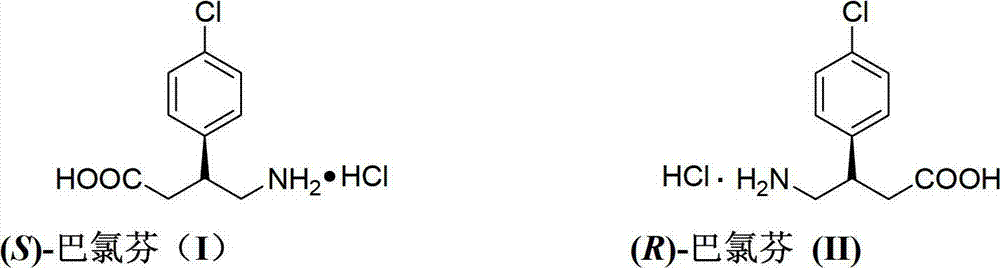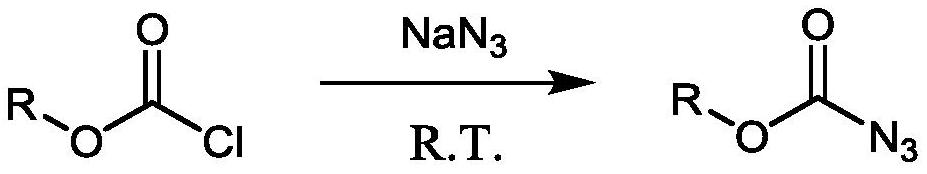Patents
Literature
57 results about "Curtius rearrangement" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Curtius rearrangement (or Curtius reaction or Curtius degradation), first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a variety of nucleophiles such as water, alcohols and amines, to yield a primary amine, carbamate or urea derivative respectively. Several reviews have been published.
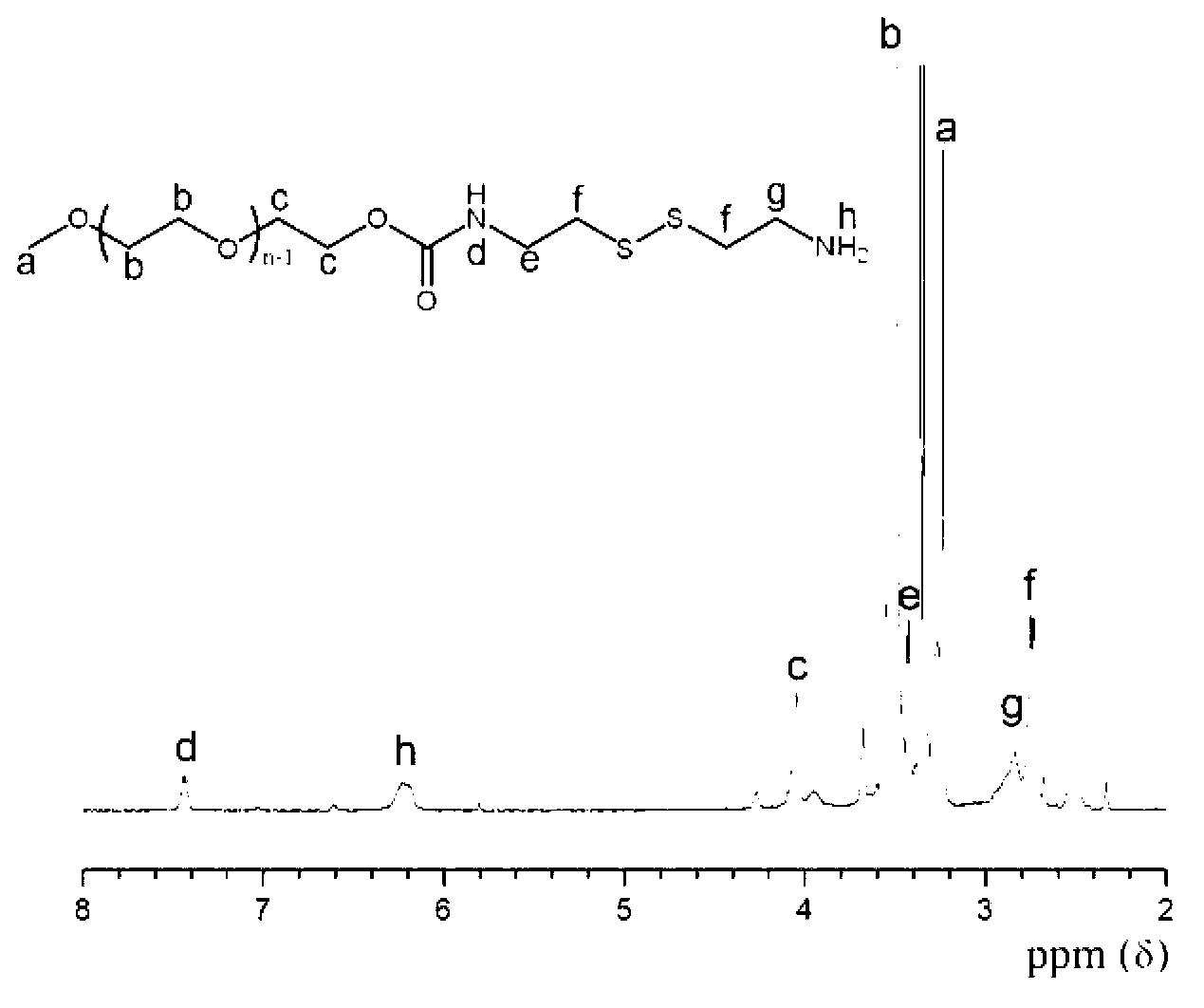
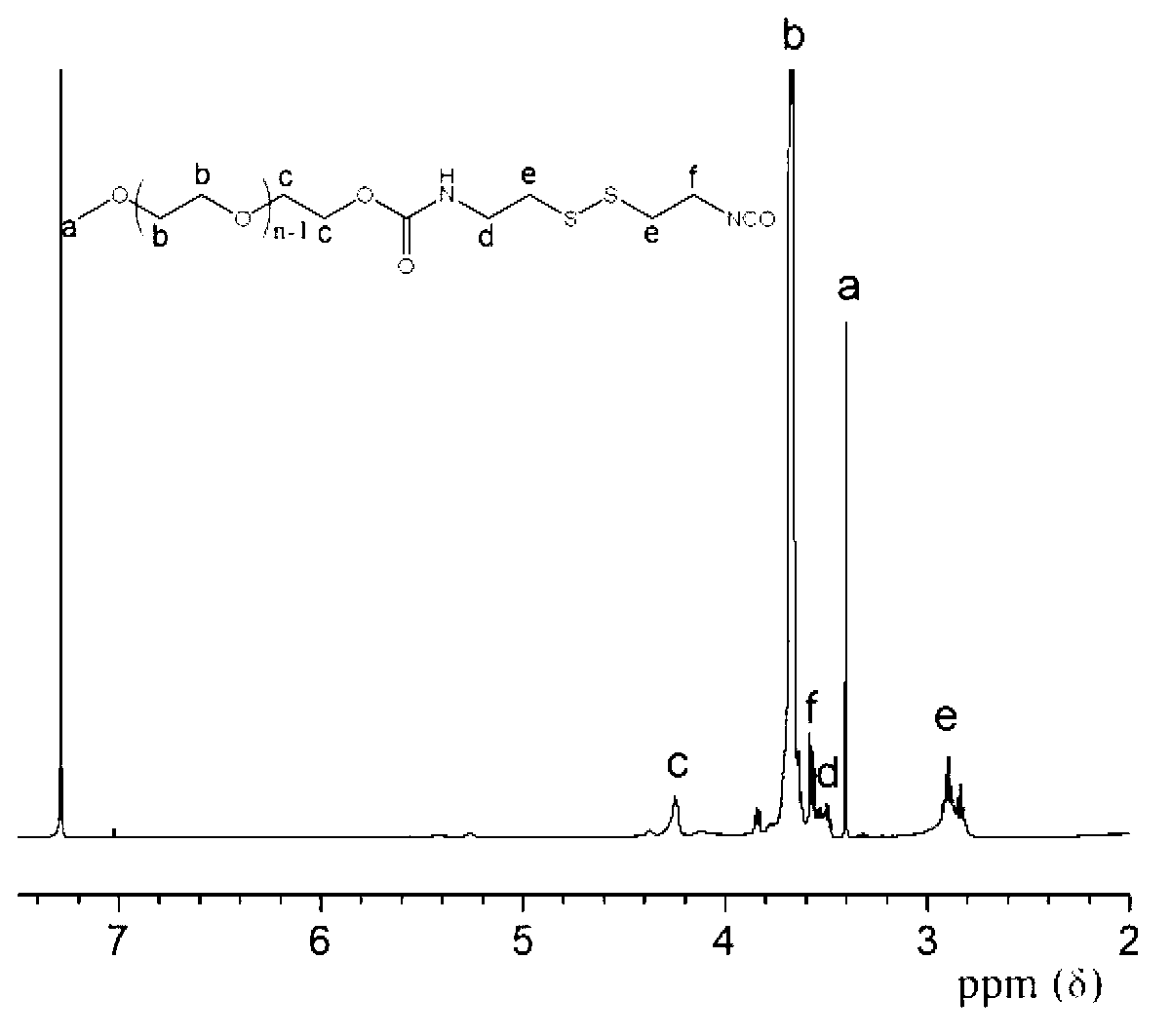
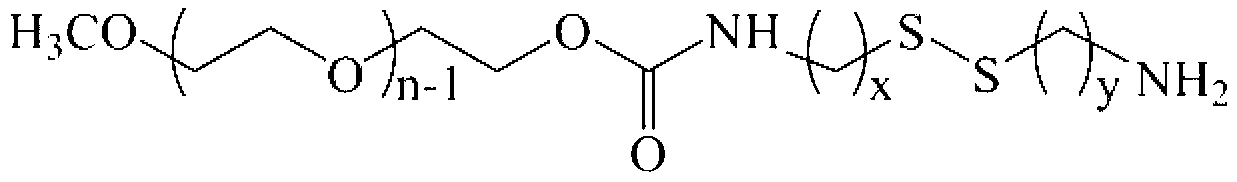
Bi-functional polyethylene glycol derivative and preparation method thereof
InactiveCN103319388AHigh reactivityRaw materials are easy to getPharmaceutical non-active ingredientsHydropoly/poly sulfide preparationPolyethylene glycolDrug release
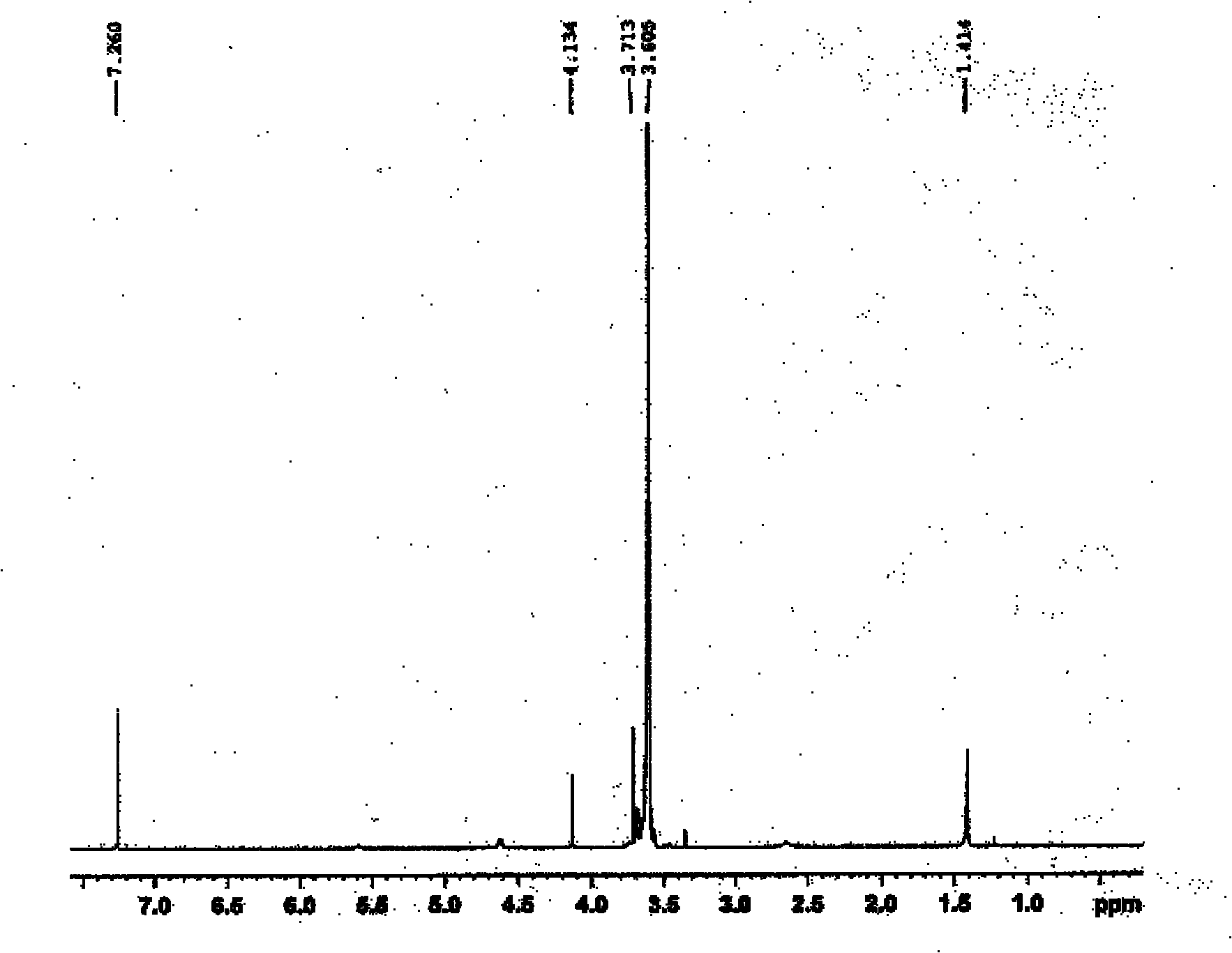
The invention relates to a bi-functional polyethylene glycol derivative and a preparation method thereof. The preparation method of the difunctional polyethylene glycol derivative comprises following steps: conducting esterification, hydrazinolysis and Curtius rearrangement in dibasic acid with disulfide bond at first to generate diisocyanate containing disulfide bond, then conducting a reaction of the diisocyanate and the polyethylene glycol to generate isocyanat-terminated polyethylene glycol and obtaining the bi-functional polyethylene glycol derivative through hydrolysis reaction of the resultant. In the invention, the preparation method has the advantages that raw materials can be gotten easily, reaction conditions are gentle, yield and product purity are high and the method is beneficial to mass production. Meanwhile, the bi-functional polyethylene glycol derivative is beneficial to both consequent reactions and controlled release of drugs and transfer of the drugs from endosome to cytoplasm. Application prospects of the bi-functional polyethylene glycol derivative are wide in surface finish of a nano-drug carrier, preparation of pro-drug and control of drug release speed etc.
