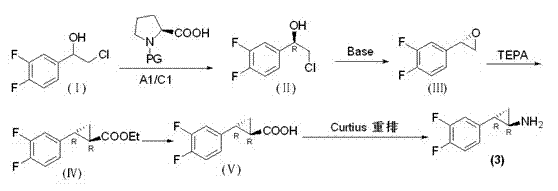
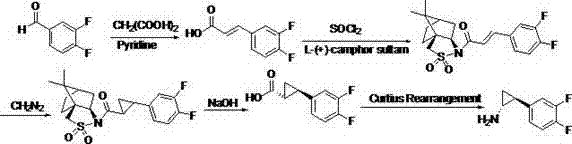
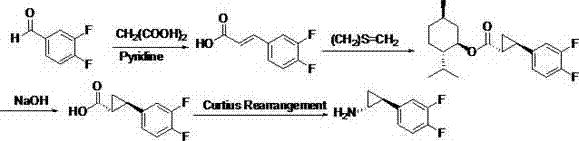
Preparation method of trans-(1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine
A technology of difluorophenyl and cyclopropylamine, applied in the field of preparation of intermediates, can solve the problems of many synthetic process routes, difficult industrialized production, cumbersome reaction conditions, etc., and achieves wide source of raw materials, easy industrialized production, and reaction conditions. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine
[0052] step 1:( R )-Preparation of chlorophenylethanol (Ⅱ)
[0053] 20 g racemic chlorophenylethanol (I) and 0.6 equivalent of Boc- R - Proline was dissolved in dichloromethane, 15 g EDCI and 2 g DMAP were sequentially added, the mixture was stirred at room temperature for 4-12 h, and detected by TLC. Boc- R -Proline disappeared, quenched the reaction with water, extracted, dried, and passed through HCl (g), TLC detected that the raw materials were completely reacted, concentrated and then column chromatographed to obtain 8 g of solids, with a yield of 40%.
[0054] Step 2: Preparation of epoxy compound (Ⅲ)
[0055] at 0 o C, Chlorohydrin (6.60g, 34.2mmol) was added to THF and 15% aqueous sodium hydroxide solution (1:1) mixture, stirred at room temperature for 1.5 hours. The reaction was monitored by TLC. After the reaction was completed, ethyl acetate was added, and after extraction...
Embodiment 2
[0062] Example 2 Preparation of trans-(1R,2S)-2-(3,4-difluorophenyl)cyclopropylamine
[0063] step 1:( R )-Preparation of chlorophenylethanol (Ⅱ)
[0064] 20 g of racemic chlorophenethyl alcohol and 0.6 equivalent of Boc- R - Proline was dissolved in dichloromethane, 15 g EDCI and 2 g DMAP were sequentially added, the mixture was stirred at room temperature for 4-12 h, and detected by TLC. Boc- R - Proline disappeared, quenched the reaction with water, extracted, dried, and passed through HCl (g), TLC detected that the raw materials were completely reacted, concentrated and then column chromatographed to obtain 7 g of solids, with a yield of 35%.
[0065] Step 2: Preparation of epoxy compound (Ⅲ)
[0066] at 0 o C, Chlorohydrin (6.60g, 34.2mmol) was added to THF and 15% aqueous sodium hydroxide solution (1:1) mixture, stirred at room temperature for 1.5 hours. The reaction was monitored by TLC. After the reaction was completed, ethyl acetate was added, and after extr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com