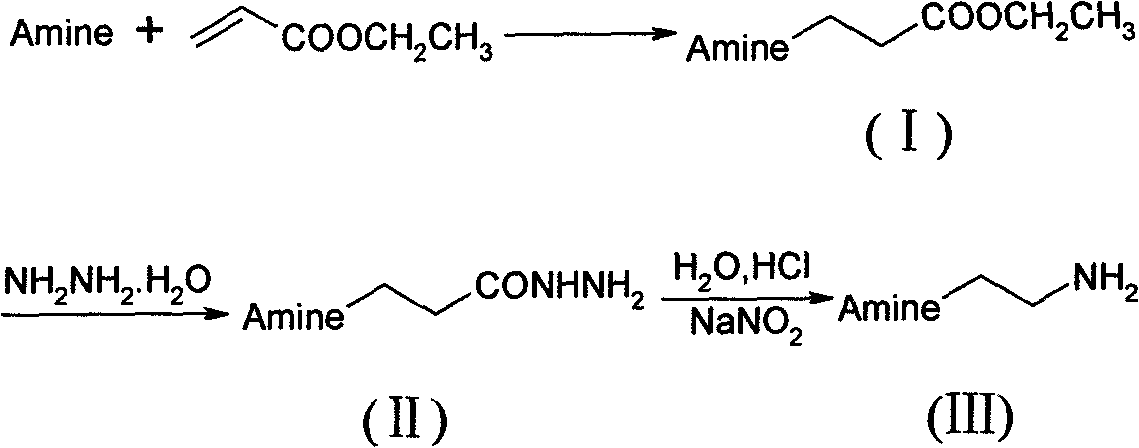
Preparation method of N-substituted ethylene diamine derivative
InactiveCN101613246AHigh yieldSmooth responseAmino group formation/introductionPreparation by rearrangement reactionsFatty amineCurtius rearrangement
The invention discloses a synthetic method of an N-substituted ethylene diamine derivative as structure fragments for various drugs. The method comprises the following steps: first performing Michael addition reaction on aromatic amine or fatty amine or heterocyclic amine and ethyl acrylate, then performing hydrazinolysis, and finally performing Curtius rearrangement reaction, thus preparing the target compound. The method has the advantages of low cost, moderate conditions, easy operation, high total yield and the like.