Owner:XI AN JIAOTONG UNIV
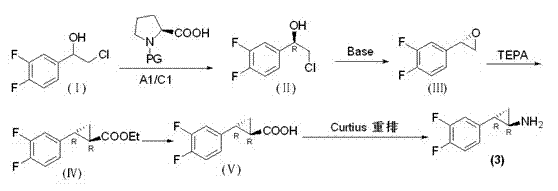
Preparation method of trans-(1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine
InactiveCN102775314AFew stepsEasy to operateHydroxy compound separation/purificationPreparation by rearrangement reactionsEpoxyPtru catalyst
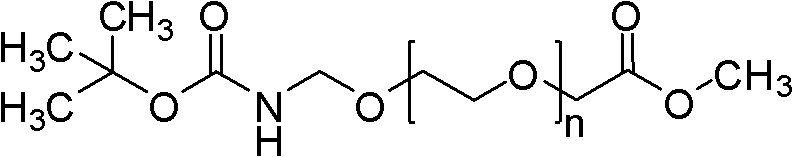
The invention provides a preparation method of trans-(1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine. The preparation method comprises the following steps: enabling racemic chloro phenethyl alcohol (I) and N-protection proline to undergo a reaction under the effects of a condensing agent A1 and a catalyst C1, and obtaining chiral chlorohydrin (II); enabling the chiral chlorohydrin (II) to generate an epoxy compound (III) under the conditions of alkalinity; enabling the epoxy compound (III) and TEPA to react and generate cyclopropyl ethyl formate (IV) under the conditions of alkalinity; removing ester from cyclopropyl ethyl formate (IV) under the conditions of alkalinity, and generating cyclopropanecarboxylic acid (V); and enabling cyclopropanecarboxylic acid (V) and azide to generate a target compound (3) by Curtius rearrangement. The preparation method has the advantages that the steps of a used synthetic process route are few, the operation is simple, and industrial production is achieved easily; a kinetic resolution method is utilized to synthesize chiral chlorohydrin (II) and is simple in reaction conditions and easy to operate; and a TEPA method is utilized synthesize the cyclopropyl ethyl formate, the product yield is high, cis-trans selectivity is good, and the purity is over 99%.
Owner:江苏富泽药业有限公司
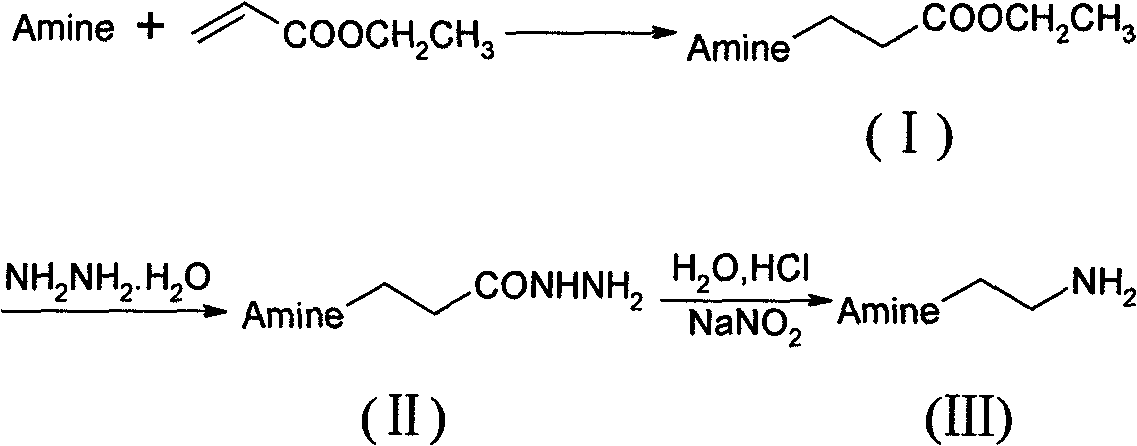
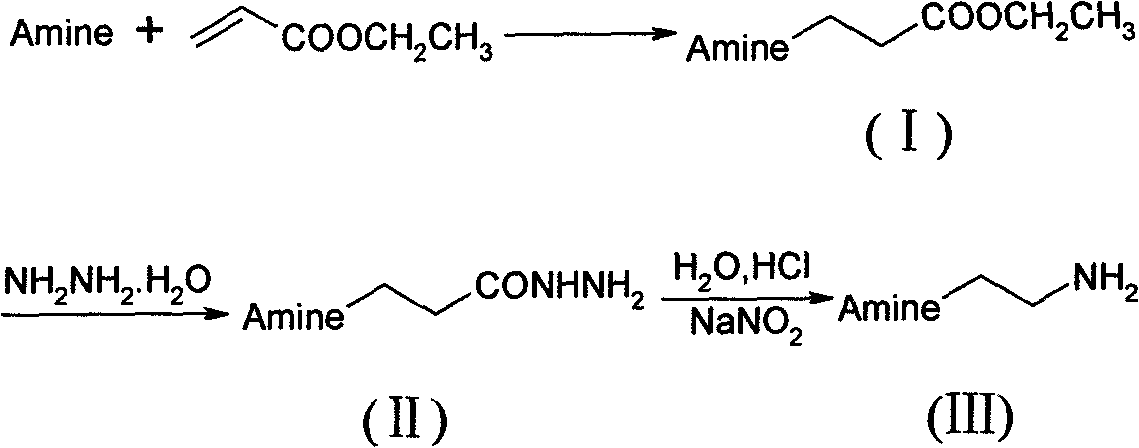
Preparation method of N-substituted ethylene diamine derivative
InactiveCN101613246AHigh yieldSmooth responseAmino group formation/introductionPreparation by rearrangement reactionsFatty amineCurtius rearrangement
The invention discloses a synthetic method of an N-substituted ethylene diamine derivative as structure fragments for various drugs. The method comprises the following steps: first performing Michael addition reaction on aromatic amine or fatty amine or heterocyclic amine and ethyl acrylate, then performing hydrazinolysis, and finally performing Curtius rearrangement reaction, thus preparing the target compound. The method has the advantages of low cost, moderate conditions, easy operation, high total yield and the like.
Owner:HEFEI UNIV OF TECH
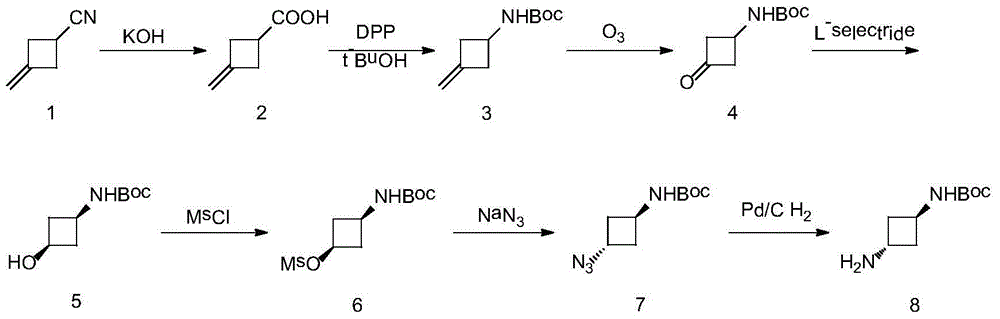
Preparation method of trans-N-Boc-1,3-cyclobutanediamine
InactiveCN104829492ASimple purification methodShort stepsCarbamic acid derivatives preparationOrganic compound preparationPurification methodsOxidative cleavage
The present invention is a preparation method of trans-N-Boc-1,3-cyclobutanediamine. The method uses easily available 3-methylene-cyclobutyl carbonitrile as a starting material, and conducts 7 steps of reaction including hydrolysis, Curtius rearrangement, oxicracking, reduction, methanesulfonyl chloride protection, substitution and hydrogenation. The invention has the technical characteristics of short reaction step, total yield of up to 49.8% and high purity of the final product (>99%) (the prior art comprises at least 8 steps, and the highest total yield is up to 19%); and N-Boc-1,3-cyclobutanediamine with transconfiguration is selectively synthesized. From the perspective of chemistry, the invention has the characteristics of few steps, simple reaction, high yield, feasible purification method of intermediate and simple operation, and has wide prospect in business application.
Owner:HEBEI UNIV OF TECH
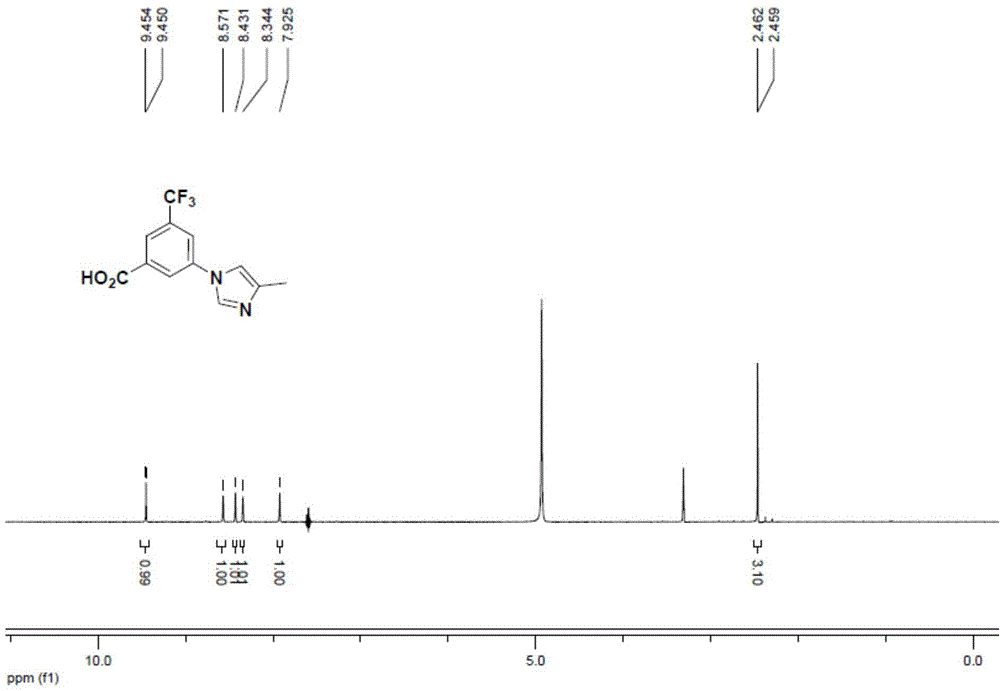
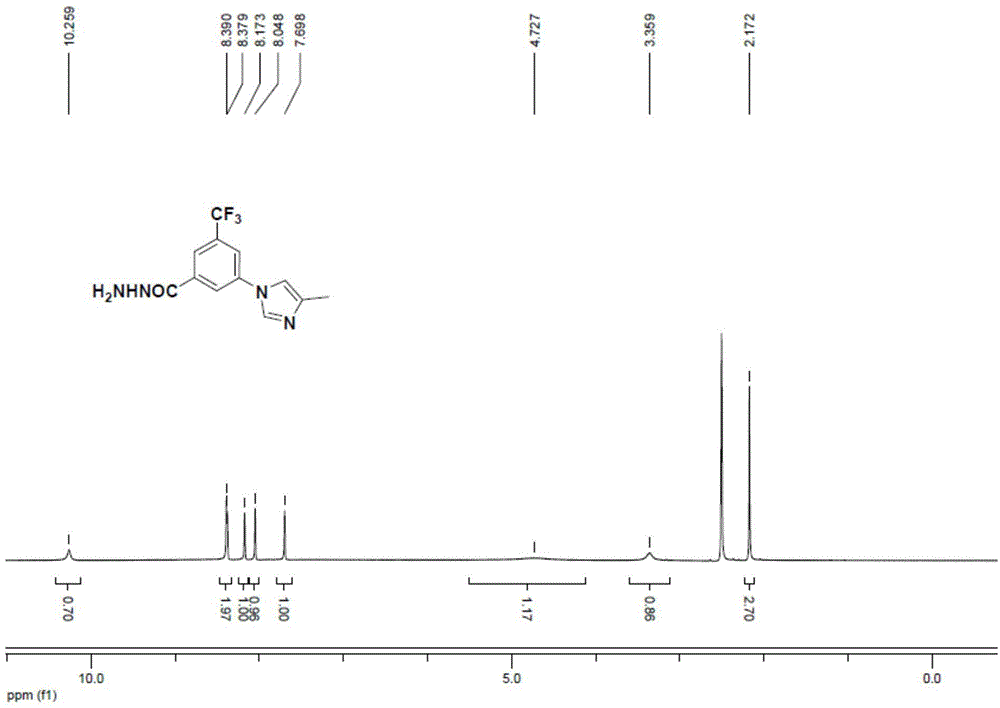
Preparation method for 3-(4-methyl-1H-imidazole-1-yl)-5-(trifluoromethyl)aniline
The invention provides a preparation method for 3-(4-methyl-1H-imidazole-1-yl)-5-(trifluoromethyl)aniline. The method includes: S1. subjecting a compound A and 4-methyl-1H-imidazole to coupling reaction to generate a coupling product; S2. carrying out hydrazinolysis reaction on the coupling product to generate a hydrazinolysis product; and S3. subjecting the hydrazinolysis product to diazotization and Curtius rearrangement reaction in order to generate 3-(4-methyl-1H-imidazole-1-yl)-5-(trifluoromethyl)aniline. Specifically, the compound A is 3-halogen-5-trifluoromethylbenzoic acid, a salt derivative of 3-halogen-5-trifluoromethylbenzoic acid or an ester derivative of 3-halogen-5-trifluoromethylbenzoic acid. The method for preparation of 3-(4-methyl-1H-imidazole-1-yl)-5-(trifluoromethyl)aniline provided by the invention has the characteristics of short synthesis route, low cost of raw materials, mild reaction conditions and high yield, thus being applicable to large-scale industrial production.
Owner:ASYMCHEM LAB TIANJIN +4
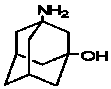
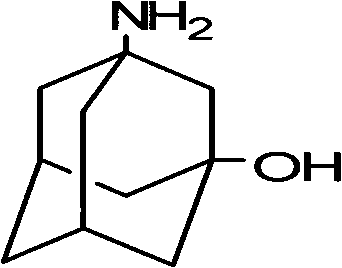
Method for synthesizing 3-amino-1-adamantanol
InactiveCN101747212AEasy to operateHigh yieldOrganic compound preparationAmino-hyroxy compound preparationBromateCurtius rearrangement
The invention discloses a method for synthesizing 3-amino-1-adamantanol. The structural formula of the 3-amino-1-adamantanol is as follows. Using adamantanecarboxylic acid as starting material, the method synthesizes the 3-amino-1-adamantanol according to the following reaction steps: bromate 3-amino-1-adamantanol is synthesized by way of bromination, modified curtius rearrangement and hydrolyzation, and finally, bromate is removed by a alkaline liquor treatment method, so that the 3-amino-1-adamantanol is obtained. The invention has the advantages of simple and easy operation, available materials, less reaction steps and high product yield.
Owner:GUANGDONG UNIV OF TECH
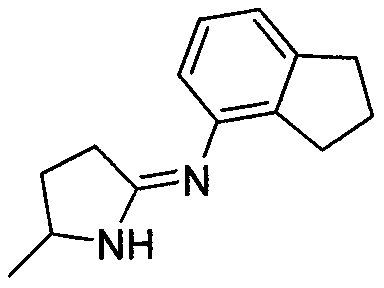
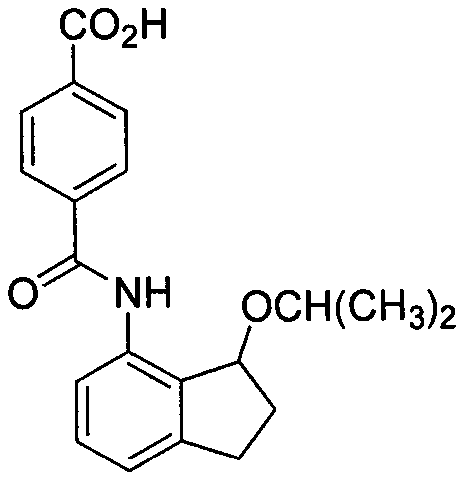
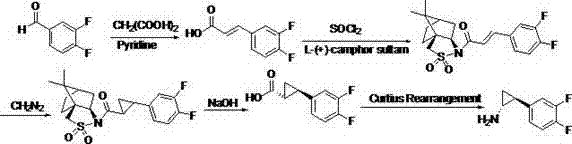
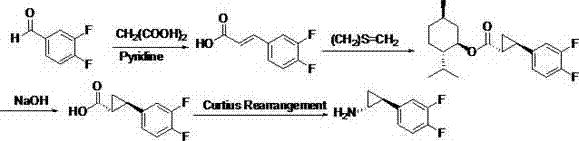
2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof
The invention belongs to the technical field of medicine and discloses a 2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative of a formula (I) as well as a preparation method thereof. The preparation method comprises the following steps: performing Witting reaction on II to obtain III, catalyzing cyclopropanation reaction of ethyl diazoacetate and the III by rhodium acetate to obtain IVand V, performing hydrolysis, performing Curtius rearrangement to obtain VI and VII; (1) removing Boc from the VI or VII and performing reduction and ammoniation to obtain a compound VIII or IX; or (2) performing substitution on the VI or VII and 2-chlorine-1-morpholine ethane-1-ketone to obtain X or XI; or (3) performing reduction and ammoniation on the VI or VII and (4-oxycyclohexyl)tert-butylcarbamate, and removing Boc to obtain a compound XII or XIII; or (4) performing Suzuki coupling on the VI or VII and substituted arylboronic acid, removing Boc to obtain XIV or XV, and performing reduction and ammoniation to obtain VIII or IX. The derivative of the formula (I) has high inhibition activity, has high selectivity on homologous enzyme such as monoamine oxidase and LSD2, and is expected to be developed into a medicine for treating diseases such as acute myelogenous leukemia.
Owner:EAST CHINA NORMAL UNIV +1
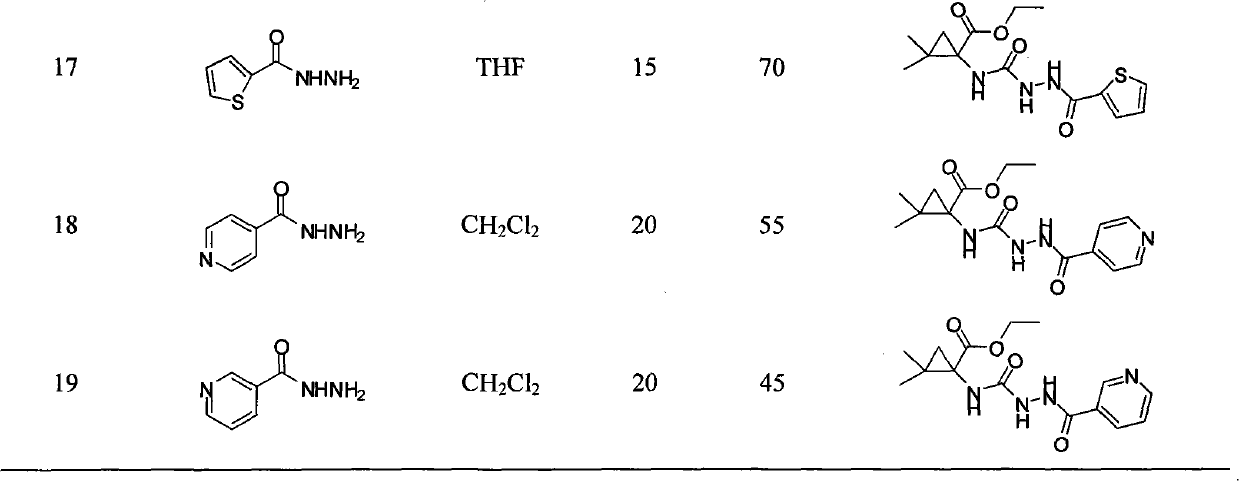
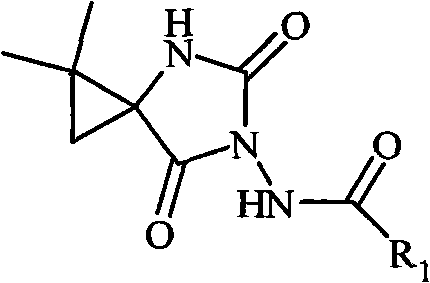
N-3-aramid-5-cyclopropane spiro hydantoin derivative, preparation method and application thereof
InactiveCN101863839AEasy to prepareEasy to manufactureOrganic active ingredientsNervous disorderEthyl chloroformateHydantoin derivatives
The invention relates to an N-3-aramid-5-cyclopropane spiro hydantoin derivative which has a structural formula described in the specification, wherein R1 is phenyl, substituted phenyl and heterocycle aryl. A preparation method comprises the steps of: reacting 1-carboxyl-2,2-dimethylcyclopropane carboxylic ethyl ester with ethyl chloroformate to generate 1-acid azide-2,2-dimethylcyclopropane carboxylic ethyl ester under the action of NaN3; carrying out Curtius rearrangement on the 1-acid azide-2,2-dimethylcyclopropane carboxylic ethyl ester to generate isocyanate, reacting the isocyanate with arylacethydrazide to obtain N'-aramid substituted carbamido cyclopropane; and then carrying out cyclization on the N'-aramid substituted carbamido cyclopropane under the alkaling condition to generate the N-3-aramid-5-cyclopropane spiro hydantoin derivative. The compound has relatively good convulsions resisting activity, simple preparation method and higher yield.
Owner:SYNCOM CHINA CO LTD WUHAN
Method for synthesizing 2-(7-(benzyloxy)benzo[d][1, 3]dioxazole-5-yl)ethylamine and hydrochloride thereof
The invention discloses a method for synthesizing 2-(7-(benzyloxy)benzo[d][1, 3]dioxazole-5-yl)ethylamine and a hydrochloride thereof. In the method, 7-methoxyl benzo[d][1, 3]dioxazole-5-formaldehyde is taken as the raw material, 3-(7-methoxyl benzo[d][1, 3]dioxazole-5-yl)propionic acid is synthesized by using a one-step method of reductive condensation and hydrolysis-decarboxylation, and a Curtius rearrangement is carried out to generate 3-(7-methoxyl benzo[d][1, 3]dioxazole-5-yl)ethylamine hydrochloride(5). The method has advantages of mild reaction conditions, simple operation, low cost and high yield.