Owner:HEFEI UNIV OF TECH
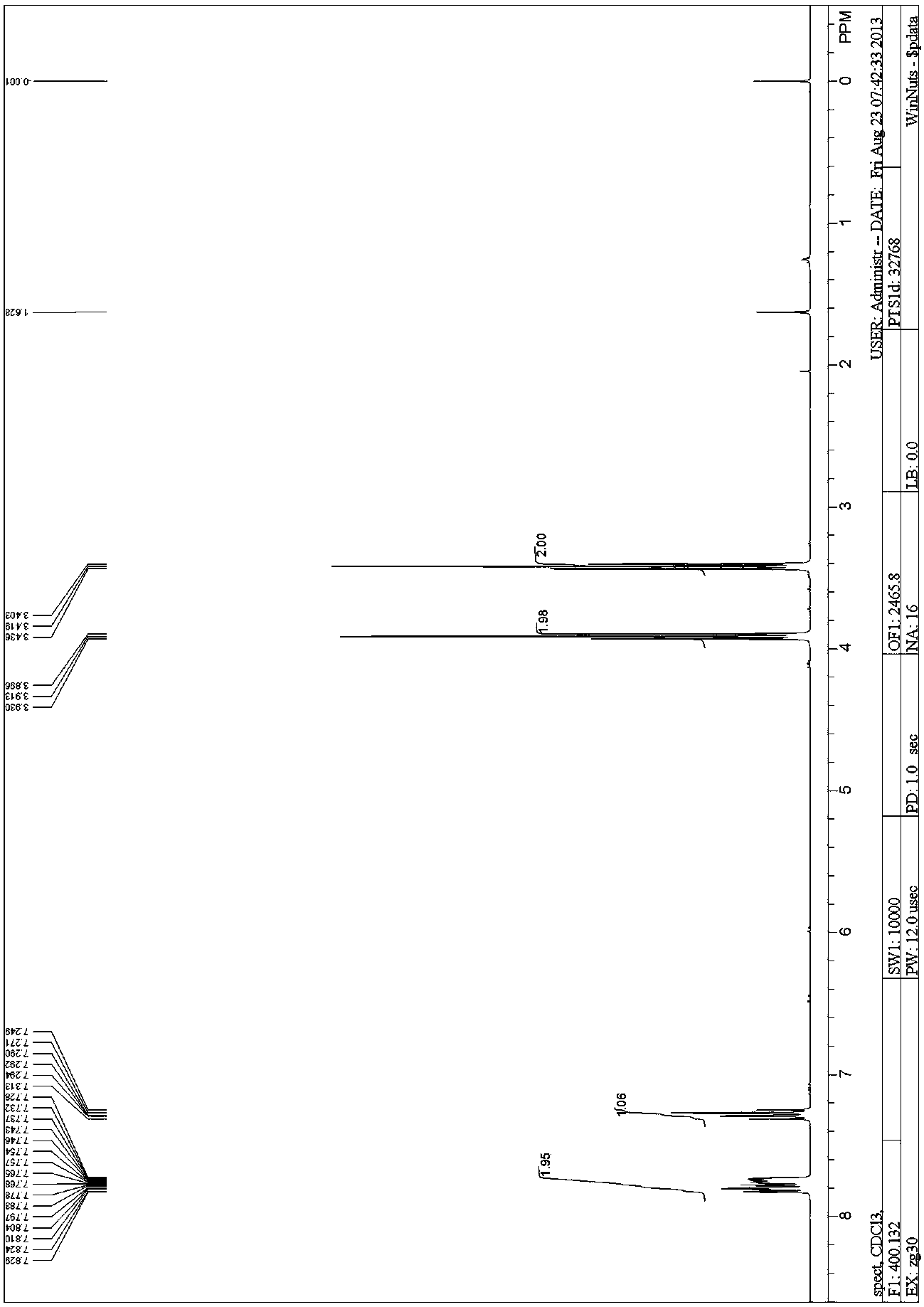
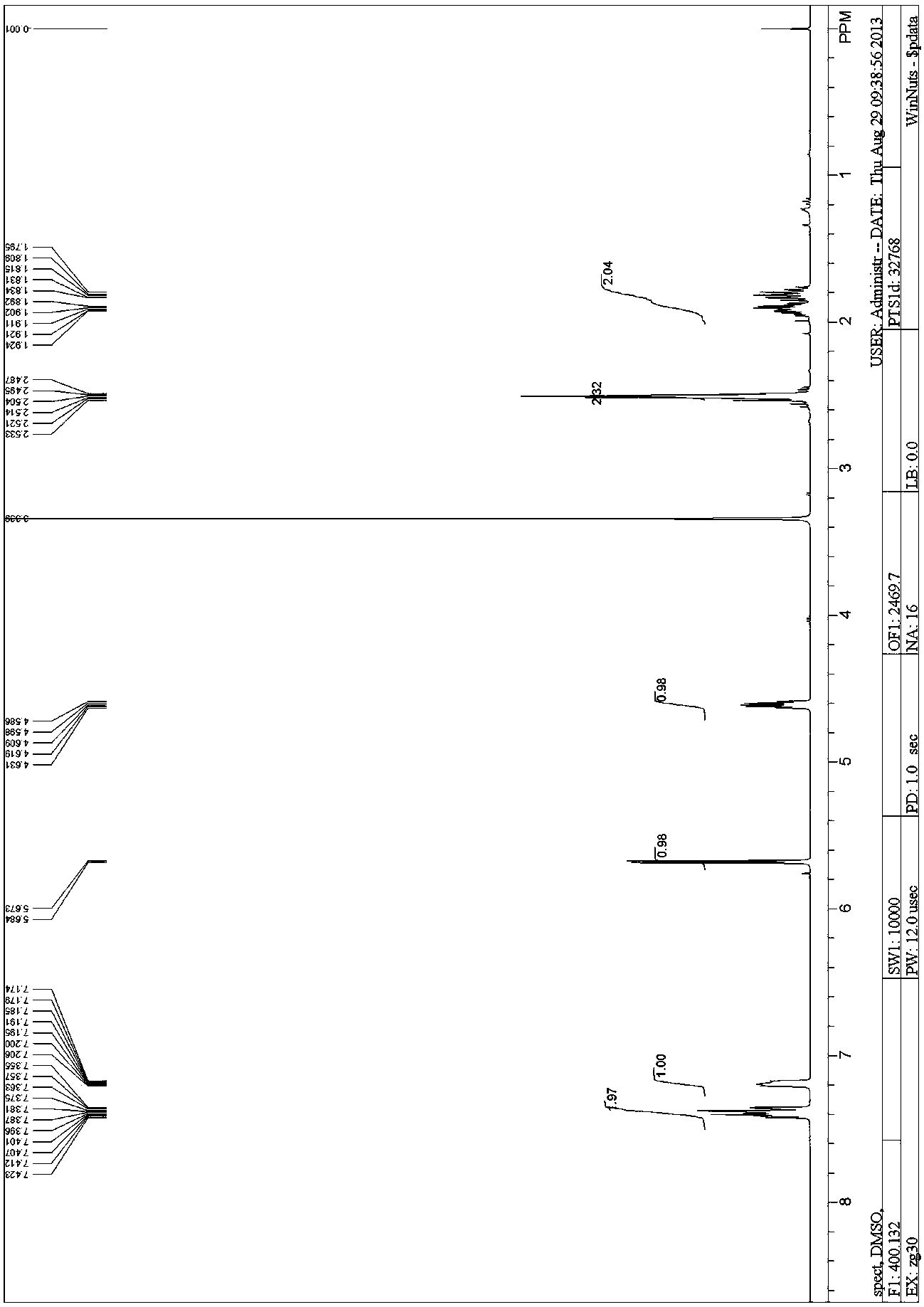
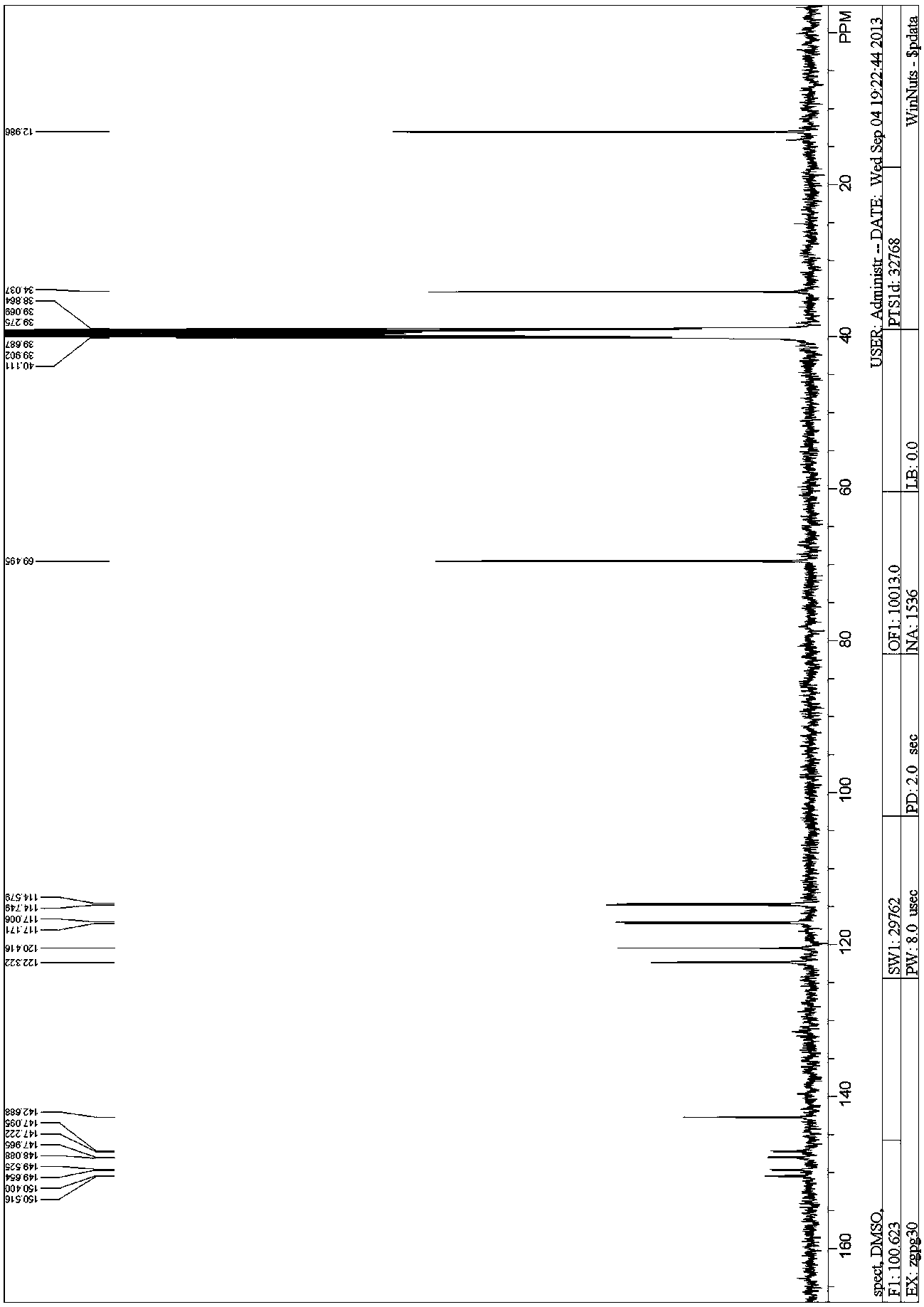
Adamantane amine derivative as well as preparation method and application of derivative
InactiveCN104693039AFew reaction stepsMild reaction conditionsNervous disorderOrganic compound preparationPtru catalystFormate
The invention provides a preparation method of an adamantane amine derivative. The preparation method comprises the following steps: performing an amidation reaction on an adamantane formic acid compound to generate an adamantane amide formate compound, and then reacting the adamantane amide formate compound with a heterocyclic aromatic halogenated substance, an aryl halogenated substance, an aliphatic alkyl halogenated substance, a halogen molecular, a metal or metalloid reagent or a high-valence iodide in the presence of additives under the catalytic action of a transitional metal catalyst, thereby generating an adamantane formamide C2 derivatization product; next, performing a hydrolysis reaction on the adamantane formamide C2 derivatization product to obtain an adamantane carboxylic acid derivative; and finally, performing a rearrangement reaction on the adamantane carboxylic acid derivative, thereby obtaining the adamantane amine derivative. The preparation method is capable of importing groups of rich types to two C atoms of the adamantane and also capable of constructing adamantane amine molecules diversified in structure on the two C atoms.
Owner:SUN YAT SEN UNIV
Popular searches
Amino-hyroxy compound preparation Carboxylic acid amides preparation Preparation from nitriles Carboxylic acid salt preparation Fermentation Organic chemistry methods Hydroxy compound preparation Carbonyl compound preparation by condensation Chemical recycling Amino compound preparation by condensation/addition reactions
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com