Owner:南京正济医药销售有限公司 +1
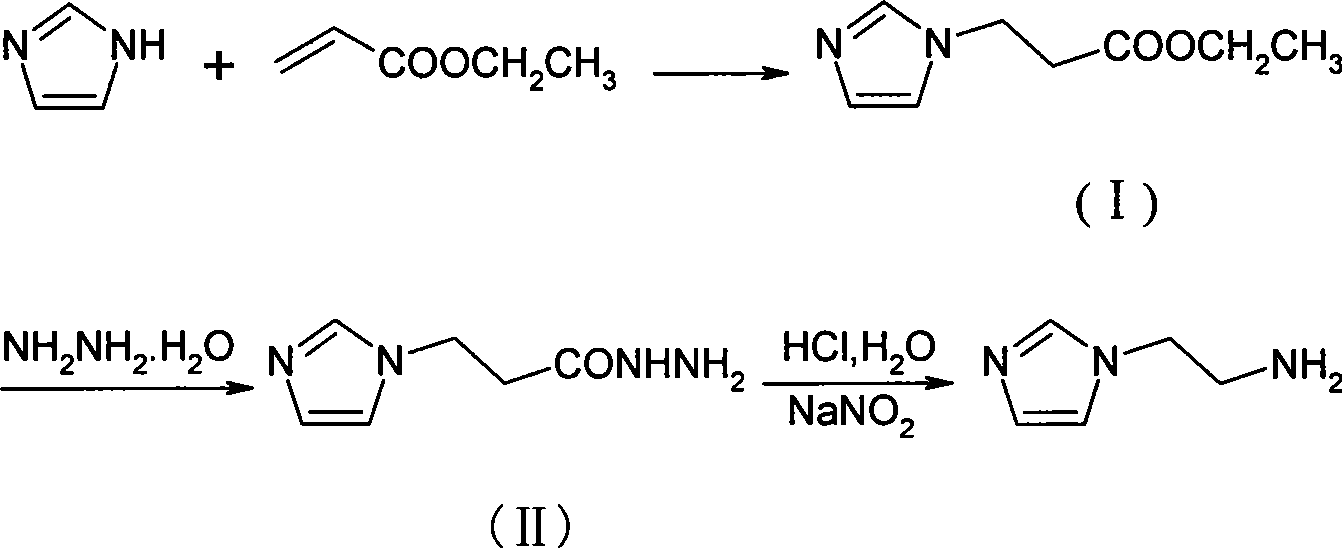
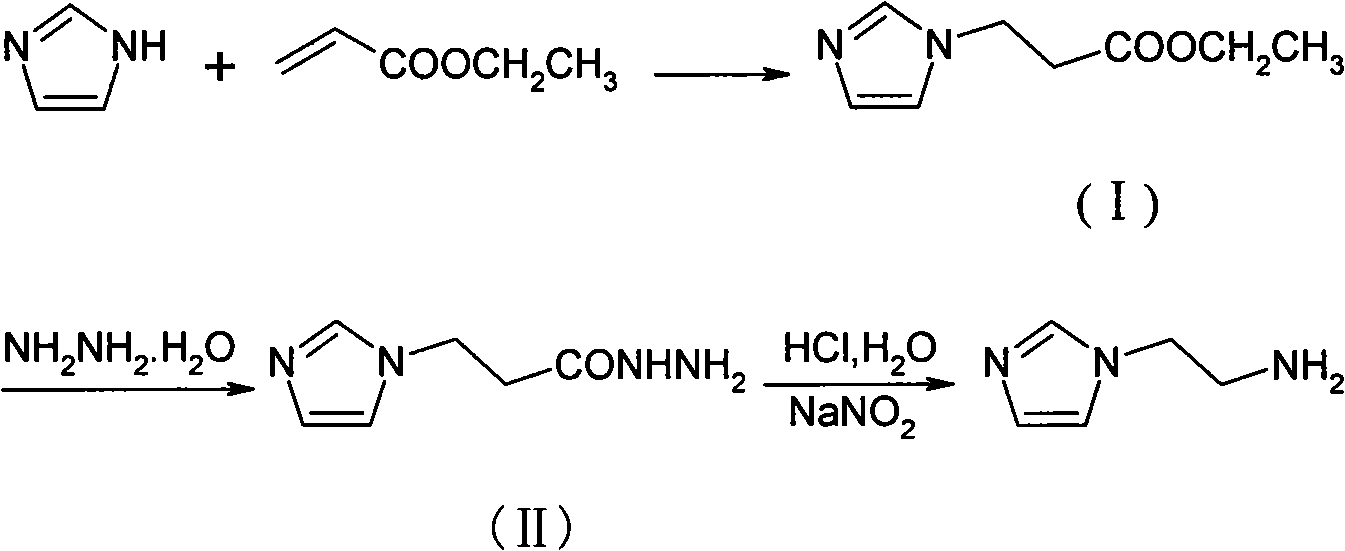
Method for synthesizing 2-(1-imidazol)ethylamine
Owner:HEFEI UNIV OF TECH
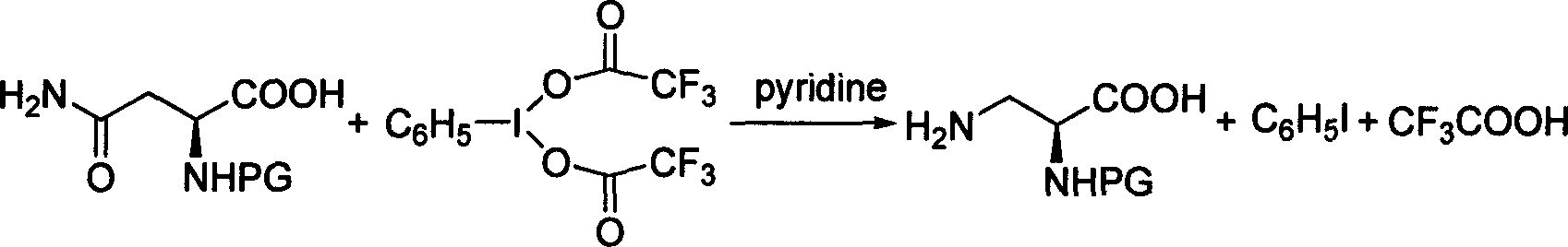
N alpha-Boc-N beta-Cbz-L-2,3 diaminopropionic acid derivative synthesis method
InactiveCN1865228AEasy to prepareHigh yieldOrganic compound preparationCarboxylic acid amides preparationEthyl chloroformateSynthesis methods
This invention relates method for synthesizing Nalpha-Boc protected ureido-alanine derivatives. Wherein: with p-toluenesulfonic acid as catalyst, conduct azeotropic dehydration of N-Boc-L-aspartate and paraformaldehyde in benzene or toluene to produce Nalpha-Boc-5-oxazolidinone; Nalpha-Boc-5-oxazolidinone reacts with ethyl chloroformate at -10--20Deg C for 20-30min under NaN3 effect to produce (S)-3-tertbutyloxycarbonyl-4-acetylazide-5-oxazolidinone which will produce corresponding isocyanate at 35-110Deg C through Curtius rearrangement, isocyanate and benzalcohol will produce (S)-3-tertbutyloxycarbonyl-4-benzyl oxylcarbony becan-5-oxazolidinone by alcoholysis, the obtained product conducts ring-opening by hydrolysis under basic condition to produce Nalpha-Boc-N beta-Cbz-L-2, 3-diaminopropionic acid derivant. This invention is characterized of simple process, and high yield.
Owner:WUHAN UNIV
Method for preparing ethyl trans-4-amino-cyclohexanecarboxylate hydrochloride
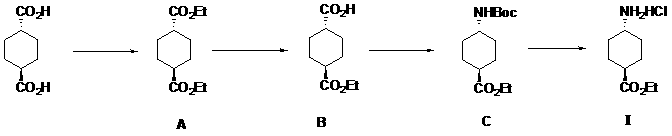
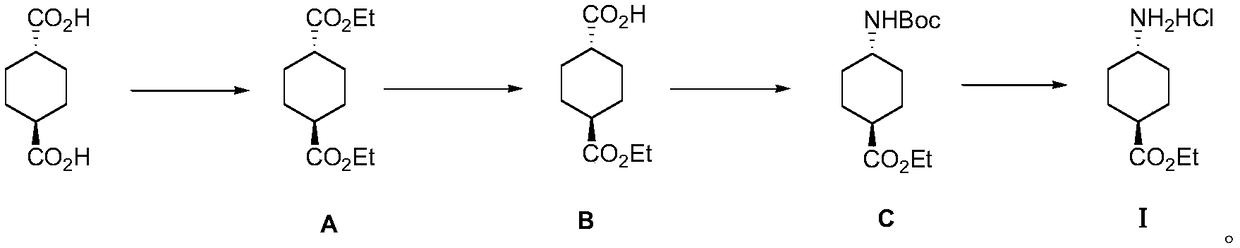
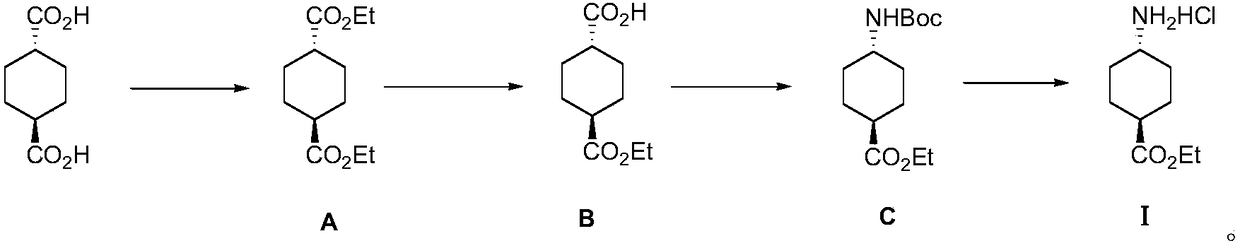
InactiveCN109280014AImprove efficiencyLow process equipment requirementsCarbamic acid derivatives preparationOrganic compound preparationEthyl acetateReaction step
The invention discloses a method for preparing ethyl trans-4-amino-cyclohexanecarboxylate hydrochloride, and belongs to the field of organic chemistry. Trans-1,4--cyclohexanedicarboxylic acid is usedas an initial material and reacts with anhydrous ethanol to produce intermediate diester A under the condition of a sulfuric acid catalyst, monoester is hydrolyzed by intermediate diester A to obtainintermediate monoacid B, the intermediate monoacid B and DPPA are subjected to Curtius rearrangement to obtain intermediate C, the intermediate C is deprotected by hydrogen chloride ethyl acetate solution to obtain final product ethyl trans-4-amino-cyclohexanecarboxylate hydrochloride I. The process adopted by the invention has the advantages of short reaction steps, cheap raw materials, mild reaction conditions and high yield, and is suitable for industrialized large-scale production.
Owner:CHENGDU BAISHIXING SCI & TECH IND
Novel preparation of fenspiride
The invention discloses a new method for preparing fenspiride. The method is characterized in that methanol is adopted as a solvent; 1-phenylethylamine and methyl acrylate as raw material are subjected to the Michae addition and the Deckman condensation to synthesizing 1-phenyl-4-piperidone raw material with high efficiency; and 1-phenyl-4-piperidone is subjected to the Reformatsky reaction and the Curtius rearrangement to obtain the fenspiride product. The method is economic, safe and environment friendly, and is suitable for the industrial production.
Owner:宁波保税区欣诺生物技术有限公司
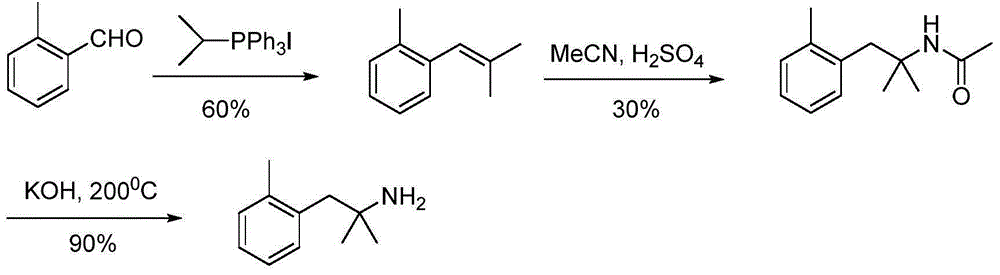
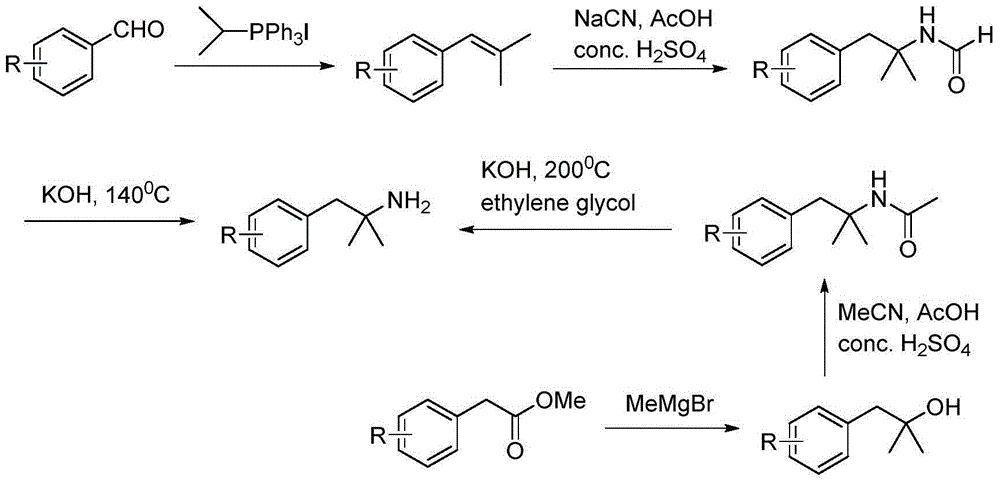
Method for preparing 2-methyl-1-substituted phenyl-2-propyl amine compound
InactiveCN105085278ATo overcome the shortcomings of low yieldReduce usageCarboxylic acid nitrile preparationOrganic compound preparationCurtius rearrangementMethyl group
The invention discloses a novel method for synthesizing a 2-methyl-1-substituted phenyl-2-propyl amine compound. The 2-methyl-1-substituted phenyl-2-propyl amine compound can be used for preparing beta2-adrenergic receptor stimulant drugs. Substituted benzyl halide is adopted as a raw material, and reacts with isobutyronitrile under the action of alkali, obtained 2-methyl-1-substituted phenyl-2-butyronitrile is subjected to hydrolysis, a Curtius rearrangement reaction and catalytic hydrogenation, and 2-methyl-1-substituted phenyl-2-propyl amine is generated. The method is easy and convenient to operate, and total yield is obviously improved.
Owner:CHANGZHOU UNIV
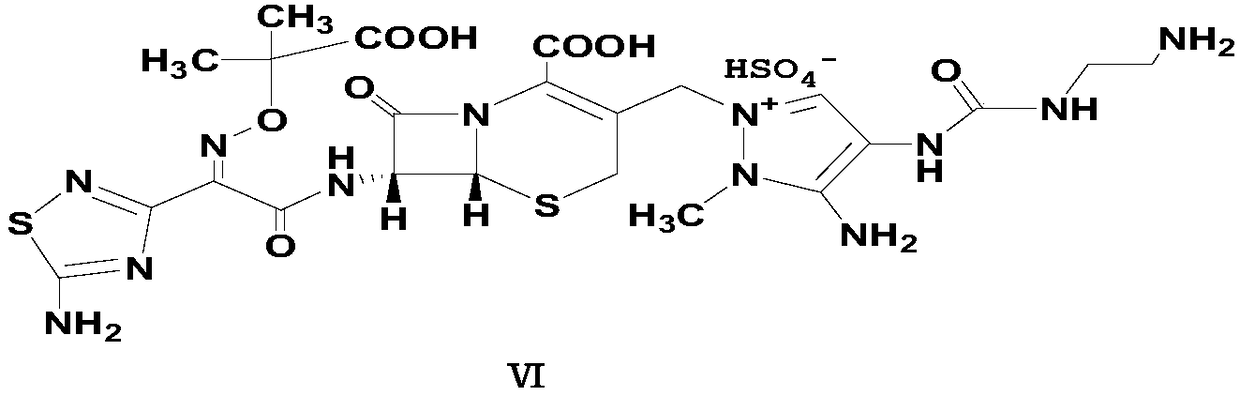
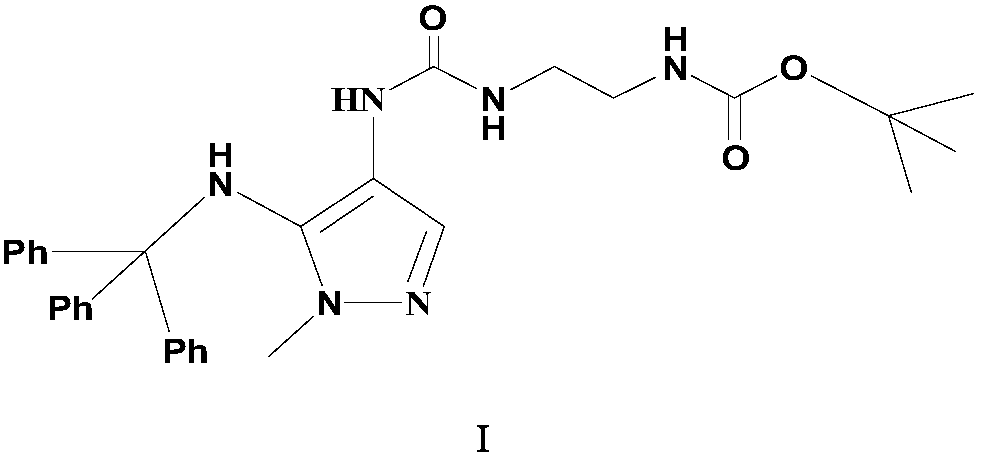
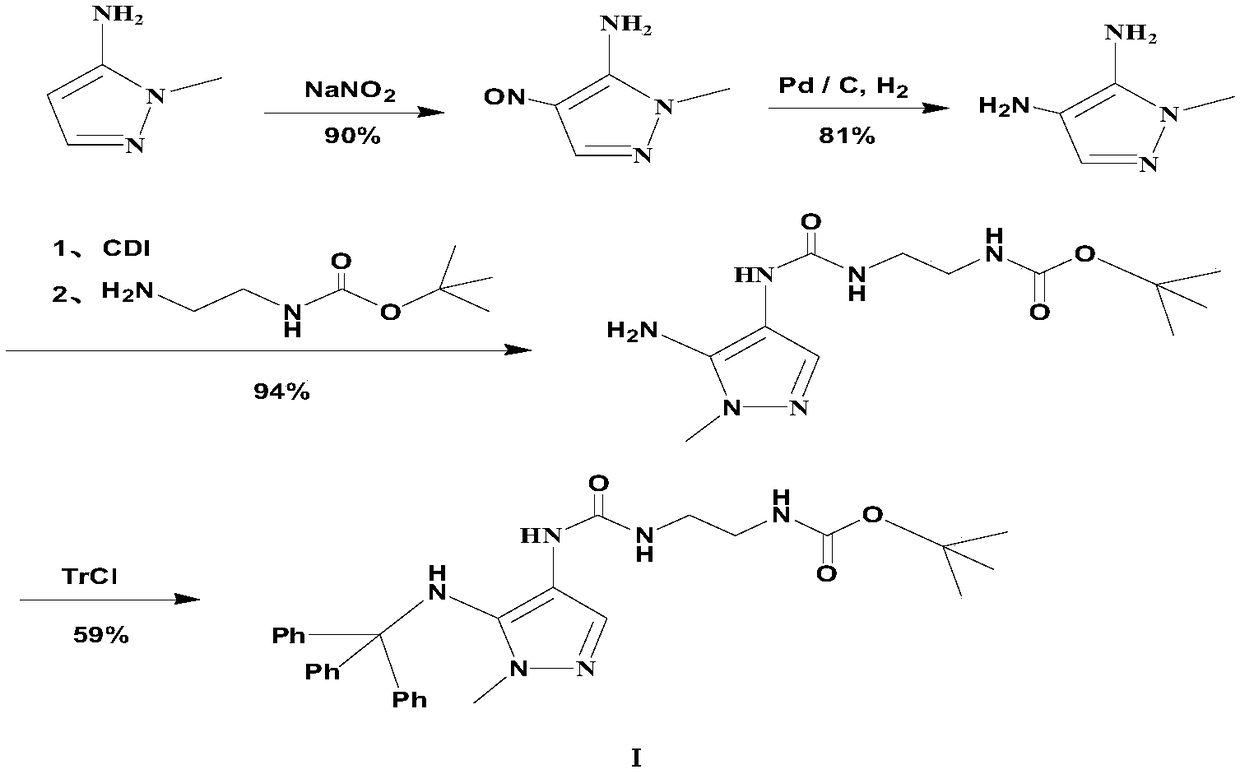
Preparation method of carbamyl amino pyrazol derived compound
The invention relates to a preparation method of a carbamyl amino pyrazol derived compound, belongs to the technical field of drug intermediates and aims to solve the problem that an existing synthetic route is complicated and is uneasy to operate. The preparation method of the carbamyl amino pyrazol derived compound comprises the steps that in the presence of an acid-binding agent, a compound shown in a formula II and triphenyl halomethane perform condensation reaction in an organic solvent to obtain an intermediate product compound shown in a formula III; then, in the presence of inorganic base, the compound shown in the formula III is subjected to ester hydrolysis reaction, and a compound shown in a formula IV is obtained; the compound and trinitride perform Curtius rearrangement reaction to obtain a compound shown in a formula V; the compound shown in the formula V and BoxEDA perform condensation reaction to obtain the carbamyl amino pyrazol derived compound. The preparation methodhas the advantages that raw materials are easy to obtain, a reaction route operation is simple, and the product yield and purity are high.
Owner:浙江东邦药业有限公司
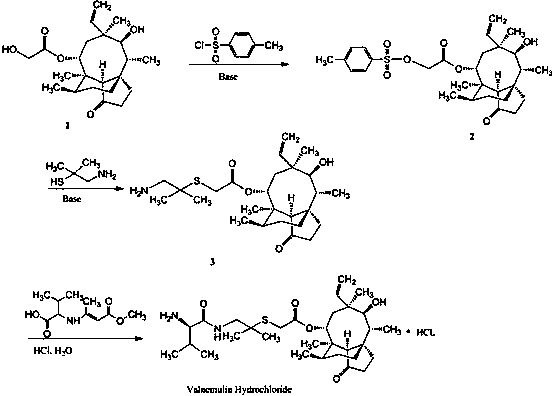
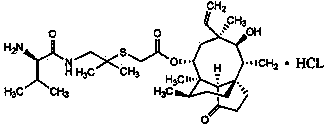
Synthesis of intermediate compound of warnemulin hydrochloride and preparation method of warnemulin hydrochloride
InactiveCN106496085BOrganic compound preparationSulfonic acid esters preparationChemical structurePropylamine
The invention discloses an intermediate compound used for synthesizing valnemulin hydrochloride. The chemical structure of the intermediate compound used for synthesizing valnemulin hydrochloride is represented by a formula in the invention. A preparation method of the intermediate compound comprises following steps: beta-hydroxyisovaleric acid is subjected to Curtius rearrangement reaction so as to obtain 2-methyl-2-hydroxy propylamine; 2-methyl-2-hydroxy propylamine and D-valine Dane salt are subjected to mixed anhydride method reaction so as to obtain an amide product; the amide product is subjected to hydroxy activation under alkaline conditions with methylsulfonyl chloride, and then is reacted with potassium thioacetate. The preparation method of valnemulin hydrochloride comprises following steps: pleuromutilin is reacted with paratoluensulfonyl chloride so as to obtain pleuromutilin p-tosylate; pleuromutilin p-tosylate is reacted with the intermediate compound, and hydrochloric acid deprotection is carried out so as to obtain valnemulin hydrochloride. In the preparation method of valnemulin hydrochloride, no dimethyl cysteamine hydrochloride is needed, production conditions are mild, no environment pollution is caused, and the preparation method is suitable for industrialized production.
Owner:盐城市优化医药化工科技有限公司
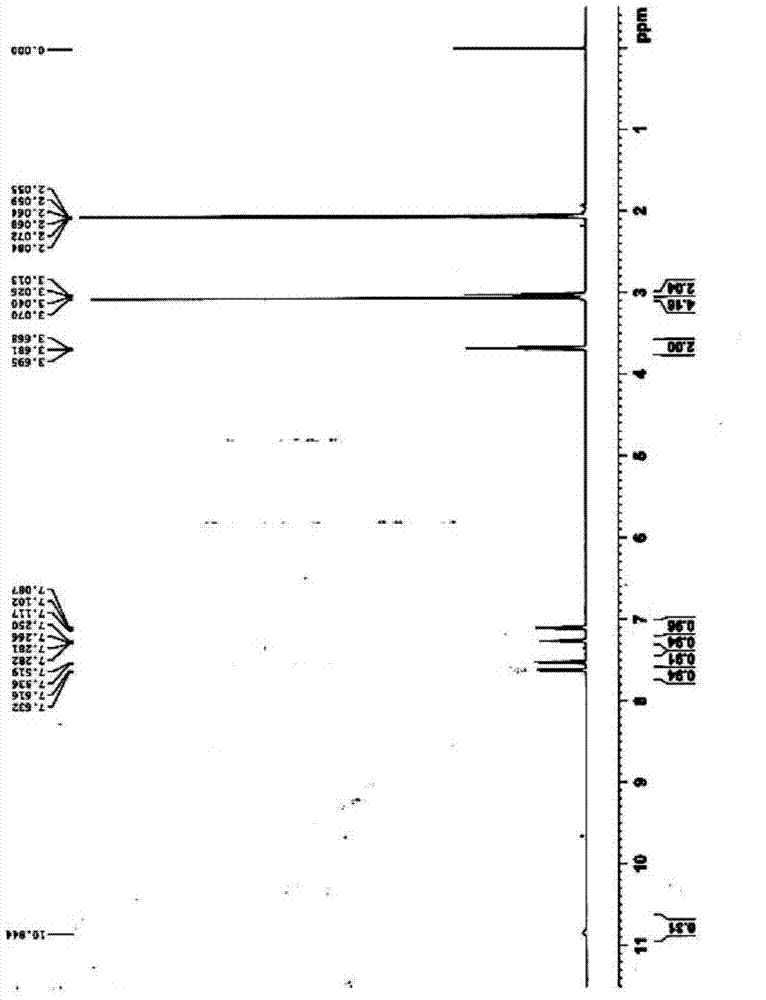
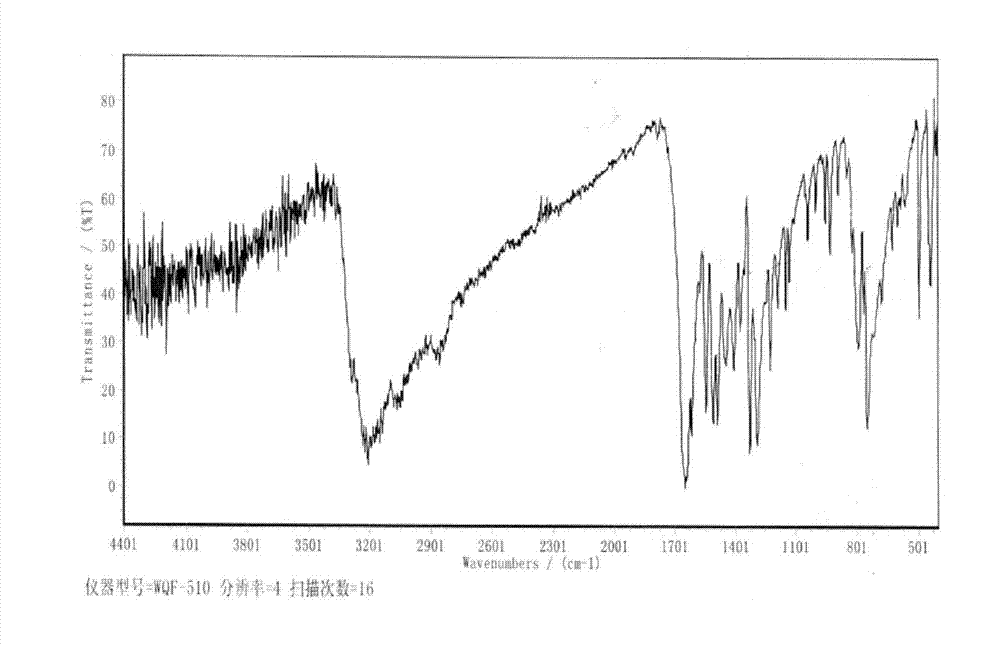
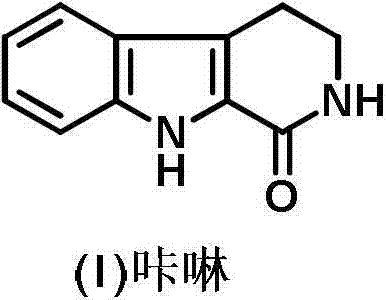
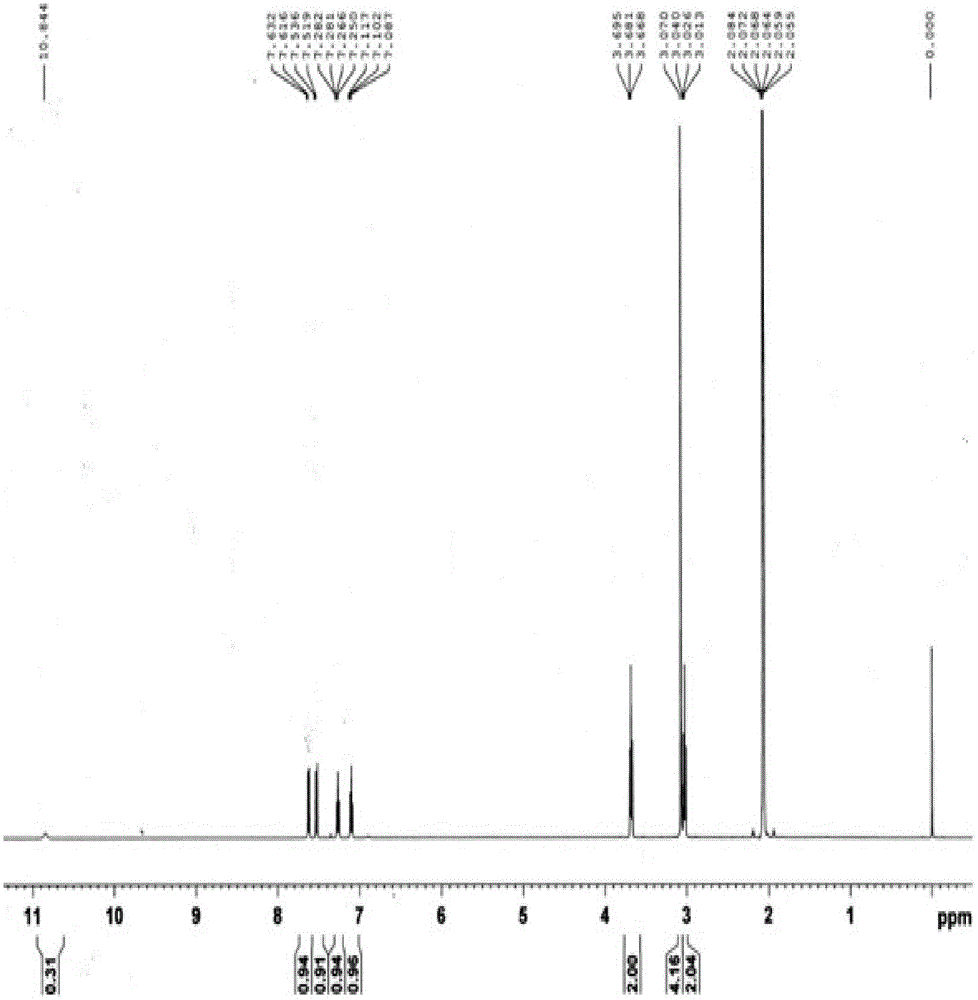
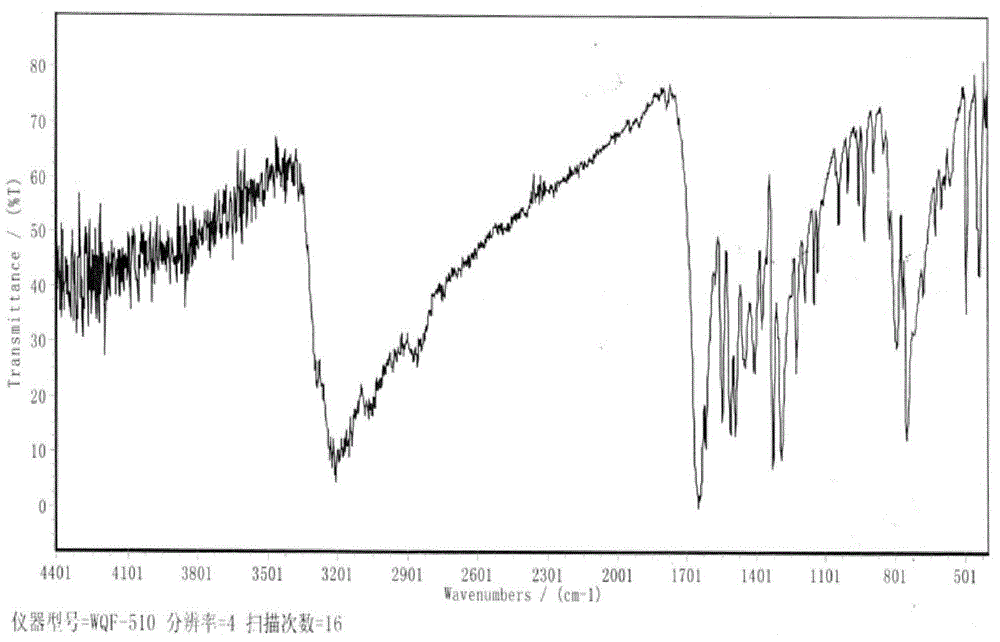
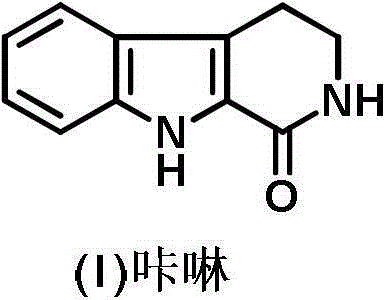
Preparation method of 2, 3, 4, 9-tetrahydro-beta-carboline-1-one
ActiveCN103319480AMild reaction conditionsSimple and fast operationOrganic chemistryPropanoic acidChloroformate
A preparation method of 2, 3, 4, 9-tetrahydro-beta-carboline-1-one comprises: chloridizing indole-3-propionic acid (III) with a chloridizing agent in an aprotic solvent to prepare indole-3- propionyl chloride (IV), or carrying out condensation reaction between indole-3-propionic acid (III) and chloro-formate in an aprotic solvent under catalysis of an acid binding agent to prepare an active mixed acid anhydride (V); then carrying out an azidation reaction with an azidation reagent to prepare indolepropionyl azide (VI); carrying out a Curtius rearrangement reaction to prepare indoleethyl isocyanate(VII); and finally preparing 2, 3, 4, 9-tetrahydro-beta-carbolin-1-one through a cyclization reaction under catalysis of an acid catalyst. The method is mild in reaction conditions, simple to operate, less in pollution, cheap and accessible in raw materials, simple in technology, high in yield, and ingenious in technology design; and the azidation reaction, Curtius rearrangement and the cyclization are carried out by one-pot synthesis; and in the reaction process, there are not complex operations such as column chromatography, repeated recrystallization and the like, and the technology scaling-up is easy to operate.
Owner:CHANGZHOU YABANG QH PHARMACHEM +1
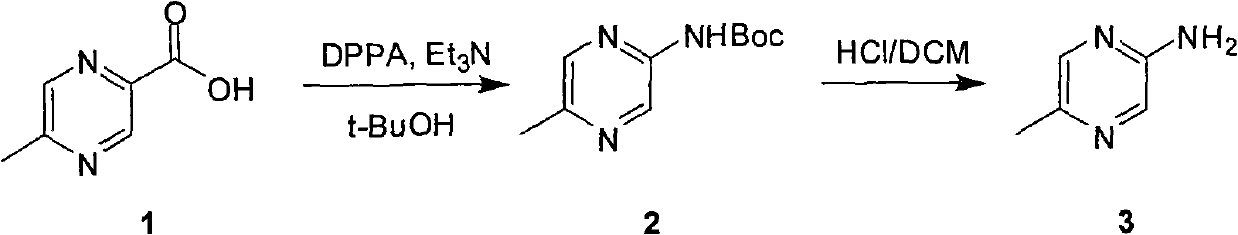
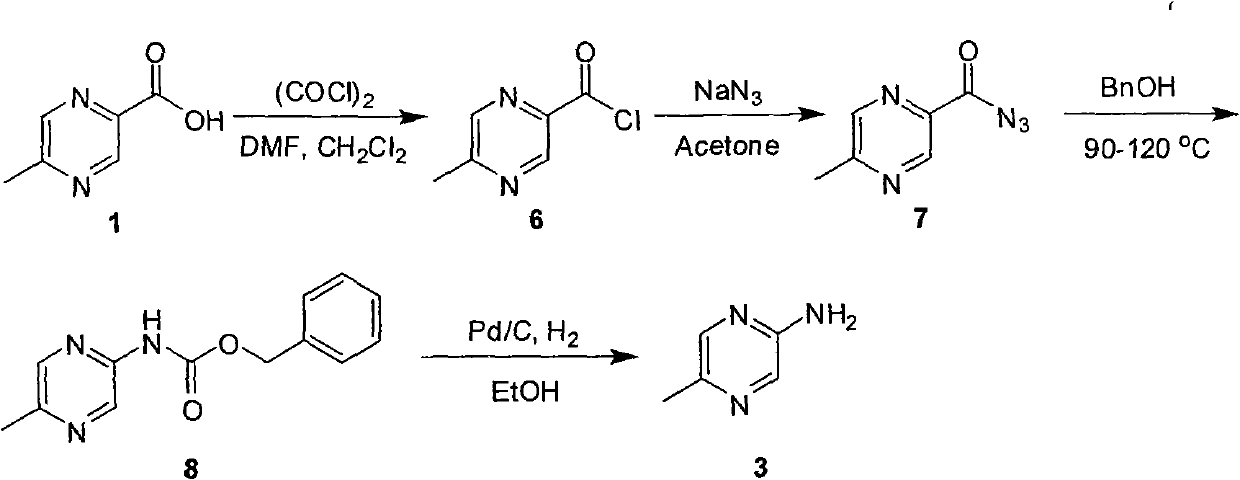
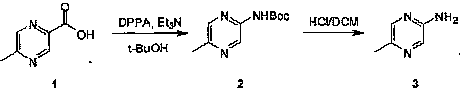
Industrial preparation method of 5-methylpyrazin-2-amine
InactiveCN101857575AReasonable choice of reaction processEasy to controlOrganic chemistryState of artChemical reaction
The invention relates to an industrial preparation method of 5-methylpyrazin-2-amine. In the invention, the 5-methylpyrazin-2-amine is prepared from general and easily obtained 5-methyl-2-pyrazinecarboxylic acid by the steps of azidation, Curtius rearrangement and Boc desorption. The chemical reaction formula is shown in the specification. The invention solves the problems of high cost, low yield, environment pollution and the like in the prior art, does not need column chromatography for purification, and can realize large-scale industrial production.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +1
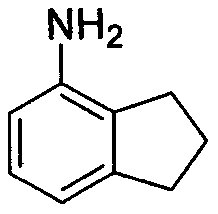
New preparation method of 4-aminoindan compound
InactiveCN110105222AAvoid problems such as separation difficultiesEasy to operateOxygen-containing compound preparationCarbamic acid derivatives preparationReduction eliminationHydrogenation reaction
The invention relates to a new preparation method of a 4-aminoindan compound. 4-Aminoindan is an important medical intermediate. With carboxybenzaldehyde as an initial raw material, through esterification, condensation, cyclization, reduction elimination, Curtius rearrangement, an amidogen deprotection reaction and a hydrogenation reaction in sequence, the 4-aminoindan is prepared. According to the method, the problems of difficult generation of isomers, difficult separation of substitution products and the like in a traditional method are solved, the whole preparation method is convenient toimplement, the yield is high, and the method has great industrial prospects.
Owner:NANKAI UNIV
Preparation method of symmetric urea compound
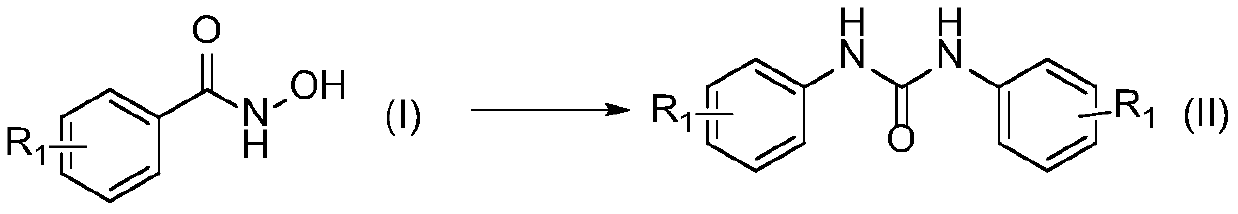
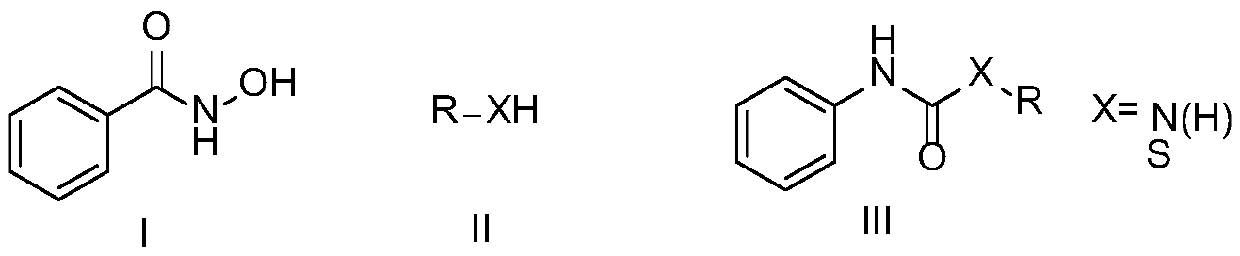
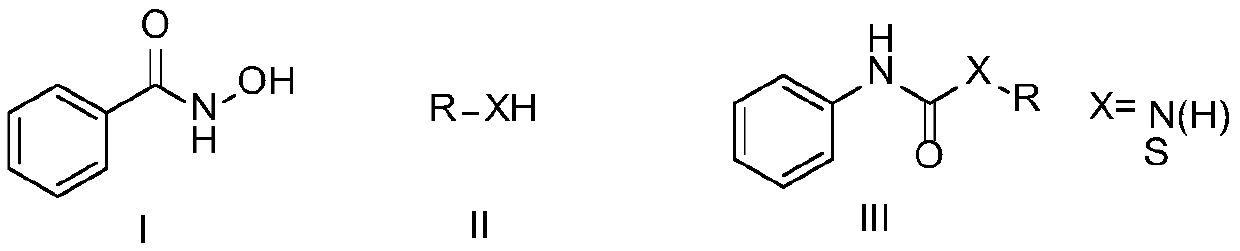
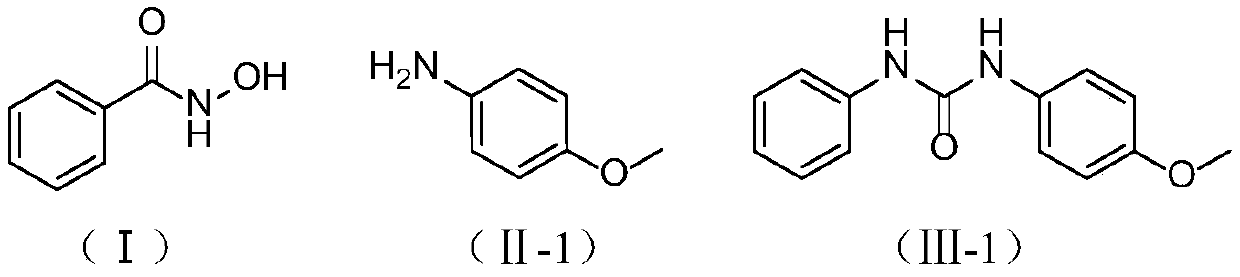
ActiveCN111116420ACheap and easy to usePromote generationUrea derivatives preparationOrganic compound preparationHydroxylamineHydroxamic acid
The invention discloses a preparation method of a symmetric urea compound, which comprises the following steps: by using a hydroxamic acid compound as a raw material, sequentially adding an alkali anda solvent, reacting at 25-50 DEG C for 1-7 hours in an SO2F2 atmosphere, and carrying out aftertreatment on the reaction solution to obtain the symmetric urea compound. According to the invention, cheap, easily available and environment-friendly SO2F2 is used as an accelerant to efficiently promote the generation of an isocyanate intermediate to form a C-N bond. The generation of isocyanate avoids the use of a large amount of halogen or azide dangerous reagents, so that the compound can be used as a green substitute for standard treatment conditions in Curtius rearrangement and Hofmann rearrangement reactions. The amine source in the final product only comes from hydroxylamine, and no additional amine needs to be added, so that the substrate is wide in applicability, and the correspondingsymmetric urea compound can be obtained at a relatively good yield. The operation process is simple, the aftertreatment only needs filtering, and the method is suitable for large-scale preparation.
Owner:ZHEJIANG UNIV OF TECH
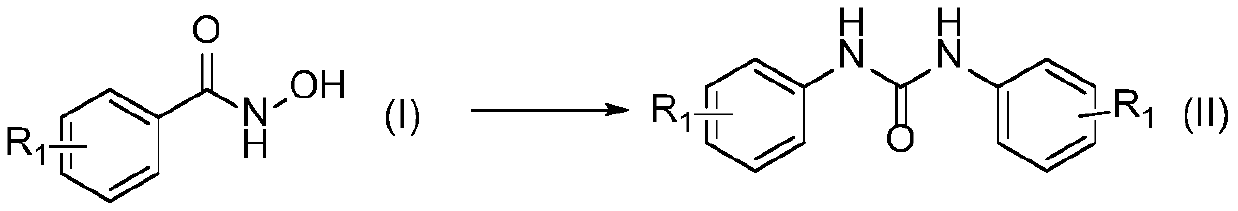
Preparation method of amide derivative
ActiveCN111116421ACheap and easy to usePromote generationUrea derivatives preparationOrganic compound preparationThiocarbamateCarbamate
The invention provides a preparation method of an amide derivative. The method comprises the following steps: taking hydroxamic acid and an amine or thiol compound as raw materials, sequentially adding alkali and a solvent, carrying out a reaction for 2-7 hours at the temperature of 25-50 DEG C in an SO2F2 atmosphere, and after the reaction is finished, carrying out aftertreatment on a reaction solution to obtain the asymmetric urea compound or thiocarbamate compound. According to the invention, cheap, easily available and environment-friendly SO2F2 is used as an accelerant to efficiently promote the generation of isocyanate intermediates and form C-N and C-S bonds. The generation of isocyanate avoids the use of a large amount of halogen or azide dangerous reagents, so that the compound can be used as a green substitute for standard treatment conditions in Curtius rearrangement and Hofmann rearrangement reactions. The substrate is wide in applicability, and the corresponding asymmetricurea and thiocarbamate compounds can be obtained at a relatively good yield. The operation process is simple and suitable for large-scale preparation.
Owner:ZHEJIANG UNIV OF TECH
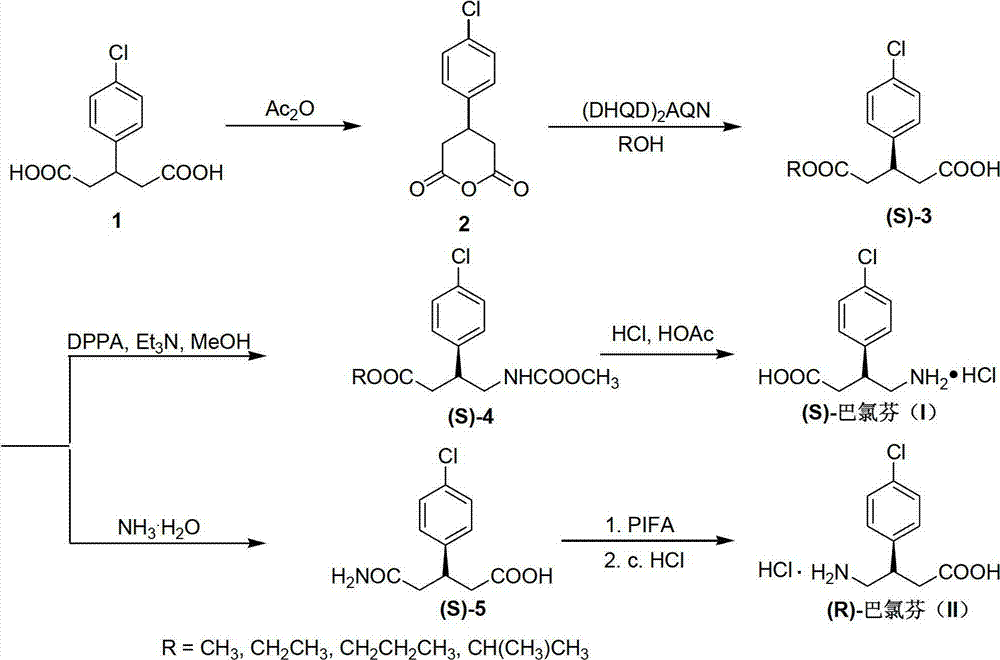
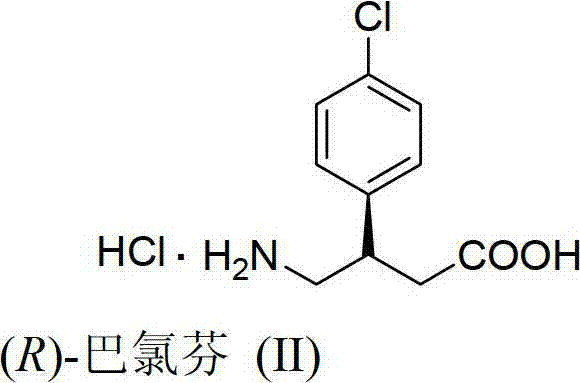
Preparation method of chiral baclofen
ActiveCN102863347AHigh selectivityOrganic compound preparationAmino-carboxyl compound preparationGlutaric anhydrideGlutaric acid
The invention relates to a preparation method of chiral baclofen, belonging to the field of synthesis of chiral compounds. The synthetic route of the method comprises the following steps: condensing the initial raw material 3-(4-chlorphenyl)glutaric acid to obtain 3-(4-chlorphenyl)glutaric anhydride; preparing the 3-(4-chlorphenyl)glutaric anhydride into a key intermediate (S)-3-(4-chlorphenyl)monoglutarate under the action of a chiral catalyst; and carrying out Curtius rearrangement (or Hofmann rearrangement) reaction to obtain the chiral baclofen. The method has the advantages of short reaction steps and is simple to operate; and the product has the advantages of high ee value, low cost and high yield.
Owner:孟坤
Method for synthesizing 2-(1-imidazol)ethylamine
The invention provides a method for synthesizing 2-(1-imidazol)ethylamine, which uses imidazole and ethyl acrylate as raw material, and includes steps of preparing intermediate (1) ethyl 2-(1-imidazol)propionate from the raw material through Michel addition reaction; preparing intermediate (2) 2-(1-imidazol)propionohydrazide from the intermediate (1) through hydrazinolysis reaction; and obtainingthe object product 2-(1-imidazol)ethylamine from the intermediate (2) through Curtius rearrangement reaction. The invention has total yield of 72% by imidazole, stable reaction, mild condition, and convenient operation and control.
Owner:HEFEI UNIV OF TECH
Polyethylene glycol omega-amino acid and preparation method thereof
InactiveCN101891886ANovel structureEasy to operateCarbamic acid derivatives preparationOrganic compound preparationEnd-groupPolyethylene glycol
The invention belongs to the technical field of macromolecular synthetic chemistry, and particularly relates to polyethylene glycol omega-amino acid and a preparation method thereof. The method comprises the following steps of: oxidizing hydroxys at two ends of polyethylene glycol into carboxyls, loading the polyethylene glycol of which the end groups are the carboxyls to resin by nucleophilic substitution reaction, and protecting the free carboxyls through methyl ester; and cracking the double-carboxyl polyethylene glycol of which one end is protected by the methyl ester from the resin, and then modifying the free carboxyls by azidation reaction and Curtius rearrangement reaction to obtain tert-butyloxycarbonyl protected amino. The method overcomes the defect that the conventional method for synthesizing the polyethylene glycol omega-amino acid has complex separation and purification. Because of the adoption of solid-phase reaction, azidation reaction and Curtius rearrangement reaction, the method has the advantages of mild condition, short synthetic route and simple separation and purification, and the yield reaches over 70 percent. The polyethylene glycol omega-amino acid can be widely applied to the fields of targeted medicament synthesis, biological sensing, immunoassay, polypeptide synthesis and the like.
Owner:CENT SOUTH UNIV
Method for synthesizing 3-amino-1-adamantanol
InactiveCN101747212BEasy to operateHigh yieldOrganic compound preparationAmino-hyroxy compound preparationBromateCurtius rearrangement
The invention discloses a method for synthesizing 3-amino-1-adamantanol. The structural formula of the 3-amino-1-adamantanol is as follows. Using adamantanecarboxylic acid as starting material, the method synthesizes the 3-amino-1-adamantanol according to the following reaction steps: bromate 3-amino-1-adamantanol is synthesized by way of bromination, modified curtius rearrangement and hydrolyzation, and finally, bromate is removed by a alkaline liquor treatment method, so that the 3-amino-1-adamantanol is obtained. The invention has the advantages of simple and easy operation, available materials, less reaction steps and high product yield.
Owner:GUANGDONG UNIV OF TECH
Preparation method of beta-homoglutamic acid
InactiveCN111825563ACarbamic acid derivatives preparationOrganic compound preparationSimple Organic CompoundsCurtius rearrangement
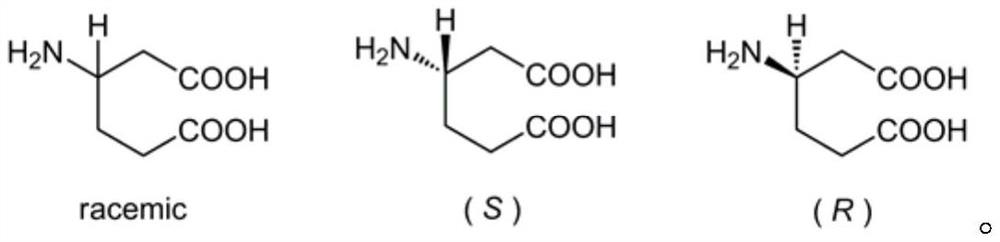
The invention belongs to the field of preparation of organic compounds, and provides a preparation method of beta-homoglutamic acid. The method is characterized in that: 3-cyclohexenecarboxylic acid is used as a raw material, beta-homoglutamic acid is synthesized at a high yield through three the steps of reactions of Curtius rearrangement, olefin oxidation and protection group removal, and beta-homoglutamic acid with high yield and high optical purity can be obtained through configuration retention by using optically pure 3-cyclohexenecarboxylic acid. The method has the advantages of easily available raw materials, mild reaction conditions and low cost, and is suitable for large-scale preparation of beta-homoglutamic acid.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
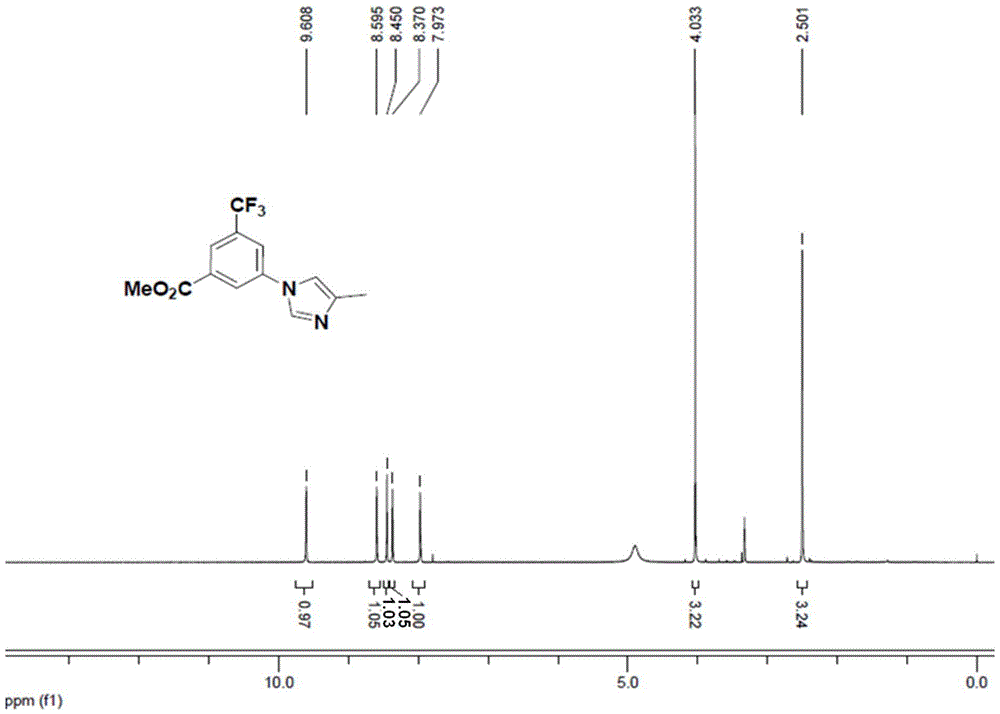
Preparation method of nilotinib intermediate
ActiveCN105985293AIncrease profitNo chromatographic purification requiredOrganic chemistryBenzoic acidCurtius rearrangement
The invention relates to a preparation method of a nilotinib intermediate, namely, 3-(trifluoromethyl)-5-(4-methyl-1H-imidazole-1-yl)aniline (I). The preparation method comprises the steps that 3-(trifluoromethyl)-5-(4-methyl-1H-imidazole-1-yl)benzoic acid (VII) is subjected to Curtius rearrangement to generate a by-product compound (III) and then subjected to further reacting to be converted into 3-(trifluoromethyl)-5-(4-methyl-1H-imidazole-1-yl)aniline (I). The preparation method has the advantages of being high in utilization rate of the raw materials and suitable for industrialized operation. Please see the formula in the description.
Owner:ESTEVE HUAYI PHARMA
A method for preparing 2,3,4,9-tetrahydro-β-carbolin-1-one
ActiveCN103319480BMild reaction conditionsSimple and fast operationOrganic chemistryPropanoic acidChloroformate
A preparation method of 2, 3, 4, 9-tetrahydro-beta-carboline-1-one comprises: chloridizing indole-3-propionic acid (III) with a chloridizing agent in an aprotic solvent to prepare indole-3- propionyl chloride (IV), or carrying out condensation reaction between indole-3-propionic acid (III) and chloro-formate in an aprotic solvent under catalysis of an acid binding agent to prepare an active mixed acid anhydride (V); then carrying out an azidation reaction with an azidation reagent to prepare indolepropionyl azide (VI); carrying out a Curtius rearrangement reaction to prepare indoleethyl isocyanate(VII); and finally preparing 2, 3, 4, 9-tetrahydro-beta-carbolin-1-one through a cyclization reaction under catalysis of an acid catalyst. The method is mild in reaction conditions, simple to operate, less in pollution, cheap and accessible in raw materials, simple in technology, high in yield, and ingenious in technology design; and the azidation reaction, Curtius rearrangement and the cyclization are carried out by one-pot synthesis; and in the reaction process, there are not complex operations such as column chromatography, repeated recrystallization and the like, and the technology scaling-up is easy to operate.
Owner:CHANGZHOU YABANG QH PHARMACHEM +1
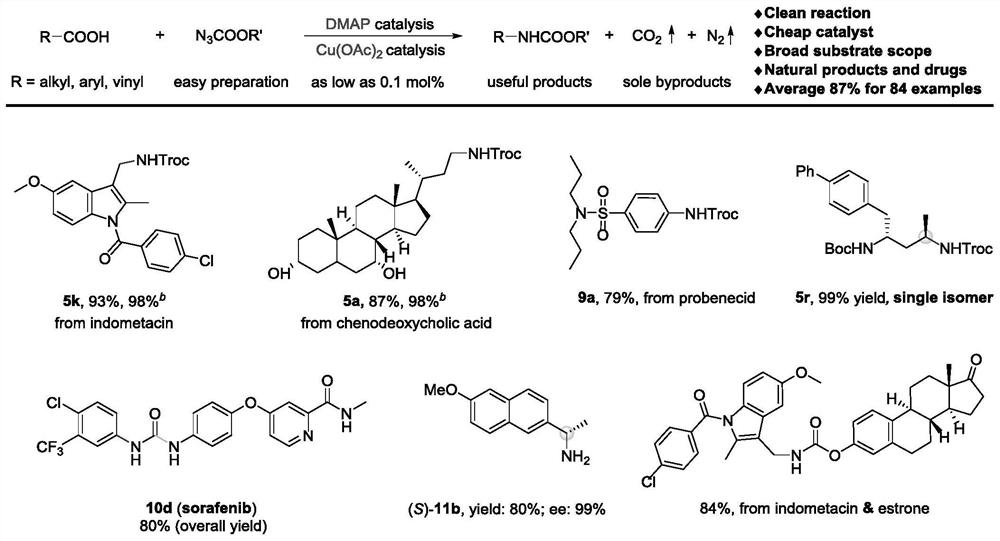
Method for preparing amine compounds based on novel catalytic curtius rearrangement reaction
ActiveCN112028814BEfficientStereospecificCarbamic acid derivatives preparationOrganic compound preparationAlkaneAcyl group
The invention prepares amine compounds based on novel catalytic Curtius rearrangement reaction. Transition metal catalyzed sp 2 C‑N bond formation is an efficient method for the synthesis of arylamines, catalyzing sp 3 The coupling reaction of C-N bond is also reported from time to time, but at the same time, sp 2 and sp 3 Methods for C‑N bond generation are relatively underdeveloped. The present invention uses resource-rich organic carboxylic acids as carbon sources and easily prepared alkane / aryloxyacyl azides as nitrogen sources, at as low as 0.1 mol% of DMAP and Cu(OAc) 2 Under catalysis, with gas N 2 and CO 2 One-pot generation of protected alkyl, alkenyl, and aryl amines as the only by-product. The reaction can be applied to the late functionalization of natural products and drug molecules, the synthesis of chiral alkylamines, and the rapid construction of different ureas and primary amines. Mechanistic studies reveal that the reaction proceeds through a cascade of carboxylic acid activation, azidation, Curtius rearrangement, and nucleophilic addition.
Owner:NANJING UNIV
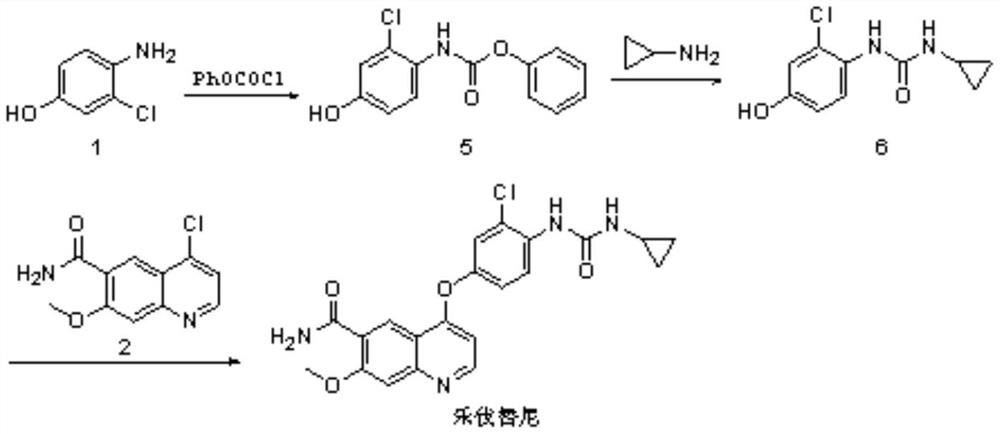
Synthesis method of lenvatinib
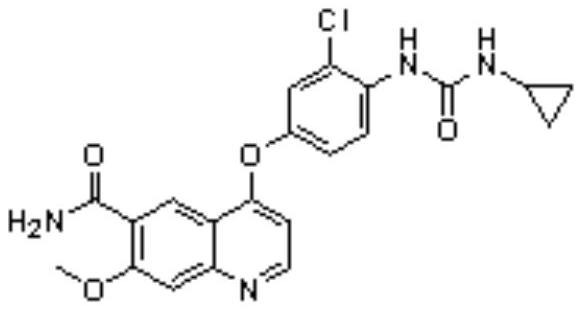
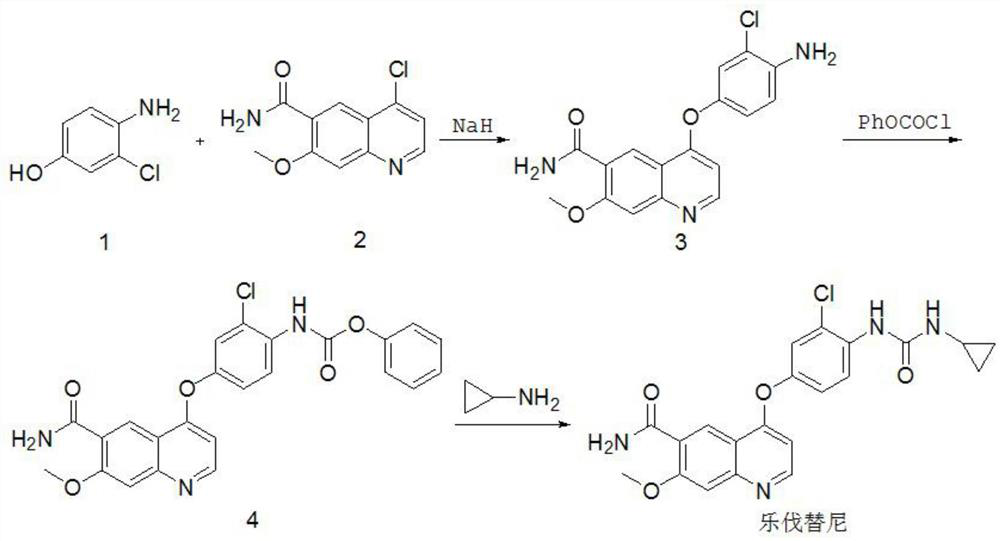
The invention relates to a systhesis method of lenvatinib. The method comprises the following steps: by taking 2-chloro-4-methyl hydroxybenzoate and 4-chloro-7-methoxyquinoline-6-amide as starting raw materials, carrying out substitution reaction to obtain 4-[3-chloro-4-methoxycarbonyl phenoxy]-7-methoxy-6-quinolinecarboxamide, carrying out an alkaline hydrolysis reaction to obtain 4-(3-chloro-4-carboxyphenoxy)-7-methoxy-6-quinolinecarboxamide, carrying out a Curtius rearrangement reaction on the 4-(3-chloro-4-carboxyphenoxy)-7-methoxy-6-quinolinecarboxamide and diphenyl azide phosphate (DPPA), and carrying out a reaction on the obtained product and cyclopropylamine to obtain lenvatinib through a one-pot method. The invention provides a novel method for synthesizing lenvatinib. The method has the advantages of simple reaction steps, simple and easily available raw materials, simple operation and low production cost.
Owner:SHANDONG HUIHAI PHARMA & CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
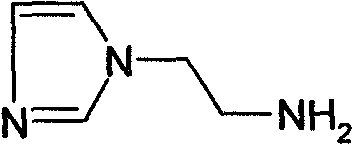
![2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof 2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/b07f705c-432f-427f-a9be-d769ee27676d/BDA0001239259360000021.png)
![2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof 2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/b07f705c-432f-427f-a9be-d769ee27676d/BDA0001239259360000031.png)
![2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof 2',3'-dihydrospiro[cyclopropane-1,1'-indene]-2-amine derivative as well as preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/b07f705c-432f-427f-a9be-d769ee27676d/BDA0001239259360000041.png)
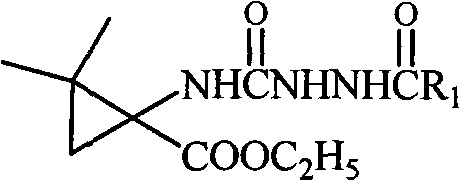
![Method for synthesizing 2-(7-(benzyloxy)benzo[d][1, 3]dioxazole-5-yl)ethylamine and hydrochloride thereof Method for synthesizing 2-(7-(benzyloxy)benzo[d][1, 3]dioxazole-5-yl)ethylamine and hydrochloride thereof](https://images-eureka.patsnap.com/patent_img/bd150bd1-e948-4925-87d6-817031e76831/BSA00000698621900011.PNG)
![Method for synthesizing 2-(7-(benzyloxy)benzo[d][1, 3]dioxazole-5-yl)ethylamine and hydrochloride thereof Method for synthesizing 2-(7-(benzyloxy)benzo[d][1, 3]dioxazole-5-yl)ethylamine and hydrochloride thereof](https://images-eureka.patsnap.com/patent_img/bd150bd1-e948-4925-87d6-817031e76831/BSA00000698621900021.PNG)
![Method for synthesizing 2-(7-(benzyloxy)benzo[d][1, 3]dioxazole-5-yl)ethylamine and hydrochloride thereof Method for synthesizing 2-(7-(benzyloxy)benzo[d][1, 3]dioxazole-5-yl)ethylamine and hydrochloride thereof](https://images-eureka.patsnap.com/patent_img/bd150bd1-e948-4925-87d6-817031e76831/BSA00000698621900022.PNG)